Chapter 15
Actuators
An actuator typically is a laminate which is composed of (1):
The polymer matrix is sometimes referred to as a solid electrolyte polymer layer. The first and the second electrode may be formed of a conductive polymer such as poly(pyrrole) (PPY), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(aniline) (PANI), poly(acetylene), poly(p-phenylene), poly(thiophene), poly(p-phenylene vinylene), or poly(thienylene vinylene) (1). The basic design of an actuator is like that of a plate condenser, as shown in Figure 15.1.
Figure 15.1 Basic design of an actuator

The electrodes may be formed of a conductive polymer such as PPY, PEDOT, PANI, poly(acetylene), poly(p-phenylene), poly(thiophene), poly(p-phenylene vinylene), or poly(thienylene vinylene). The polymer may be a crosslinked poly(vinylidene fluoride) (PVDF)-based polymer alone or in combination with a non-cross-linked PVDF-based polymer (1,2). The radical initiator includes dicumyl peroxide and dibenzoyl peroxide, with amine accelerators such as methylenediamine, ethylenediamine, isopropylethylenediamine, 1,3-phenylenediamine, 1,5-naphthalenediamine, or 2,4,4-trimethyl-1,6-hexanediamine.
The mechanistic bending model of a three-layer polymer actuator is shown in Figure 15.2.
Figure 15.2 Mechanistic bending model (3)

The normal strains in the different symmetric layers are assumed to be distributed linearly and continuously and have different constant slopes (3). More sophisticated models and designs have been developed and are explained in other sections.
The operating principle of a polymer actuator is as follows (1): As a voltage is applied across the electrodes, the solid electrolyte polymer layer is oxidized, and charged with positive charges. In addition, negative charges in the solid electrolyte polymer layer migrate towards the first electrode or the second electrode. For this reason, the solid electrolyte polymer layer swells and bends so that the polymer actuator starts to operate. The direction in which the polymer actuator bends may be selectively varied according to the direction in which a voltage is applied.
15.1 Mathematical Model
The basic principle of a dielectric elastomer actuator is shown in Figure 15.3. The device is basically an electric capacitor filled with the polymer.
Figure 15.3 Basic principle of a dielectric elastomer actuator (4)
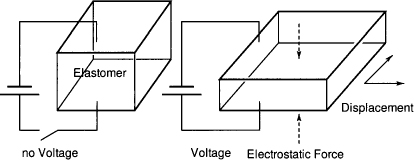
Only a few mathematical models that describe the action of electrostatic actuators are available.
An early model by Pelrine assumes the conservation of the volume of the elastomer membrane with a Poisson’s ratio of 0.5 (5). Assuming equal strain in the orthogonal directions by the voltage the strain, parallel to the strain can be related to the electrostatic strain. Details are beyond of the scope of this text can be found in the original literature (5). In addition, the performance of various dielectric polymers have been measured. The results are collected in Table 15.1.
Table 15.1 Performance of dielectric polymers (5)

From Table 15.1 is obvious that silicone polymers exhibit the highest strain performance of 32%. In contrast, a polyurethane polymer shows the highest actuation pressures of 1.9 MPa.
In addition, Hooke’s law can be used to relate the strains caused by the applied voltage (6). The overall stress Tzz perpendicular to the plain by an applied electric field E is:
(15.1) ![]()
Here, a1 and a2 are experimentally determined coefficients that characterize the response of the film to an applied electric field. Similarly, the stresses parallel to the plain are:
(15.2) ![]()
Using Young’s modulus Y and Poisson’s ratio v, the strain Szz can be calculated as:
(15.3) ![]()
The lateral strains are given as:
(15.4) ![]()
Using these equations, the model has been tested by experiments with a 24 factorial design. The factors were silicone type, silicone thickness, electrode thickness, and applied electric field. The measured quantity was the strain. The most significant factor turned out to be the thickness of the polymer (6).
A multilayer bending model has been presented that can describe actuators which are composed of an arbitrary number of polymer layers. In this model parameters like bending curvature, strain, stress, and work density are introduced as explicit functions of the thickness and modulus of each individual layer (7).
Further, theoretical models for use in numerical methods, such as finite elements (8,9), for the prediction of the behavior of electroactive polymer actuators has been presented (10). Another model of this type uses an array of masses coupled by an electrostatic force. The motion is governed by Hooke’s law. The model allows the prediction of the response of actuators of various sizes (4).
15.2 Fields of Application and Special Designs
Actually, the principle of actuators has been used in living organisms for a long time. For example, myosin motor proteins are responsible for muscular contraction and enable motion on a macroscopic scale (11).
Only recent research has been focussed on synthetic stimuli-responsive polymers. Some recent developments in actuator materials based on stimuli-responsive polymers have been reviewed (12). Stimuli-responsive polymers are suitable for actuators and active elements in microfluidic devices.
A polymer chain can reversibly change its conformation depending on a particular state in its environment. An electroresponsive actuator was designed by a diblock copolymer of a positively charged dendrimer and a negatively charged linear chain (13).
Another concept is the one side modification of atomic force microscopy cantilevers with stimuli-responsive polymer brushes. When these brushes are swelling, e.g., by some analyte, the cantilever is bent. This can be detected by deflection of a laser beam (14).
In biomedical applications, polymer actuators find use for micro-valves, cell biology, and microrobotics (15). Hydrogel actuators have been integrated into a microfluidic system that serves as a container for a liquid droplet. The hydrogel reacts to stimuli by adjustment of the shape (16).
A reversible swelling and deswelling of a hydrogel results in changes of the periodicity of its lattice that causes a change in its optical properties. Mechanical treatment, temperature, light, magnetic and electrical fields are stimuli for switching the optical properties (12).
Besides single polymer actuators, grafted polymer layers and hydrogels, liquid crystals and shape-memory polymers are of interest.
Controlled bending of free polymer films can be effected in a liquid crystal network with an azobenzene chromophore by exposure to linearly polarized light (17). This is addressed as photomechanical effect. It results from a photoselective volume contraction and is of interest for high-speed actuators in microrobotics in medicine or for optical microtweezers (12).
Carbon nanotubes (CNTs) with macroscopically ordered structures, e.g., aligned or patterned mats, fibers, and sheets with large surface areas have proven promising as electroactive polymer materials for the development of advanced chemical and biological sensors. In addition, controlled mechanical deformation for actuation has been demonstrated in CNT mats, fibers, sheets, and individual nanotubes (18).
15.2.1 Electroactive Polymers
The use of electroactive polymers in actuators (19), as well as using ionic liquids therein as electrolytes has been reviewed (20). Electroactive polymers change their shape by electrical stimulation. Examples of electroactive materials include ferroelectric polymers, e.g., PVDF and Nylon 11, dielectric electroactive polymers, electrorestrictive polymers, electro-viscoelastic elastomers, and liquid crystal elastomer composite network structures (21).
Ionic electroactive polymers include ionic polymer gels, ionomeric polymer metal composites, conductive polymers and CNTs. The induced displacement can be geometrically designed to bend, stretch or contract (21).
Common polymer materials such as poly(ethylene), poly(styrene), or poly(propylene) can be made electrically conductive through the addition of conductive fillers by various compounding techniques. The above mentioned polymers are thermoplastic, but thermosetting materials such as epoxies, may also be employed.
Conductive properties are imparted to the polymer by the formation of conductive through the polymer matrix. In fact, the polymer matrix remains an insulator. Suitable conductive fillers include metals, carbon black, or carbon fibers. Ionic polymer gels are activated by a chemical modification. These polymers swell as the pH changes.
Ionomeric polymer metal composites bend as the mobility of cations changes. Suitable base compounds for ionomers include perfluorosulfonate and perfluorocarboxylate (21). Commonly used electroactive polymers are summarized in Table 15.2.
Table 15.2 Electroactive polymers (21)
| Material | Remarks |
| Poly(pyrrole) | |
| Poly(aniline) | |
| Poly(thiophene) | |
| Poly(ethylenedioxythiophene) | |
| Poly(p-phenylene) | |
| Poly(p-phenylene vinylene) | |
| Poly(sulfone) | |
| Poly(pyridine) | |
| Poly(quinoxaline) | |
| Poly(acetylene) | |
| Poly(anthraquinone) | |
| Poly(N-vinylcarbazole) | |
| Poly(phosphazene) |
Some of the monomers for these polymers are shown in Figure 15.4. The elements of an actuator with an electroactive polymer are (21):
Figure 15.4 Monomers for electroactive polymers

