Chapter 4: Column Distillation: Internal Stage-by-Stage Balances
4.1 Internal Balances
In Chapter 3 we introduced column distillation and developed the external balance equations. In this chapter we start looking inside the column. For binary systems the number of stages required for the separation can conveniently be obtained by use of stage-by-stage balances. We start at the top of the column and write the balances and equilibrium relationship for the first stage, and then once we have determined the unknown variables for the first stage we write balances for the second stage. Utilizing the variables just calculated we can again calculate the unknowns. We can now proceed down the column in this stage-by-stage fashion until we reach the bottom. We could also start at the bottom and proceed upwards. This procedure assumes that each stage is an equilibrium stage, but this assumption may not be true. Ways to handle nonequilibrium stages are discussed in Section 4.11.
In the enriching section of the column it is convenient to use a balance envelope that goes around the desired stage and around the condenser. This is shown in Figure 4-1. For the first stage the balance envelope is shown in Figure 4-1A. The overall mass balance is then
(4-1, stage 1)
![]()
The more volatile component mass balance is
(4-2, stage 1)
![]()
For a well-insulated, adiabatic column, the energy balance is
(4-3, stage 1)
![]()
Figure 4-1 Enriching section balance envelopes; (A) stage 1, (B) stage 2, (C) stage j

Assuming that each stage is an equilibrium stage, we know that the liquid and vapor leaving the stage are in equilibrium. For a binary system, the Gibbs phase rule becomes
Degrees of freedom = C − P + 2 = 2 − 2 + 2 = 2
Since pressure has been set, there is one remaining degree of freedom. Thus for the equilibrium stage the variables are all functions of a single variable. For the saturated liquid we can write
(4-4a, stage 1)
![]()
and for the saturated vapor,
(4-4b, stage 1)
![]()
The liquid and vapor mole fractions leaving a stage are also related:
(4-4c, stage 1)
![]()
Equations (4-4) for stage 1 represent the equilibrium relationship. Their exact form depends on the chemical system being separated. Equations (4-1) to (4-4c) are six equations with six unknowns: L1, V2, x1, y2, H2, and h1.
Since we have six equations and six unknowns, we can solve for the six unknowns. The exact methods for doing this are the subject of the remainder of this chapter. For now we will just note that we can solve for the unknowns and then proceed to the second stage. For the second stage we will use the balance envelope shown in Figure 4-1B. The mass balances are now
(4-1, stage 2) or (4-1, stage 2)
![]()
(4-2, stage 2)
![]()
while the energy balance is
(4-3, stage 2)
![]()
The equilibrium relationships are
(4-4, stage 2)
![]()
Again we have six equations with six unknowns. The unknowns are now L2, V3, x2, y3, H3, and h2.
We can now proceed to the third stage and utilize the same procedures. After that, we can go to the fourth stage and then the fifth stage and so forth. For a general stage j (j can be from 1 to f − 1, where f is the feed stage) in the enriching section, the balance envelope is shown in Figure 4-1C. For this stage the mass and energy balances are
(4-1, stage j)
![]()
(4-2, stage j)
![]()
and
(4-3, stage j)
![]()
while the equilibrium relationships are
(4-4, stage j)
![]()
When we reach stage j, the values of yj, Qc, D, and hD will be known, and the unknown variables will be Lj, Vj+1, xj, yj+1, Hj+1, and hj. At the feed stage, the mass and energy balances will change because of the addition of the feed stream.
Before continuing, we will stop to note the symmetry of the mass and energy balances and the equilibrium relationships as we go from stage to stage. A look at Eqs. (4-1) for stages 1, 2, and j will show that these equations all have the same structure and differ only in subscripts. Equations (4-1) or (4-1) can be obtained from the general Eq. (4-1stage j) by replacing j with 1 or 2, respectively. The same observations can be made for the other Eqs. (Equations 4-2, Equations 4-3, 4-4a, 4-4b, and 4-4c). The unknown variables as we go from stage to stage are also similar and differ in subscript only.
In addition to this symmetry from stage to stage, there is symmetry between equations for the same stage. Thus Eqs. (4-1 stage j), (4-2 stage j), and (4-3 stage j) are all steady-state balances that state
In all three equations the output (of overall mass, solute, or energy) is associated with streams Lj and D. The input is associated with stream Vj+1 and (for energy) with the cooling load, Qc.
Below the feed stage the balance equations must change, but the equilibrium relationships in Eqs. (4-4a, b, c) will be unchanged. The balance envelopes in the stripping section are shown in Figure 4-2 for a column with a partial reboiler. The bars over flow rates signify that they are in the stripping section. It is traditional and simplest to write the stripping section balances around the bottom of the column using the balance envelope shown in Figure 4-2. Then these balances around stage f + 1 (immediately below the feed plate) are
(4-5, stage f + 1)
![]()
(4-6, stage f + 1)
![]()
(4-7, stage f + 1)
![]()
Figure 4-2 Stripping section balance envelopes; (A) below feed stage (stage f + 1), (B) stage k, (C) partial reboiler