- A source of electrical potential,
- An active region, which comprises the electroactive polymer,
- A counter electrode and
- An electrolyte in contact with both the active region and the counter electrode.
PPY is one of the most stable polymers under physiological conditions (21).
15.2.2 Membrane Actuators
A membrane actuator has a structure including an electrically conductive polymer membrane laminated on a solid electrolyte membrane, either on both faces or only on one face.
When the polymer membrane is laminated on only one face of the solid electrolyte membrane, a metal electrode thin film is formed as the counter electrode on the other face of the solid electrolyte membrane for applying a voltage. Further, by applying a certain voltage between the polymer membrane and the counter electrode, a bending of the laminated film results. The applied voltage allows the electrically conductive polymer to be oxidatively reacted.
The electrically conductive polymer actuator utilizes the migration of the ion to and from the electrically conductive polymer membrane, which is caused concomitantly by an electrical oxidation and reduction of the electrically conductive polymer, according to the motion principle. Therefore, an electrolyte is required as an ion supply source for executing a motion, and a solid electrolyte having a sufficient ionic conductivity at a temperature around the room temperature is required.
By this reaction, ions are incorporated into the polymer membrane, or taken out, depending on the nature of the voltage. This process alters the volume of the polymer membrane and results in a bending.
15.2.3 Light Weight Actuators
Compact and lightweight actuators, are of the class of electrostatic attraction type, piezoelectric type, ultrasonic type, and shape-memory alloy type (22). When an inorganic material is used, the actuators typically are not highly flexible. Therefore, lightweight and highly flexible actuator use an organic material such as a polymer.
For example, a high electric voltage applied between dielectric elastomer thin films such as silicone elastomers, and acrylic elastomers produces strains of 30–40%. However, prestraining of the film improves the performance of these devices. Strains up to 117% have been experienced with silicone elastomers, and up to 215% with acrylic elastomers, when biaxially and uniaxially prestrained films are examined (23). However, a high voltage in the range of kV is not desirable for application in robots for household use, because of the risk of an electric shock.
Another principle uses an oxidative-reductive reaction in an electrically conductive polymer (24). An electrically conductive polymer has a comparatively simple structure, is easy in miniaturization and weight saving, and is highly flexible. Such an actuator can be driven at a voltage as low as several volts, and is also characterized by sufficiently high initiation stress (22).
15.2.4 Microelectromechanical Systems
Polymers find wide applications in microelectromechanical systems. This area has been extensively developed in the past (25,26). Initially the technology was based on wet anisotropic chemical etching processes for forming three-dimensional silicon geometries. Subsequently, the metal oxide semiconductor was used for polycrystalline silicon micromachining. In this way, surface micromachined devices can be fabricated, such as drives (27).
Meanwhile, many commercially successful products based on microelectromechanical principles were developed, such as a digital light processor and ink jet printer nozzles (28). Several polymer types can be used for microelectromechanical devices. These polymers are summarized in Table 15.3.
Table 15.3 Polymers for microelectromechanical devices (28)
| Material | Processing | Usage |
| Parylene | Chemical vapor deposition | Micropumps and valves, pressure and shear stress sensors, micro air vehicle wings, microfluidis |
| Poly(imide) | Spin coating, extrusion | Sensor substrates, microfluidics, acrylics molding, microfluid channels |
| Poly(dimethylsiloxane) | Molding | Microfluidic channels, pumps, and valves |
| Liquid crystal polymers | Chemical etching | Flow sensors |
| Biodegradable polymers | Molding | Drug delivery devices |
| Epoxy resins | Photo patterning, spin casting | Artificial haircell sensors |
| Polyurethane | Molding | Structures |
| Nanocomposite elastomers | Screen printing, molding | Conductors, sensors, actuators |
For example, electroactive polymers are dispensed onto metallized poly(carbonate) substrates by ink jet printing. Multiple layers can be printed with a film thickness of 5–10 μm. After printing, the polymer layers are annealed thermally at 130°C. Also the top electrodes can be deposited by ink jet printing of a silver nanoparticle ink. An electric field across the polymer layer induces a piezoelectric strain in the polymer. Driving voltages of 200 V effect displacements of 20 μm and blocking forces of 3 mN.
The method of fabrication has been described in detail and claimed to be a commercially viable method (29). Also, photolithography can be used for the fabrication of microelectromechanical devices (30).
15.2.5 Biaxial Bending
An actuator that allows biaxial bending has been designed. The device is shaped with a square cross-section and four insulated electrodes on its surface. By the application of different voltages to these four electrodes, a biaxial bending motion can be induced (31).
15.3 Materials
15.3.1 Rotaxanes
Rotaxanes are supramolecules. They consist of a threadlike molecule that is also addressed as axle and a ring. To the axle, recognition moieties are attached. The ring and the axle are mechanically interlocked. Bistable rotaxanes have at least two recognition stations. They are molecular shuttles (32). Rotaxanes have potential uses in molecular elements and as molecular actuators (33). Photoswitchable rotaxanes have been described (32).
15.3.1.1 Host-guest Complexes Including Pseudorotaxanes
Pseudorotaxanes of crown ethers and rotaxanes of cyclic viologens with organic axle molecules can form gels. This occurs by the bonding of the functional end groups of the axle component (34). In particular, host-guest complexes and the pseudorotaxane of such macrocyclic compounds can form hydrogels.
The gels can be reverted into sols by heating or by the addition of competing substances. The gelation can be controlled by using these supramolecules as a hydrogelator.
The first typical example of a two-component gelation system was reported in 1993 (35). An equimolar mixture of triaminopyrimidine and dialkylbarbituric acid can form a gel in N,N-dimethylformamide (DMF), chloroform, carbon tetrachloride, and cyclohexane. The formation of such a physical gel is shown in Figure 15.5.
Figure 15.5 Formation of a physical gel (35)

15.3.2 Poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA)-clay nanocomposite laminates have been prepared as transparent thermal actuators that can be used in automatic aeration and ventilation or as thermal switches.
When low levels of smectite clay are added the thermal expansion coefficient of PMMA is reduced, however, its optical clarity and the reduced water absorption is retained. The individual layers in the laminate exhibit different coefficients of thermal expansion, which causes the bending upon a chance in temperature (36).
Quaternary ammonium-modified montmorillonite types can be used as clays. The PMMA-clay nanocomposites are fabricated by melt processing at 170°C on a twin-roll mill. At room temperature the fabricated laminates exhibit a curvature toward the polymer side resulting from cooling from the heated press. With increasing temperature the laminates become straight (36).
15.3.3 Dendritic Poly(styrene sulfonate)
A polymer actuator with high stability for operating in air is based on fluoropolymers onto which dendritic poly(styrene sulfonate) is grafted as ionomeric moiety. The fluoropolymer is a copolymer from vinylidene fluoride and hexafluoropropylene, and imidazolium-based compounds serve as ionic liquids (37).
The grafting is achieved by a γ-radiation grafting method (38). A molded film is irradiated with γ-rays at room temperature using a 60Co source with an irradiation rate of 6.8 kGyh−1 until a total absorbed dose of 50 k Gy is reached. The actuators exhibit a greatly enhanced bending displacement in comparison to Nafion-based actuators (37).
15.3.4 Fluoropolymers
To allow the operation of electron-conducting or ion-conducting polymer actuators outside of an electrolyte solution, i.e., in air, the conjugated polymer can be disposed on a gel membrane saturated with a high-boiling organic solvent. However, there are problems with the drying of a solvent and its usually low ion conductivity as in the case of propylene carbonate.
Studies have been performed concerning conductive polymers in an ionic liquid and of a totally solid state element using a complex comprising polymer from pyrrole and an ionic liquid of PVDF (39,40). However, it was criticized by other authors because problems with the slow response, preparation process and service life obviously have not yet been solved (41).
A substantial improvement is the use of ionic liquids in such devices. The synthesis of a suitable polymer and the use of an ionic liquid for such devices has been described (41). In an autoclave, 9H,9H-perfluoro-2,5-dimethyl-3,6-dioxa-8-nonenoic acid is copolymerized with vinylidene fluoride and hexafluoropropylene in 1,1-dichloro-1-fluoroefhane and methanol solution using bis(n-propyl)-peroxydicarbonate as a radical initiator.
To get actuators, a film with a three-layer structure is prepared by sandwiching the copolymer with butylmethylimidazolium tetrafluoroborate as an ionic liquid between single-wall CNTs. The ionic liquid is shown in Figure 15.6.
Figure 15.6 1-Butyl-3-methylimidazolium tetrafluoroborate and 9H,9H-perfluoro-2,5-dimethyl-3,6-dioxa-8-nonenoic acid

Eventually, a CNT paper is used. Here, a single-wall CNT is dispersed by ultrasonic waves in an aqueous solution of Triton X-100 at a pH value of 10. The dispersion is filtered and collected on a poly(tetrafluoroethylene) (PTFE) filter and the film is peeled off after some cleaning and rinsing steps to get the CNT paper (41).
15.3.4.1 Triblock Copolymers
Triblock copolymers from styrene and methyl methacrylate with two different poly(styrene) block have been synthesized using the atom transfer radical polymerization technique (42).
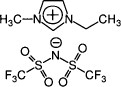
Ion gels consisting of the triblock copolymers and an ionic liquid, i.e., 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide were subsequently prepared by the cosolvent evaporation method with tetrahydrofuran (THF) as solvent. The ionic liquid is shown in Figure 15.7.
Figure 15.7 Ionic liquid (42)
The styrene blocks form phase-separated sphere domains, which act as physical crosslinking sites. However, the PMMA blocks serve as conduction paths for ions. The viscoelastic properties of the gels can be controlled by the amount of the hydrophobic styrene blocks.
These gels exhibit high ionic conductivities and can be used for an ionic polymer actuator. The application of low voltages to the electrodes, results in a soft bending motion toward the anodic side (42).
On the basis of the difference in the direction of motion of the actuator, a simple model of the actuation mechanisms was proposed by taking the difference in ionic transport properties into consideration. This model discriminates the behavior of the actuators in terms of the products of transference numbers and ionic volumes. The experimentally observed behavior of the actuators was successfully explained by this model (43).
15.3.5 Poly(pyrrole)
PPY can be doped with dodecylbenzene sulfonate anions. In contact with an electrolyte that contains small and mobile cations, when a current is passed through an active region, these cations are inserted or removed by a reduction or oxidation reaction, of the polymer, respectively. This results in an expansion or contraction of the polymer. This process occurs as:
(15.5) ![]()
PPY: poly(pyrrole), DBS: dodecylbenzene sulfonate.
When the PPY is oxidized, the positive charges on the backbone are at least partially compensated by the dodecylbenzene sulfonate anions. By the reduction of the PPY, the dodecylbenzene sulfonate ions cannot exit the polymer to maintain charge neutrality. So the smaller, more mobile, sodium cations enter the polymer, and expand the volume of the polymer. When this structure is back oxidized, the sodium ions again exit the polymer into the electrolyte, thus reducing the volume of the polymer.
A nine-layer multilayer conducting polymer actuator based on PPY has been design for use for household assistance robotic application. For those applications, special properties are needed, such as air-stable ionic liquid gels. Research revealed that multilayered conducting polymer actuators are feasible for household robotics, as they provide a substantial practical work density in a compact structure and can be easily scaled as required (44).
15.3.5.1 Metal Ion Implantation
In cantilevered PPY actuators gold ions have been implanted near the outer surfaces. A filtered vacuum cathode arc ion source was used for implantation of the gold particles. By such a modification, the conductivity of the layers is increased and consequently the overall conductivity of the device increases.
Assessment of the properties of thus modified devices reveals that the implanting causes a higher mechanical stiffness and thus a smaller displacement. However, their response time becomes smaller. The gold-implanted actuators generate a 15% higher mechanical work (45).
15.3.6 Poly(phosphazene)s
Poly(phosphazene)s can be synthesized by a variety of methods, including, ring opening, condensation, and living cationic polymerization (46). For example, poly(dichlorophosphazene) can be formed by a ring opening polymerization of a phosphonitrilic chloride trimer (46). The reaction is shown in Figure 15.8. The polymers may contain additives, such as plasticizers, antioxidants, and high dielectric constant particles.
Figure 15.8 Ring opening polymerization to get poly(dichlorophosphazene) (47)

Poly(phosphazene)s can be cast into films from solution. Appropriate solvents are acetone, fluorinated hydrocarbons, THF, toluene, benzene, dimethyl sulfoxide (DMSO), DMF, hexafluoroisopropanol, and mixtures of these solvents. To the casting solution a curing agent for crosslinking can be added, e.g., 2,2′-azobisisobutyronitrile or various peroxides (46).
Crosslinked poly(phosphazene)s can be made piezoelectric (48). The non-crosslinked poly(phosphazene)s must be placed in an electric field by applying high voltage and then crosslinked in the field. In this way, materials are obtained that display an excellent piezoelectric effect but also rubber elasticity.
15.3.7 Poly(thiophene)s
For the synthesis of monomers, bromo thiophene is formylated with DMF and the aldehyde groups are protected. Then the bromo groups are substituted by iodo groups. Afterwards the aldehyde groups are deprotected and reacted with benzenedithiol. Finally, in the presence of 2,3-dichloro-5,6-dicyano-p-benzoquinone, boron tetrafluoride is added. The dimerization and bromination is done with zinc and nickel catalysts (47). The synthesis is schematically shown in Figure 15.9.
Figure 15.9 Synthesis of a bithiophene derivative (47)

Bithiophene is a group having bonds that function as rotation axes, and is capable of rotation with the bonds as axes. Liquid crystallisable functional groups are made from 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]boronic acid. 2,3-Difluorobenzene is reacted with n-butyllithium, and then treated with trimethyl borate, thereby yielding 2,3-difluoro boronic acid. Then, the 2,3-difluoro boronic acid is reacted with a 4-alkoxy-1-bromobenzene.
Further, the thus synthesized 4-(4-alkoxyphenyl)-2,3-difluorobenzene is again reacted with n-butyllithium, then with trimethyl borate, thereby obtaining 4-(4-alkoxyphenyl)-2,3-difluoro boronic acid. Then, the obtained 4-(4-alkoxyphenyl)-2,3-difluoro boronic acid is reacted with 1,4-dibromobenzene in the presence of palladium, to get 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-dmuorophenyl]benzene. Then, the 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]-benzene is reacted with n-butyllithium, which was then treated with trimethyl borate, thereby obtaining 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]boronic acid (47).
Eventually, the stimuli-responsive compound is synthesized by coupling the liquid crystal compound to the dithiophene compound. The switching by light is a rotation and is shown in Figure 15.10.
Figure 15.10 Switching by light (R = liquid crystallisable moieties) (47)

An actuator can be fabricated with the stimuli-responsive compound polymer in between electrodes. When a voltage to the electrodes in the actuator is applied, an oxidation reduction reaction occurs that causes inhomogeneous swelling. For this reason, the actuator bends in the direction where the reduction reaction occurs (47).
15.3.8 Poly(ether imide)
An electric-stimulus-responsive bending actuator based on an ionic exchangeable membrane of biocompatible sulfonated poly(ether imide) has been designed (49). The synthesis of a a sulfonated poly(ether imide) is shown in Figure 15.11.
Figure 15.11 Synthesis of a sulfonated poly(ether imide) (49)
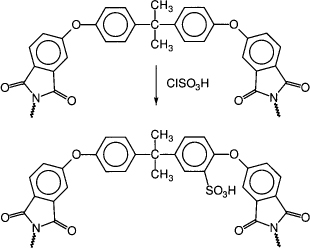
The sulfonation is effected with chlorosulfonic acid. The membrane was prepared from the polymer by the solution casting method. A 10% solution in dimethylacetamide (DMAC) is poured into a Teflon mold and kept in an oven at 80°C overnight, and at 120°C for 4 h to evaporate the solvent (49).
Experiments for the characterization revealed that the water uptake ratio of the sulfonated poly(ether imide) membrane is higher than that of a Nafion® 117 membrane. Poly(ether imide)-based membranes are biocompatible, and are low cost in comparison to perfluorosulfonic polymeric membranes.
15.3.9 Sulfonated Poly(ether ether ketone)
A blended polymer membrane of sulfonated poly(ether ether ketone) (PEEK) and PVDF yields a cost-effective polymer actuator with high performance, controllable stiffness and ionic networking (50). The response of the membrane composition using PVDF exhibits a much larger tip displacement relative to an actuator composed from sulfonated PEEK alone. Excellent electromechanical responses of the membrane arise due to the tailored stiffness and nanochannels of the ionic networking membrane.
A pure sulfonated PEEK has a very high amount of water uptake. This arises due to its high degree of sulfonation. On the other hand, pure sulfonated PEEK can be easily prepared and exhibits and excellent chemical resistance. PEEK can be easily sulfonated by the treatment with concentrated sulfuric acid (50).
The ion conductivity and mobility of sulfonated PEEK is dependent on the degree of sulfonation. This can be adjusted by the conditions of reaction, such as the concentration of the sulfuric acid, reaction time, and reaction temperature (51).
A blend membrane can be prepared by a casting technique from a solution of a mixture of sulfonated PEEK and PVDF in DMAC. The membrane is casted on a glass plate and can be peeled off after drying (50). The properties of such films are shown in Table 15.4.
Table 15.4 Properties of sulfonated PEEK films (50)
| Membrane type → Property | Sulfonated PEEK | Sulfonated PEEK/PVDF |
| Thickness/[μm] | 197 | 244 |
| Degree of Sulfonation/[%] | 95 | 86 |
| Ratio PEEK/PVDF | 1:0 | 2:0.2 |
| Water uptake/[%] | 40 | 28 |
| Swelling/[%] | 25 | 11 |
| Proton conductivity/[mS(cm)−1] | 6.2 | 9.9 |
| Young’s modulus/[MPa] | 1830.06 | 1436.51 |
| Membrane Tensile strength/[MPa] | 55.75 | 52.06 |
| Yield stress/[MPa] | 51.24 | 49.31 |
| Yield strain/[%] | 3 | 1.9 |
| Break stress/[MPa] | 41.31 | 40.52 |
| Break strain/[%] | 21 | 11.79 |
15.3.10 Poly(amic acid)
A high performance electroactive polymer actuator based on a sulfonated poly(imide) can be easily fabricated by a self-metallization reaction. This method if fabrication reduces both time and cost. No cation exchange process is needed. Further/the self-metallized silver electrodes exhibit a very good adhesion between metal and polymer and a high electrical conductivity. In this way, larger tip displacements are achieved in comparison to Nafion-based actuators (52).
For the synthesis of a membrane, 3,3′,4,4′-benzophenone tetracarboxylic dianhydride is reacted with 4,4′-oxydianiline and the lithium salt of benzidinedisulfonic acid.
After an ion exchange process with a silver precursor, the membrane formed undergoes a thermal treatment. This results in the imidization of the sulfonated poly(amic acid) and a simultaneous reduction of the silver cations. In this way, a native metal layer is formed. The method of synthesis is shown in Figure 15.12. The imidization process is shown in detail in Figure 15.13.
Figure 15.12 Synthesis of a poly(amic acid)-based actuator (52)

Figure 15.13 Imidization (52)