The equilibrium relationships are Eqs. (Equations 4-4) written for stage f + 1.
(4-4, stage f + 1)
![]()
These six equations have six unknowns: Lf, ![]() , xf, yf+1, Hf+1, and hf. xB is specified in the problem statement; B and QR were calculated from the column balances; and yf (required for the last equation) was obtained from the solution of Eqs. (4-1 stage j) to (4-4 stage cj) with j = f − 1. At the feed stage we change from one set of balance envelopes to another.
, xf, yf+1, Hf+1, and hf. xB is specified in the problem statement; B and QR were calculated from the column balances; and yf (required for the last equation) was obtained from the solution of Eqs. (4-1 stage j) to (4-4 stage cj) with j = f − 1. At the feed stage we change from one set of balance envelopes to another.
Note that the same equations will be obtained if we write the balances above stage f + 1 and around the top of the distillation column (use a different balance envelope). This is easily illustrated with the overall mass balance, which is now
![]()
Rearranging, we have
![]()
However, since the external column mass balance says F − D = B, the last equation becomes
![]()
which is Eq. (4-5,. Similar results are obtained for the other balance equations.
Once the six Eqs. (4-4a) to (4-7) for stage f + 1 have been solved, we can proceed down the column to the next stage, f + 2. For a balance envelope around general stage k as shown in Figure 2-2B, the equations are
(4-5, stage k)
![]()
(4-6, stage k)
![]()
(4-7, stage k)
![]()
the equilibrium expression will correspond to Eqs. (4-4, stage f + 1) with k − 1 replacing f as a subscript. Thus,
(4-4, stage k)
![]()
A partial reboiler as shown in Figure 4-2C acts as an equilibrium contact. If we consider the reboiler as stage N + 1, the balances for the envelope shown in Figure 4-2C can be obtained by setting k = N + 1 and k − 1 = N in Eqs. (4-5k), (4-6k) and (4-7k).
If xN+1 = xB, the N + 1 equilibrium contacts gives us exactly the specified separation, and the problem is finished. If xN+1 < xB while xN > xB, the N + 1 equilibrium contacts gives slightly more separation than is required.
Just as the balance equations in the enriching section are symmetric from stage to stage, they are also symmetric in the stripping section.
4.2 Binary Stage-by-Stage Solution Methods
The challenge for any stage-by-stage solution method is to solve the three balance equations and the three equilibrium relationships simultaneously in an efficient manner. This problem was first solved by Sorel (1893), and graphical solutions of Sorel’s method were developed independently by Ponchon (1921) and Savarit (1922). These methods all solve the complete mass and energy balance and equilibrium relationships stage by stage. Starting at the top of the column as shown in Figure 4-1A, we can find the liquid composition, x1, in equilibrium with the leaving vapor composition, y1, from Eq. (4-4c). The liquid enthalpy, h1, is easily found from Eqs. (4-4a). The remaining four Eqs. (4-1) to (4-3) and (4-4b) for stage 1 are coupled and must be solved simultaneously. The Ponchon-Savarit method does this graphically. The Sorel method uses a trial-and-error procedure on each stage.
The trial-and-error calculations on every stage of the Sorel method are obviously slow and laborious. Lewis (1922) noted that in many cases the molar vapor and liquid flow rates in each section (a region between input and output ports) were constant. Thus in Figures 4-1 and 4-2,
(4-8)

and
(4-9)