The sulfonated electroactive polymer described above is believed to be suitable for use for artificial muscles (52).
15.3.11 Ionic Polymer Metal Composites
Ionic polymer metal composites are very popular types of electroactive polymer actuators, because of their low electric driving potential, large deformation range, and their light weight. These types have been used as actuators or sensors in many areas of biomedical and robotic engineering (31).
There are ionic conducting polymer actuators which can only operate in a liquid electrolyte, but some types that can also operate both in air and liquids. The bending in such an actuator is based on ion and water flux into and from a polyelectrolyte that is covered with two metal electrode layers. The driving force is due to electroosmosis (53).
The devices are synthesized by electroless plating (54). However, electroless plating has some drawbacks, including a low reproducibility of electrode performance and a complicated method of fabrication. There are alternative techniques of fabrication, such as direct assembly plating, replication techniques, and microfabrication technologies (55, 56).
The fabrication process described by Chung (56) uses silver nanoparticles that are dissolved in a diluted Nafion® solution, followed by casting, embossing, nontoxic electroless plating, and eventually microelectroforming of nickel. The actuators exhibit bending angles of more than 90° at a driving voltage of only 3 V.
Instead of precious metals, conducting polymers can be used as electrodes. Further, Nafion® multi-walled CNT webs were fabricated by electrospinning and used as supporting electrodes of an ionic polymer transducer (57).
A plasma treatment using Cl2 and SF6 can improve the actuation properties. This is effected by a modification of the surface morphology of the ionic polymer metal composite. In particular, the treatment changes the surface appearance of the electroactive polymer. Round and cone-shaped microstructures are developed that contribute significantly to an enhanced electric conductivity after electroless plating. In this way, the changes in the electrical properties enhance the performance of actuation. Actuators with lower surface resistance generate a large deflection and actuators with higher capacitance generate a large actuation force (58).
15.3.12 Crosslinked Poly(vinylidene fluoride)
In a solid electrolyte polymer crosslinked PVDF has been used (1). Crosslinking enhances the thermal stability and the resistance to chemicals. The monomers are vinylidene fluoride, trifluoroethylene, and chlorotrifluoroethylene or fluoroethylene. Thus a terpolymer is formed.
Crosslinking is achieved by organic peroxides with amines as accelerator. Examples include, dicumyl peroxide, dibenzoyl peroxide, ethylenediamine, isopropylethylenediamine, 1,3-phenylenediamine, 1,5-naphthalenediamine, and 2,4,4-trimethyl-1,6-hexanediamine. Crosslinking occurs by heating to 160–170°C.
The extent of crosslinking can be determined with thermal mechanical analysis (DMA), differential scanning calorimetry (DSC), or a solubility test. The glass transition temperatures of polymer is raised due to crosslinking. Also, the loss modulus and the storage modulus become greater. This can be found out using DMA. In contrast, DSC gives information about the amount of crystallinity that becomes less, because the chains become entangled.
Further, most simply, to determine whether the polymer is cross-linked or not, a solubility test may be performed. When the polymer is crosslinked, the polymer is not dissolved in the solvent used for performing crosslinking. When the polymer after crosslinking reaction is added to a solvent, such as methyl isobutyl ketone or methyl ethyl ketone, the resulting crosslinked polymer shows a decreased solubility.
The electrolytic material consists of n-butyl-3-methyl imidazolium tetrafluoroborate, n-butyl-3-methyl imidazolium hexafluorophosphate and n-butyl-3-methyl imidazolium bis(trifluoromethanesulfonyl)imide (1). If two or more kinds of cations or anions are used, the melting point is lowered and thus the range of operation temperature can be increased if desired (59). The amount of the electrolytic material in the crosslinked fluorine-containing polymer may be 90–50% (1). In order to inject an electrolytic material into the polymer layer the polymer layer laminate is immersed into a liquid electrolyte.
The liquid electrolyte is prepared by dissolving the electrolytic material in a solvent. Suitable solvents are propylene carbonate, acetonitrile, methyl benzoate, and ethylene carbonate. Heat or pressure may be applied during the immersion to enhance the soaking of the liquid electrolyte. Finally, the solvent is evaporated (1).
15.3.13 Stimuli-responsive Polymer
15.3.13.1 Synthesis of a Stimuli-responsive Compound
A precursor for a stimuli-responsive polymer is a iodine compound with bithiophene bonded to 1,3-benzodithiolyl groups.
The dimerization and bromination of thiophene is achieved by zinc and nickel catalysts. Formylation is done in DMF. Then, the protection of the aldehyde groups is done, and the bromine group is exchanged by iodine. After deprotection of the aldehyde groups a reaction with benzenedithiol is conducted, further a reaction with 2,3-dichloro-5,6-dicyano-p-benzoquinone occurs, and finally boron tetrafluoride is added. As a result, a iodine compound with bithiophene and 1,3-benzodithiolyl groups is synthesized (47).
1-Bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]boronic acid is suitable for liquid crystallisable functional groups. This compound is synthesized as follows:
2,3-Difluorobenzene is reacted with n-butyllithium, then treated with trimethyl borate, thereby 2,3-difluoro boronic acid is obtained. Then, this compound is reacted with 4-alkoxy-1-bromobenzene in the presence of palladium catalyst, thereby yielding 4-(4-alkoxyphenyl)-2,3-difluorobenzene.
In the next step, 4-(4-alkoxyphenyl)-2,3-difluorobenzene is again reacted with n-butyllithium, and subsequently treated with trimethyl borate. In this way, 4-(4-alkoxyphenyl)-2,3-difluoro boronic acid is obtained. Then, this compound is reacted with 1,4-dibromobenzene.
This results in the formation of 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]benzene. In the last step, this compound is treated with n-butyllithium, and then with trimethyl borate, to get 1-bromo-4-[4-(4-alkoxyphenyl)-2,3-difluorophenyl]boronic acid (47).
Actually the polymerization reaction consists of a coupling reaction. The iodine compound described above undergoes a coupling reaction with 1-bromo-4-[4-(4-alkoxyphehyl)-2,3-difluorophenyl]-boronic acid in the presence of palladium catalyst.
Then, the reaction product is reacted with benzenedithiol in the presence of an acid catalyst, and then treated with 2,3-dichloro-5,6-dicyano-p-benzoquinone and boron tetrafluoride. In this way the stimuli-responsive compounds are obtained. When polymerized, the stimuli-responsive compound exists in a state in which long molecules extend in a structure; when oxidized.
15.3.13.2 Mechanism of Action
The application of a voltage causes a reduction reaction. The molecules rotate with bithiophene as axes, adjacent ones of the 1,3-benzodithiolyl groups mutually bond to each other by the oxidation reduction reaction. Further, the liquid crystallisable functional groups are oriented, causing the long molecules to be in a folded state. For this reason, the degree of deformation can be made great and an orientation occurs (47).
15.3.14 Silicone and Acrylate Elastomers
Silicones and acrylics are commonly used as dielectric materials for electroactive polymers. They should exhibit large strain, high stress, high energy density, good efficiency and high response speed. In a systematic study, the relevant properties of silicones and acrylics have been compared (60).
The silicone elastomer turned out to have a fast electromechanical response of around 3 s with a good reproducibility. Further, the dissipated work is negligible and not dependent on the applied electric frequency. It also exhibits a stable mechanical behavior over a wide temperature range. However, the strains are comparatively small.
On the other hand, the acrylic elastomer shows a slow electromechanical response with poor reproducibility. Further, dissipated work is significant and a strong frequency and temperature dependency of the dissipated work is observed. In summary, the silicone elastomer is more advantageous, however, when there exists a demand of high strains, acrylic elastomer might be preferred (60).
15.3.15 Other Electrically Conductive Polymers
An electrically conductive polymer has a conjugate double bond, whereby the π-electrons are spread through the entire polymer to contribute to the electronic conductivity. The electric conduction by an electrically conductive polymer has been believed to be caused via polaron and bipolaron, which are generated upon interaction of an oxidizing agent doped in the polymer and π-electrons in the polymer, and serve as charging carriers.
Electrically conductive polymers include PANI, PPY, and poly-(thiophene) (22). However, these polymers exhibit low adhesive properties to solid electrolyte membranes.
So, polymers with better adhesive properties have been developed. PVDF and copolymers, such as from vinylidene fluoride and hexafluoropropylene, may be used. Sulfonic acid may be introduced into the polymers.
For example, poly(ethylene glycol) (PEG) is added to a colloid dispersion liquid of PEDOT and poly(ethylenesulfonate), or poly(styrene sulfonic acid). Poly(styrene sulfonic acid) is strongly bound to PEDOT via ionic bonds in the mixture. PEDOT undergoes less likely an oxidative deterioration since the β-position of its chemically active five-membered ring is previously inactivated by modification with oxygen.
When PEDOT is used, its monomer can be polymerized in advance, and thus an electrically conductive polymer membrane can be formed by merely coating a dispersion of this polymer on a substrate. Therefore, a polymer membrane having a uniform thickness can be readily obtained on a substrate having a great area by employing a spin coating, slit coating, bar coating, dipping or casting method. In addition, it is suited for mass production technologies owing to the simple process for production (22).
15.3.16 Ionic Compounds
15.3.16.1 Electrolytes
Electrolyte polymer actuators using a liquid electrolyte necessitates a chamber for containing the liquid electrolyte, and thus the volume of the electrolyte polymer actuator may be increased. In addition, the reliability of sealing the chamber may be problematic (1).
As alternatives to such electrolyte polymer actuators, solid electrolyte polymer actuators using acrylonitrile butadiene rubber nitril butadiene rubber and PPY are known (1).
15.3.16.2 Organogelators
Dialkylammonium and dibenzo[24]crown-8–ether form a stable pseudorotaxane in solvents with low polarity. The structure is shown in Figure 15.14.
Figure 15.14 Pseudorotaxanes structures based on crown ethers (34)

A related pseudorotaxane from [MeNH2(CH2)3NH2Me]2+(PF6−)2 and dibenzo[24]crown-8-ether has been functionalized with two cholesterol groups (61).
This substance was used as an organogelator. Due to the intermolecular interaction of the cholesterol groups, fibrous aggregates in organic solvents such as cyclohexane, methylcyclohexane, benzene, toluene, p-xylene and diphenyl ether are formed. In this way, the solution turns into a gel.
When branched poly(3-caprolactone) with dibenzo[24]crown-8-ether end groups and linear poly(3-caprolactone) with dibenzylammonium end groups are mixed, supramolecular polymers are formed (62). The crown-8-ether dibenzylammonium end groups are forming complexes.
15.3.16.3 Ion Gels
An ion gel is prepared by gelatinizing a polymer or a monomer dispersed in an ionic liquid, and allowing the ionic liquid to be retained in the three-dimensional network structure of the gel. Thus, it has flexibility, and achieves a conductivity value of 10−2 S cm−1 at room temperature, which is 100 times or higher than that of conventional poly(ether)-type polymer solid electrolytes (63).
In the ionic liquid for producing the ion gel, ethylmethyl imidazolium is used as a cation and bis(trifluoromethanesulfonyl)imide is used as an anion. As the polymer to be mixed, a copolymer from vinylidene fluoride and hexafluoropropylene copolymer is used.
15.3.16.4 Three-layer Structure
A three-layer structure of the electrically conductive polymer membrane, electrolyte ion gel and the electrically conductive polymer membrane was formed by overlaying the electrically conductive polymer membrane on both faces of the electrolyte ion gel.
This three-layer structure was into pieces with a width of 2.5 mm and a length of 15 mm. A platinum electrode with a width of 2 mm and a length of 10 mm was mounted on this device.
When a voltage of ± 1.0 V was applied to this actuator, the bending motion was effected in response to the applied voltage without causing detachment of the boundary surface at the electrolyte ion gel-electrically conductive polymer membrane. The displacement magnitude observed upon driving with a rectangular pulse of 1 Hz is shown in Table 15.5.
Table 15.5 Bending properties of an actuator (22)
| Number of driving times | Displacement/[mm] |
| 1 | 0.59 |
| 60 | 0.59 |
| 120 | 0.59 |
| 600 | 0.59 |
| 1800 | 0.60 |
| 5400 | 0.60 |
15.3.16.5 Epoxy Hydrogel Polymers
A particular class of polymer-based actuators are based on elastic epoxy hydrogel polymers. Ionic imbalances near the electrodes are created in the materials that cause an increase in the swelling pressure in the hydrogel. This is achieved by the use of polymeric amido amine and ether dendrimers, poly(propylene imine) dendrimers, or amino functionalized dendrimers.
Preferred ethers for such hydrogels are PEG dodecylether or cyclohexanedimethanol diglycidyl ether. The ethers produce a very clear and strong hydrogel that reacts hydrpphobically to high pH aqueous solutions and swells when exposed to low pH or acidic solutions.
The density and the porosity of the hydrogel can be controlled by adding an amount of oxidizing agent to the polymer during the polymerization reaction. The swelling of the ionic hydrogel is achieved by a change in pH, ions, cations or protons between a solution outside of the hydrogel and a solution inside of the hydrogel or the polymer composition of the hydrogel.
The performance characteristics can be tailored in the course of polymerization. Adding acid during polymerization creates a hydrogel that has a higher pH-swelling property.
It is possible to create an electro-activated hydrogel by hydrating the epoxy hydrogel in an electrolyte, inserting an electrode into the gel, and spacing a second electrode a short distance from the hydrogel and running low amounts of current through the electrodes. The swelling may be increased in the region of a platinum electrode using saline as an electrolyte fluid. When the polarity is reversed, the hydrogel will contract by deswelling. Also the hydrophobic and hydrophilic properties can be switched by such methods.
Gases produced in a polymer actuator are undesirable. Gas creates a significant problem of compressible pressure in closed or sealed actuator systems, and can actually outpace the polymer actuation or volume change thereby creating unreliable results in actuation cycles. An actuator assembly with the electrodes separated by a porous membrane in an electrolyte can avoid the problems with gas production (64).
To manufacture a high surface type of electrode, a PTFE aqueous emulsion, is mixed with a high surface area carbon, e.g., activated carbon. The high surface area material is mixed with the emulsion and pressed or coated onto the electrode and baked to the correct processing temperature to bind the mixture together and to the electrode. This process is well known in the art of battery and capacitor manufacturing. The electrode substrate can be perforated, expanded or plated with solid metals (64).
15.4 Carbon-based Conductive Materials
15.4.1 Graphene
When graphene is dispersed within an ion conductive polymer membrane of a polymer actuator, reverse ion migration due to an osmotic pressure can be reduced. Thus the drivability of the polymer actuator can be improved (65).
An ionic polymer metal composite is derived from Nafion® membrane and a conductive metal. Both surfaces of the Nafion® membrane are electroplated with metal electrodes.
The electric conductivity can be increased by about a factor of 105 times by reducing graphene oxide to graphene. However, it is difficult to directly apply the reduction of the graphene oxide in the presence of an ion conductive polymer membrane because of the possible damage of the polymer membrane, because high temperature and strong sulfuric acid is needed. Recently, methods have been developed that allow the reduction of the graphene oxide under more mild conditions (65).
After the graphene oxide is dispersed within the polymer composite. it is heated in an aqueous solution of hydrazine, NH2–NH2 at 80°C. for 3 h. Afterwards, a thermal treatment is performed at 200–220°C for 15 min (65).
15.4.2 Carbon Nanottibes and Nanohorns
An ion conductivity-type polymer actuator has been described with a conventional structure, i.e., a pair of electrode layers and an electrolyte layer between the electrodes. However, in the electrode layers there are carbon nanoparticles which are a mixture of CNTs and carbon nanohorns (59).
A carbon nanohorn is a carbon-based material that is fabricated by wrapping graphene into a cone (59). The use of carbon nanohorns should result in a higher driving force and higher displacement.
A mixing ratio of CNTs to carbon nanohorns of 42:23 has been found to be preferable. In such mixing ratios, the characteristics of the actuator are dramatically enhanced. The properties of an actuator with varying amounts of nanotubes and nanohorns are shown in Table 15.6.
Table 15.6 Properties of an actuator with varying amounts of nanotubes and nanohorns (59)

15.4.3 Metal Nanoparticle-Polytner Composites
Conventional techniques of manufacturing nanoparticle-polymer composites mainly use nanoparticles that are synthesized in a previous step before further application.
Therefore an additional process of preparing nanoparticles is needed. The aggregation and precipitation occurs when the nanoparticle-polymer composite is mixed with another a polymeric liquid.
A more recent development of synthesizing a metal nanoparticle-polymer composite includes the following steps (66):
In other words, an organometallic compound may be dissolved as a raw metal material in a molecular state in a polymer melt solution without additionally preparing metal nanoparticles, and reduced to metal nanoparticles in a polymer liquid state in situ so that highly uniform metal nanoparticle-polymer composites can be manufactured in uniform distribution with high efficiency using an inexpensive process. The process has been demonstrated with a platinum and palladium-based organometallic compound, with dimethyl sulfoxide (DMSO):

The synthesis of the platinum compound starts from an aqueous solution of potassium tetrachloroplatinate to which DMSO is added. After 5 h stirring at room temperature, cis-Pt(DMSO)2Cl2 was formed as a pale yellow solid. After some conventional steps of purifying and isolation, the compound was dissolved and CH2Cl2 and ethanolamine was added.
After 3 h, a white powdered precipitate was formed, yellow crystals started to be generated within 10 h hours. The white powdered precipitate was removed by filtering. The remaining filtrate was concentrated by vacuum distillation and recrystallized into n-hexane, thereby obtaining a yellow solid (66). The synthesis of the palladium-based compound proceeds in a similar manner.
Next, the organometallic compound is dissolved in DMF together with Nafion®. Alternatively, a polymer melt solution may be mixed with the solution of the organometallic compound, thereby preparing a solution mixture.
The mixture should contain the organometallic compound in the range of 0.001–10 phr (parts per 100 parts of weight). When the solution mixture contains less than 0.001 phr, the efficiency of formation of the metal nanoparticles may be reduced. In contrast, when the solution mixture contains more than 10 phr, it may be difficult to form nanoparticles to a uniform size with a uniform distribution, and part of the organometallic compound may leak during a thermal reduction process to form a metal film. As a result, the electrical properties of the surface of the final metal nanoparticle-polymer film could become non-uniform (66). To form the final desired film, the mixture is dried as it is subjected to elevated temperatures and in a vacuum. The conditions of drying have been elucidated in preliminary experiments conveniently with thermogravimetry (TG).
Inspection of the TG of trans–Pt(DMSO)(NH2CH2CH2OH)Cl2 shows relatively high changes of 59.67 J g−1 and 315.7 J g−1 at temperatures of 159°C and 203°C. Thus, the masses of the precursors are largely varying at these temperatures, which indicates a decomposition process.
In practice, to obtain desired physical and chemical properties, shape, and thickness of the final composite layer a certain program with regards to the rate of temperature and vacuum applied, must be used. Eventually, from this metal nanoparticle Nafion® composite film an ionic polymer metal composite actuator can be manufactured. The formation of such an actuator has been demonstrated in detail (66). The metal nanoparticles have a high particle uniformity and can be used in a wide concentration range so that various metal nanoparticle composite materials may be easily manufactured at low cost (66).
15.4.4 Metal Salts in CNT Devices
The effects of alkali and earth alkali metal salts on the performance of an actuator made from a single-walled CNT ionic liquid have been investigated. As polymer, poly(vinylidene fluoride-co-hexafluoropropylene) is used and as ionic liquid, 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide are used. The ionic liquids are shown in Figure 15.15.
Figure 15.15 Ionic liquids (67)

In the presence of the salts, a much better performance of the actuator was observed. It is assumed that the higher ionic conductivity of the gel electrolyte layer containing the alkaline metal salt effects a quick response actuator. Further, the enhanced capacitance of the actuator results in a larger strain (67–69).
The dependence of the anionic counterions of ionic liquids on the performance of actuators has been systematically investigated (70). 1-Ethyl-3-methylimidazolium was selected as the common cation. The following anions were used: trifluoromethyltrifluoroborate, pentafluoroethyltrifluoroborate, fluorosulfonyl(trifluoromethylsulfonyl)imide, and pentafluoroethylsulfonyl(trifluoromethylsulfonyl)imide.
The dependence of the performance on a series of properties were investigated, including ionic conductivity, generated strains, van der Waals volumes, Young’s modulus, and viscosity. The results revealed that trifluoromethyltrifluoroborate and pentafluoroethyltrifluoroborate anions show superior performance in comparison to the other ionic fluids investigated (70).
15.5 Medical Applications
15.5.1 Blood Pressure Sensor
An apparatus for measuring arterial blood pressure has been developed. The method consists of (71):
- Measurement of a plethysmographic signal of a plethysmographic sensor that is disposed on the extremity relative to the heart,
- Using an externally applied pressure, and
- Processing a signal from a continuous arterial blood pressure waveform and the value of the externally applied pressure.
Photoplethysmographs are instruments which use light for determining and registering variations in a patient’s blood volume. They can instantaneously track arterial blood volume changes during the cardiac cycle. Photoplethysmographs operate in a noninvasive manner and much work has been done to use them successfully to measure blood pressure (72).
Obviously, the modern use of such methods for determining arterial blood pressure traces back to 1983 (73). However, the plethysmograph according to Mosso has already been described in 1876 (74).
An improved photoplethysmograph can discriminate light traversing the extremity from ambient light on the basis of differential response (71). A pulsatile waveform from the photoplethysmographic signal may be obtained at a plurality of externally applied pressures to calibrate the photoplethysmograph.
While measuring the plethysmographic signal, externally a focal pressure to the extremity is applied. Thus, the apparatus may have a conducting polymer actuator for applying pressure to the extremity.
A cuff is coupled with the conducting polymer actuator for improving and stabilizing the sensor results (71). Each polymer actuator is encapsulated in a flexible tube filled with an electrolyte. When electrically charged, the conducting polymers shrink or expand to create a strain of 5–15%. These conducting polymer actuators are used as active bands, which are wrapped around the finger or the wrist. When the conducting polymer actuators shrink, the pressure of the finger tissue under the sensor unit increases (71).
15.5.2 Medical Balloons
Balloon catheters are employed in a variety of medical procedures. Percutaneous transluminal coronary angioplasty, or balloon angioplasty, is used for treating peripheral and coronary arteries. This technique consists of inserting an uninflated balloon catheter into the affected artery. Dilation of the diseased segment of artery is accomplished by inflating the balloon which pushes the atherosclerotic lesion outward, thereby enlarging the arterial diameter. The balloon catheter is guided through the vascular system until the balloon is positioned across the vessel obstruction. The balloon is then inflated to apply pressure to the obstruction whereby the vessel is opened for improved flow (21).
An expandable medical balloon may be actuated by an electroactive polymer. An actuator made from an electroactive polymer may be placed in the inner and outer surfaces of the catheter shafts, or the balloon cones, or other parts that have been discussed in detail (21).
15.5.3 Biomedical Application
For the biomedical application of an ionic polymer-based actuator, the device should generate a fast and large tip displacement at low direct current and alternating current voltages of 1–2 V (75). The tip displacement of composites made from PVDF, poly(N-vinyl-2-pyrrolidone) (PVP) and poly(styrene sulfonic acid) have been tested. In an ionic membrane, PVDF is the hydrophobic polymer, PVP is the basic, water-soluble polymer, and poly(styrene sulfonic acid) is the strong, water-soluble polyelectrolyte, which provides the free charge carrier. Platinum particles were embedded in such a composite-blended membrane by electroless plating (76).
The results were compared with a Nafion-based actuator. Some compositions were found to have a higher ion exchange capacity and a higher water uptake than a Nafion® membrane (75).
15.5.4 Hybrid Bio-derived Conducting Polymer Actuator
Recent developments in smart materials use the principle of biological ion transport. Biological systems utilize the ion transport across a barrier membrane for energy conversion. Among smart materials, ionic active materials demonstrate electromechanical coupling using ion transport across the thickness of the polymer. There is a close relation between the ionic interaction in a conducting polymer and in biological membranes.
Actuators that use the ion transport through a biological membrane to produce shape changes in a conducting polymer have been designed. The basic architecture, the physics of transduction and the analysis of actuation in a hybrid biopolymer actuator have been presented (77).
15.5.5 Controlled Release of Rhodamine B
The synthesis of a dual-responsive supramolecular polymer gel has been reported. The gel is made from a heteroditopic monomer by making use of the reversible host-guest interactions between dibenzo[24]crown-8-ether and its complementary molecule, a dibenzylammonium salt.
The dibenzo[24]crown-8-ether forms a 1:1 threaded complex with the dibenzylammonium salt. The complex is responsive to pH and temperature. Long pending alkyl chains allow the formation of supramolecular polymers. Reversible sol-gel transitions are observed by heating cooling cycles, or by acidification and neutralization. Thus, a thermo-responsive and pH-responsive sol-gel transition process was successfully employed for the controlled release of rhodamine B (78).
15.6 Optical Applications
15.6.1 Variable-focal Lens
Optical systems often require variable focal length to allow the field of view or the magnification to be altered. Conventional mechanical systems use focusing mechanisms which are complicated and consist of many moving parts such as gears, motors and sliders to image a moving object or objects with highly irregular shape. Such systems become particularly difficult to use in small systems (79).
The study of variable focal length microlens has been an area of activity for many years. Variable focal length is a necessary attribute in many optical applications if the object being imaged is not in a fixed position (79).
There are various approaches using liquid crystal methods (80) and electrowetting methods (81,82). On the other hand, a circular diaphragm actuator has been designed made from poly(dimethylsiloxane). Compliant circular electrodes are attached on each side of poly(dimethylsiloxane) film. The outside edge of the thin film is fixed tightly to prevent any movement. After applying the voltage on the electrodes, the thin film can be deformed in the central non-electrode part (79). The actuator is fabricated by a mold casting process.
A recently introduced lens device is composed of an elastic membrane upon a circular lens chamber, a reservoir of liquid, and a channel between them. The shape and curvature can be controlled by hydraulic pressure. An electroactive polymer is used as an actuator (83).
Three layers of poly(dimethylsiloxane) are used for its design as a carbon polymer composite. The actuator is mechanically attached to the liquid reservoir to compress or decompress the liquid, thus controlling the shape of the lens. Depending on the pressure the lens can be shaped from convex to concave. It is also possible to fabricate biconvex and biconcave lenses. The method of fabrication is claimed to be simple and cost effective (83).
15.6.2 Variable Mirrors
Polymer actuator arrays have been reported that are based on environmentally responsive hydrogels. Their optical properties change in response to the environment e.g., with humidity or pH.
Arrays of micrometer scale plates are patterned using reactive ion etching of silicon. These plates are suitable as master structures for replica molding. An UV-curable epoxy was cast in a metal-sputtered poly(dimethylsiloxane) mold and a micromirror array is formed by a thin metal film placed onto the microplate.
On these arrays, a polyelectrolyte hydrogel, such as poly(acrylamide-co-acrylic acid), is patterned, which acts as the actuator. The micromirrors are deformed in response to humidity or pH. It is believed that such devices have a wide field of application for environmentally responsive adaptive and active optics (84).
15.6.3 Lens Positioning System
An electroactive polymer actuator has been described for use in camera diaphragms and lenses (85). The actuator converts electrical energy to mechanical energy.
The device consists of two flexible electrodes and a transparent elastic nonconductive polymer layer. This layer can effect a compression orthogonally to its thickness. The compression occurs by an electric field.
The flexible electrodes may be coupled to the polymer by painting or coating the polymer material, or by graphite powder. Two coupled electroactive polymer actuators are in use (85). Electroactive polymers are new and interesting types of actuators. The motion is generated by changing the shape of the polymer or its mechanical properties (85).
15.7 Pumping Applications
15.7.1 Micropump
A valveless micropump has been developed that is driven by two conducting polymer soft actuators that open and close (86,87). This is effected by oxidation and reduction reactions. These reactions cause the polymer to swell or shrink which results in the desired change in properties and thus effecting the pumping.
The PPY polymer used is created by electrochemical polymerization. The two actuators are composed of a cation-sensitive and an anion-sensitive layer. The actuator completely closes on reduction and opens perfectly by oxidation.
As ionic liquid, Lithium bis(trifuoromethanesulfonyl)imide is used. At the reduction reaction, Li+ is doped, which effects an expansion of the cation-sensitive layer. The bis(trifuoromethanesulfonyl)imide anion is used for doping the anion-sensitive layer to contract. The micropump can transport fluids in one direction without suffering from backflow.
15.7.2 Electroosmotic Pump
A polymer actuator has been developed based on an electroosmotic pumping principle to create hydraulic pressure. The actuator is made from poly(dimethylsiloxane). Soft lithographic techniques were used to fabricate prototype devices. Into the structure, microscale channels and reservoirs are embedded. By the application of an electric potential, one reservoir expands as a fluid is pumped into it, but another reservoir contracts as the membrane above the expansion reservoir rises (88).
15.7.3 Microfluidic Pump for Electrophoresis
Microfluidic systems for analysis are significantly developing, which causes an increased complexity of the devices. A common issue of all types of systems, such as liquid chromatography or electrophoresis is the introduction of a sample mixture into the part of the device where the actual separation of the sample occurs. Here the reproducibly of the process is of unique importance. Nowadays, electrophoretic micro total analysis systems have been developed (89).
Siloxane-based polymers can be used as smart materials because their shapes can be modified in the presence of an electric field. The energy of this electric field can be transduced into mechanical energy and is directly coupled with a small multi-channel network and will thus initiate a motion of fluids.
When an electrical potential is applied across the electroactive polymer film, a change in shape also affects the volume of the microfluidic channel. Using this principle it is possible to inject plugs of a sample on an electrophoresis cross chip (90).
In addition, the amount of sample injected in one stroke can be varied as a function of the electric field, the active area of the actuation unit and the softness of the electroactive material (90).
This example of pressure injection has been esteemed as an attractive alternative to the traditional gated injection schemes reported for continuous sampling since it does not introduce any sample bias and offers the potential of adding additional valves for flow control with no additional processing steps (89).
References
1. J.-O. Kwon, S.-T. Choi, Y.-K. Lee, J.-C. Koo, and S.-J. Park, Solid electrolyte polymer, polymer actuator using cross-linked polyvinylidene fluoride-based polymer, and method of manufacturing the polymer actuator, US Patent Application 20 100 148 635, assigned to Samsung Electronics Co., Ltd., Suwon-Si KR Sungkyunkwan University Foundation for Corporate Collaboration. Suwon-Si KR, June 17, 2010.
2. S.-T. Choi, Y.-K. Lee, J.-C. Koo, J.-O. Kwon, and S.-J. Park, Polymer and polymer actuator comprising the same, US Patent Application 20 100 201 227, assigned to Samsung Electronics Co., Ltd., Suwon-Si (KR) and Sungkyunkwan University Foundation for Corporate Collaboration, Suwon-Si (KR), August 12, 2010.
3. A. Kaynak, C. Yang, Y.C. Lim, and A. Kouzani, Electrochemical fabrication and modelling of mechanical behavior of a tri-layer polymer actuator, Materials Chemistry and Physics, 125(1–2):113–117, January 2011.
4. M. Potter, K. Gouder, and J.F. Morrison, A numerical model for electro-active polymer actuators with experimental validation, Sensors and Actuators A: Physical, 170(1–2):121–130, November 2011.
5. R.E. Pelrine, R.D. Kornbluh, and J.P. Joseph, Electrostriction of polymer dielectrics with compliant electrodes as a means of actuation, Sensors and Actuators A: Physical, 64(1):77–85, January 1998.
6. R. Trujillo, J. Mou, P.E. Phelan, and D.S. Chau, Investigation of electrostrictive polymers as actuators for mesoscale devices, The International Journal of Advanced Manufacturing Technology, 23(3–4):176–182, February 2004.
7. P. Du, X. Lin, and X. Zhang, A multilayer bending model for conducting polymer actuators, Sensors and Actuators A: Physical, 163(1):240–246, September 2010.
8. A. Deodhar, A. York, M. Hodgins, and S. Seelecke, Finite element modeling of electromechanical behavior of a dielectric electroactive polymer actuator, Proceedings of SPIE, 7978(D), May 2011.
9. M. Hodgins, A. York, and S. Seelecke, Modeling and experimental validation of a bi-stable out-of-plane DEAP actuator system, Smart Materials and Structures, 20(9), September 2011.
10. M. Wissler and E. Mazza, Modeling of a pre-strained circular actuator made of dielectric elastomers, Sensors and Actuators A: Physical, 120(1): 184–192, April 2005.
11. F. Tama and C.L. Brooks, Symmetry, form, and shape: Guiding principles for robustness in macromolecular machines, Annual Review of Biophysics and Biomolecular Structure, 35(1):115–133, June 2006.
12. L. Ionov, Actively-moving materials based on stimuli-responsive polymers, Journal of Materials Chemistry, 20(17):3382, 2010.
13. P.M. Welch, A tunable dendritic molecular actuator, Nano Letters, 5(7): 1279–1283, July 2005.
14. J.Z. Hilt, A.K. Gupta, R. Bashir, and N.A. Peppas, Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels, Biomedical Microdevices, 5(3):177–184, 2003.
15. E. Smela, “Microfabricated conjugated polymer actuators for micro-valves, cell biology, and microrobotics,” in F. Carpi and E. Smela, eds., Biomedical Applications of Electroactive Polymer Actuators, chapter 12, pp. 249–264. John Wiley & Sons, Inc., New York, 2009.
16. L. Dong, A.K. Agarwal, D.J. Beebe, and H. Jiang, Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature, 442 (7102):551–554, August 2006.
17. Y. Yu, M. Nakano, and T. Ikeda, Directed bending of a polymer film by light, Nature, 425(6954):145, 2003.
18. L. Qu, Q. Peng, L. Dai, G.M. Spinks, G.G. Wallace, and R.H. Baughman, Carbon nanotube electroactive polymer materials: Opportunities and challenges, MRS Bulletin, 33(03):215–224, March 2008.
19. Y. Bar-Cohen, “Electroactive polymers as actuators,” in K. Uchino, ed., Advanced Piezoelectric Materials: Science and Technology, chapter 8, pp. 287–317. Woodhead Publishing Ltd., Cambridge, UK, 2010.
20. K. Asaka, “Actuators,” in H. Ohno, ed., Electrochemical aspects of ionic liquids, chapter 18, pp. 271–278. Wiley, New York, 2nd edition, 2011.
21. T. Eidenschink, D. Wise, D. Sutermeister, Y. Alkhatib, D. Gregorich, A. Jennigs, M. Heidner, D. Godin, R.C. Gunderson, J. Blix, K.A. Jagger, and A. Kornkven Volk, Medical balloon incorporating electroactive polymer and methods of making and using the same, US Patent 7919910, assigned to Boston Scientific Scimed, Inc. (Maple Grove, MN), April 5, 2011.
22. Y. Kudoh, Electrically conductive polymer actuator, and method for manufacturing the same, US Patent Application 20 100 039 001, assigned to Panasonic Corporation, Osaka (JP), February 18, 2010.
23. R. Pelrine, R. Kornbluh, Q. Pei, and J. Joseph, High-speed electrically actuated elastomers with strain greater than 100%, Science, 287(5454): 836–839, February 2000.
24. K. Takakuwa, M. Oshima, and S. Motoge, Conductive high-polymer actuator, JP Patent 2 006 050 780, assigned to Japan Carlit Co. Ltd., 2006.
25. M.J. Madou, Fundamentals of Microfabrication: The Science of Miniaturization, CRC Press, Boca Raton, 2002.
26. G. De Micheli, Nanosystems design and technology, Springer, Dordrecht, New York, 2009.
27. W.C. Tang, M.G. Lim, and R.T. Howe, Electrostatic comb drive levitation and control method, Journal of Microelectromechanical Systems, 1 (4):170–178, 1992.
28. C. Liu, Recent developments in polymer MEMS, Advanced Materials, 19(22):3783–3790, November 2007.
29. O. Pabst, J. Perelaer, E. Beckert, U.S. Schubert, R. Eberhardt, and A. Tünnermann, Inkjet printing of electroactive polymer actuators on polymer substrates, Proceedings of SPIE, 79762(H), March 2011.
30. N. Lange, F. Wippermann, R. Leitel, C. Bruchmann, E. Beckert, R. Eberhardt, and A. Tünnermann, First results on electrostatic polymer actuators based on UV replication, Proceedings of SPIE, 7926(9), January 2011.
31. G.-Y. Lee, J.-O. Choi, M. Kim, and S.-H. Ahn, Fabrication and reliable implementation of an ionic polymer-metal composite (IPMC) biaxial bending actuator, Smart Materials and Structures, 20(10):105026, October 2011.
32. Y. Duo, S. Jacob, and W. Abraham, Photoswitchable rotaxanes on gold nanoparticles, Org. Biomol. Chem., 9:3549–3559, 2011.
33. C.A. Schalley, K. Beizai, and F. Vögtle, The way to rotaxane-based molecular motors: Studies in molecular mobility and topological chirality, Acc. Chem. Res., 34(6):465–476, 2001.
34. Y. Suzaki, T. Taira, and K. Osakada, Physical gels based on supramolecular gelators, including host-guest complexes and pseudorotaxanes, J. Mater. Chem., 21:930–938, 2011.
35. K. Hanabusa, T. Miki, Y. Taguchi, T. Koyama, and H. Shirai, Two-component, small molecule gelling agents, Journal of the Chemical Society, Chemical Communications, 18:1382–1384, 1993.
36. B. Chen, S. Liu, and J.R.G. Evans, Polymeric thermal actuation using laminates based on polymer–clay nanocomposites, Journal of Applied Polymer Science, 109(3):1480–1483, August 2008.
37. J.Y. Lee, H.S. Wang, B.R. Yoon, M.J. Han, and J. Y. Jho, Radiation-grafted fluoropolymers soaked with imidazolium-based ionic liquids for high-performance ionic polymer-metal composite actuators, Macromolecular Rapid Communications, 31(21):1897–1902, November 2010.
38. M.J. Han, J.H. Park, J.Y. Lee, and J.Y. Jho, Ionic polymer-metal composite actuators employing radiation-grafted fluoropolymers as ion-exchange membranes, Macromolecular Rapid Communications, 27(3):219–222, February 2006.
39. W. Lu, Use of ionic liquids for pi -conjugated polymer electrochemical devices, Science, 297(5583):983–987, July 2002.
40. D. Zhou, G.M. Spinks, G.G. Wallace, C. Tiyapiboonchaiya, D.R. Mac-Farlane, M. Forsyth, and J. Sun, Solid state actuators based on polypyrrole and polymer-in-ionic liquid electrolytes, Electrochimica Acta, 48(14–16):2355–2359, June 2003.
41. Y. Komatsu, K. Hirata, H. Mohri, and H. Aoyama, Actuator element, US Patent 8123983, assigned to Daikin Industries, Ltd. (Osaka, JP), February 28, 2012.
42. S. Imaizumi, H. Kokubo, and M. Watanabe, Polymer actuators using ion-gel electrolytes prepared by self-assembly of ABA-triblock copolymers, Macromolecules, 45(1):401–409, January 2012.
43. S. Imaizumi, Y. Kato, H. Kokubo, and M. Watanabe, Driving mechanisms of ionic polymer actuators having electric double layer capacitor structures, The Journal of Physical Chemistry B, 116(16):5080–5089, April 2012.
44. K. Ikushima, S. John, K. Yokoyama, and S. Nagamitsu, A practical multilayered conducting polymer actuator with scalable work output, Smart Materials and Structures, 18(9):095022, September 2009.
45. G. Alici, A. Punning, and H.R. Shea, Enhancement of actuation ability of ionic-type conducting polymer actuators using metal ion implantation, Sensors and Actuators B: Chemical, 157(1):72–84, September 2011.
46. R.A. Basheer and R.-M.L. Mercado, Elastomeric polyphosphazene transducers, methods of making, and methods of use thereof, US Patent 6 876 125, assigned to Delphi Technologies, Inc. (Troy, MI), April 5, 2005.
47. T. Otake, Stimuli responsive compound, stimuli responsive compound polymer, actuator and method for manufacturing stimuli responsive compound, US Patent Application 20 110 196 120, assigned to Seiko Epson Corporation, Tokyo (JP), August 11, 2011.
48. T. Kotaka and K. Adachi, Crosslinked polyphosphazenes as piezoelectric materials, US Patent 4 933 479, assigned to Idemitsu Petrochemical Co., Ltd. (Tokyo, JP), June 12, 1990.
49. M. Rajagopalan, J.-H. Jeon, and I.-K. Oh, Electric-stimuli-responsive bending actuator based on sulfonated polyetherimide, Sensors and Actuators B: Chemical, 151(1):198–204, November 2010.
50. J.-H. Jeon, S.-P. Kang, S. Lee, and I.-K. Oh, Novel biomimeric actuator based on SPEEK and PVDF, Sensors and Actuators B: Chemical, 143(1):357–364, December 2009.
51. T. Kobayashi, M. Rikukawa, K. Sanui, and N. Ogata, Proton-conducting polymers derived from poly(ether-etherketone) and poly(4-phenoxybenzoyl-1,4-phenylene), Solid State Ionics, 106(3–4):219–225, February 1998.
52. J. Song, J.-H. Jeon, I.-K. Oh, and K.C. Park, Electro-active polymer actuator based on sulfonated polyimide with highly conductive silver electrodes via self-metallization, Macromolecular Rapid Communications, 32(19):1583–1587, October 2011.
53. M. Shahinpoor, Y. Bar-Cohen, J.O. Simpson, and J. Smith, Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles - a review, Smart Materials and Structures, 7(6):R15–R30, December 1998.
54. M. Shahinpoor and K.J. Kim, Ionic polymer-metal composites: I. Fundamentals, Smart Materials and Structures, 10(4):819–833, August 2001.
55. B.J. Akle, M.D. Bennett, and D.J. Leo, High-strain ionomeric–ionic liquid electroactive actuators, Sensors and Actuators A: Physical, 126(1): 173–181, January 2006.
56. C. Chung, P. Fung, Y. Hong, M. Ju, C. Lin, and T. Wu, A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders, Sensors and Actuators B: Chemical, 117(2):367–375, October 2006.
57. J.-W. Lee and Y.-T. Yoo, Preparation and performance of IPMC actuators with electrospun Nafion®–MWNT composite electrodes, Sensors and Actuators B: Chemical, 159(1):103–111, November 2011.
58. S. Saher, W. Kim, S. Moon, H. Jin Kim, and Y.H. Kim, Electro-actuation characteristics of Cl2 and SF6 plasma-treated IPMC actuators, Smart Materials and Structures, 19(10):105013, October 2010.
59. Y. Ono, K. Suzuki, and K. Asaka, Polymer actuator, US Patent Application 20 110 156 538, June 30, 2011.
60. S. Michel, X.Q. Zhang, M. Wissler, C. Löwe, and G. Kovacs, A comparison between silicone and acrylic elastomers as dielectric materials in electroactive polymer actuators, Polymer International, 59(3):391–399, March 2010.
61. S. Kawano, N. Fujita, and S. Shinkai, Novel host-guest organogels as stabilized by the formation of crown-ammonium pseudo-rotaxane complexes, Chem. Commun., 12:1352–1353, 2003.
62. Z. Ge, J. Hu, F. Huang, and S. Liu, Responsive supramolecular gels constructed by crown ether based molecular recognition, Angewandte Chemie, 121(10):1830–1834, 2009.
63. H. Ohno, ed., Electrochemical aspects of ionic liquids, Wiley, New York, 2nd edition, 2011.
64. M. Banister, R. Clark, E. Coiner, Y.M. Geronov, M.D. Mcwilliams, and M.A. van Veen, High surface area polymer actuator with gas mitigating components, US Patent Application 20 120 029 430, February 2, 2012.
65. H.K. Lee, N.J. Choi, K.S. Yang, S.K. Jung, K.H. Park, and J.D. Kim, Polymer actuator containing graphene and method of preparing the same, US Patent Application 20 110 133 607, assigned to Electronics and Telecommunications Research Institute, Daejeon (KR), June 9, 2011.
66. K.S. Yang, N.J. Choi, H.K. Lee, S.K. Jung, K.H. Park, and J.D. Kim, Metal nonparticle-polymer composites, method of manufacturing the same, and polymer actuator using the same, US Patent Application 20 110 140 580, assigned to Electronics and Telecommunications Research Institute, Daejeon (KR), June 16, 2011.
67. N. Terasawa, I. Takeuchi, K. Mukai, and K. Asaka, The effects of alkaline and alkaline earth metal salts on the performance of a polymer actuator based on single-wal led carbon nanotube-ionic liquid gel, Physics Procedia, 14:73–86, January 2011.
68. N. Terasawa, I. Takeuchi, K. Mukai, and K. Asaka, The effects of Li salts on the performance of a polymer actuator based on single-walled carbon nanotube-ionic liquid gel, Polymer, 51(15):3372–3376, July 2010.
69. N. Terasawa, I. Takeuchi, K. Mukai, and K. Asaka, The effects of alkaline earth metal salts on the performance of a polymer actuator based on single-walled carbon nanotube-ionic liquid gel, Sensors and Actuators B: Chemical, 150(2):625–630, October 2010.
70. N. Terasawa, I. Takeuchi, H. Matsumoto, K. Mukai, and K. Asaka, High performance polymer actuator based on carbon nanotube-ionic liquid gel: Effect of ionic liquid, Sensors and Actuators B: Chemical, 156 (2):539–545, August 2011.
71. H.H. Asada, P. Shaltis, D.B. McCombie, and A.T. Reisner, Wearable blood pressure sensor and method of calibration, US Patent 7 641 614, assigned to Massachusetts Institute of Technology (Cambridge, MA), January 5, 2010.
72. P.H. Jones and W.-M. Wang, Method of measuring blood pressure with a photoplethysmograph, US Patent 5 140 990, assigned to SpaceLabs, Inc. (Redmond, WA), August 25, 1992.
73. G. Warner, Method and apparatus for measurement of heart-related parameters, US Patent 4 418 700, assigned to S. Warner (St. Laurent, CA) and T. S. Sankar (Brossard, CA), December 6, 1983.
74. Plethysmographs, selected instruments by year, [electronic:] http://vlp.mpiwg-berlin.mpg.de/technology/search?-max=10&-title=1&-op_varioid=numerical&varioid=9, 2012. Max Planck Institute for the History of Science, Berlin.
75. V. Panwar, K. Cha, J.-O. Park, and S. Park, High actuation response of PVDF/PVP/PSSA based ionic polymer metal composites actuator, Sensors and Actuators B: Chemical, 161(1):460–470, January 2012.
76. V. Panwar, B.-S. Kang, J.-O. Park, and S.-H. Park, New ionic polymer-metal composite actuators based on PVDF/PSSA/PVP polymer blend membrane, Polymer Engineering & Science, 51(9):1730–1741, September 2011.
77. V.B. Sundaresan and H. Zhang, Chemomechanical transduction in hybrid bio-derived conducting polymer actuator, in ASME 2010 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Vol. 1, pp. 695–701. ASME International, 2010.
78. S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao, and F. Huang, A dual-responsive supramolecular polymer gel formed by crown ether based molecular recognition, Angewandte Chemie International Edition, 50(8):1905–1909, 2011.
79. J. Fang, J. Chen, W. Wang, K. Varahramyan, R.A. Gunasekaran, and M. Agarwal, Wide-angle variable focal length lens system, US Patent 7 359 124, assigned to Louisiana Tech. University Research Foundation as a division of the Louisiana Tech. University Foundation (Ruston, LA) N/A (April 15, 2008).
80. S. Sato, Applications of liquid crystals to variable-focusing lenses, Optical Review, 6(6):471–485, November 1999.
81. B. Berge and J. Peseux, Variable focal lens controlled by an external voltage: An application of electrowetting, The European Physical Journal E, 3(2):159–163, October 2000.
82. S. Kwon and L. Lee, Focal length control by microfabricated planar electrodes-based liquid lens (μPELL), in Proceedings Volume, Vol. 1, pp. 10–14, Munich, Germany, 2001. 11th International conference on solid-state sensors and actuators: Transducers.
83. V. Vunder, A. Punning, and A. Aabloo, Variable-focal lens using electroactive polymer actuator, Proceedings of SPIE, 79771(E), May 2011.
84. P. Kim, L.D. Zarzar, M. Khan, M. Aizenberg, and J. Aizenberg, Environmentally responsive active optics based on hydrogel-actuated deformable mirror arrays, in W.V. Schoenfeld, J.J. Wang, M. Loncar, and T.J. Suleski, eds., Advanced Fabrication Technologies for Micro/Nano Optics and Photonics IV, Vol. 792705, pp. 1–7, San Francisco, CA, 2011. International Society for Optical Engineering.
85. B. Verhaar, B. Dirkx, M. Bauer, and F. Sahin Nomaler, Camera diaphragm and lens positioning system employing a dielectrical polymer actuator, US Patent Application 20 090 161 239, assigned to Koninklijke Philips Electronics, N.V. Eindhoven nl, June 25, 2009.
86. Y. Naka, M. Fuchiwaki, and K. Tanaka, A micropump driven by a polypyrrole-based conducting polymer soft actuator, Polymer International, 59(3):352–356, March 2010.
87. M. Fuchiwaki, Y. Naka, and K. Tanaka, Performance of a micro pump driven by conducting polymer soft actuator based on polypyrrole, Advanced Materials Research, 93–94:615–618, January 2010.
88. M.E. Piyasena, R. Newby, T.J. Miller, B. Shapiro, and E. Smela, Electroosmotically driven microfluidic actuators, Sensors and Actuators B: Chemical, 141(1):263–269, August 2009.
89. J.M. Karlinsey, Sample introduction techniques for microchip electrophoresis: A review, Analytica Chimica Acta, 725:1–13, May 2012.
90. A.K. Price, K.M. Anderson, and C.T. Culbertson, Demonstration of an integrated electroactive polymer actuator on a microfluidic electrophoresis device, Lab on a Chip, 9(14):2076, 2009.