For each additional column section there will be another set of equations for constant flow rates. Note that in general ![]() and
and ![]() . Equations (4-8) and (4-9) will be valid if every time a mole of vapor is condensed a mole of liquid is vaporized. This will occur if:
. Equations (4-8) and (4-9) will be valid if every time a mole of vapor is condensed a mole of liquid is vaporized. This will occur if:
1. The column is adiabatic.
2. The specific heat changes are small compared to latent heat changes.
(4-10)
![]()
3. The heat of vaporization per mole, λ, is constant; that is, λ does not depend on concentration. Condition 3 is the most important criterion. Lewis called this set of conditions constant molal overflow (CMO). An alternative to conditions 2 and 3 is
4. The saturated liquid and vapor lines on an enthalpy-composition diagram (in molar units) are parallel.
For some systems, such as hydrocarbons, the latent heat of vaporization per kilogram approximately is constant. Then the mass flow rates are constant, and constant mass overflow should be used.
The Lewis method assumes before the calculation is done that CMO is valid. Thus Eqs. (4-8) and (4-9) are valid. With this assumption, the energy balance, Eqs. (4-3) and (4-7), will be automatically satisfied. Then only Eqs. (4-1), (4-2), and (4-4c), or (4-5), (4-6), and (4-4c) need be solved. Eqs. (4-1 stage j) and (4-2 stage j) can be combined. Thus,
(4-11)
![]()
(4-12a)
![]()
Since L and V are constant, this equation becomes
(4-12b)
![]()
Eq. (4-12b) is the operating equation in the enriching section. It relates the concentrations of two passing streams in the column and thus represents the mass balances in the enriching section. Eq. (4-12b) is solved sequentially with the equilibrium expression for xj, which is Eq. (4-4cj).
To start we first use the column balances to calculate D and B. Then L0 = (L0/D)D and V1 = L0 + D. For a saturated liquid reflux, L0 = L1 = L2 = L and V1 = V2 = V. At the top of the column we know that y1 = xD. The vapor leaving the top stage is in equilibrium with the liquid leaving this stage (see Figure 4-1A). Thus x1 can be calculated from Eq. (4-4cj) with j = 1. Then y2 is found from Eq. (4-12) with j = 1. We then proceed to the second stage, set j = 2, and obtain x2 from Eq. (4-4cj) and y3 from Eq. (4-12b). We continue this procedure down to the feed stage.
In the stripping section, Eqs. (4-5k) and (4-6k) are combined to give
(4-13)
![]()
With CMO, ![]() and
and ![]() are constant, and the resulting stripping section operating equation is
are constant, and the resulting stripping section operating equation is
(4-14)
![]()
Once we know ![]() /
/![]() we can obviously alternate between the operating Eq. (4-14) and the equilibrium Eq. (4-4ck).
we can obviously alternate between the operating Eq. (4-14) and the equilibrium Eq. (4-4ck).
The phase and temperature of the feed obviously affect the vapor and liquid flow rates in the column. For instance, if the feed is liquid, the liquid flow rate below the feed stage must be greater than liquid flow above the feed stage, ![]() > L. If the feed is a vapor, V >
> L. If the feed is a vapor, V > ![]() . These effects can be quantified by writing mass and energy balances around the feed stage. The feed stage is shown schematically in Figure 4-3. The overall mass balance and the energy balance for the balance envelope shown in Figure 4-3 are
. These effects can be quantified by writing mass and energy balances around the feed stage. The feed stage is shown schematically in Figure 4-3. The overall mass balance and the energy balance for the balance envelope shown in Figure 4-3 are
(4-15)
![]()
and
(4-16)
![]()
(Despite the use of “hF” as the symbol for the feed enthalpy, the feed can be a liquid or vapor or a two-phase mixture.) If we assume CMO neither the vapor enthalpies nor the liquid enthalpies vary much from stage to stage. Thus Hf+1 ˜ Hf and hf−1 ~ hf. Then Eq. (4-16) can be written as
![]()
The mass balance Eq. (4-15) can be conveniently solved for ![]() − V,
− V,
![]()
Which can be substituted into the energy balance to give us
![]()
Combining terms, this is
![]()
or
(4-17)
![]()
In words, the “quality” q is
(4-18)

This result is analogous to the use of q in flash distillation. Since the liquid and vapor enthalpies can be estimated, we can calculate q from Eq. (4-17). Then
(4-19)
![]()
The quality q is the fraction of feed that is liquid. For example, if the feed is a saturated liquid, hF = h, q = 1, and ![]() = L + F. Once
= L + F. Once ![]() has been determined,
has been determined, ![]() is calculated from either Eq. (4-15) or Eq. (4-5, stage f + 1) or from
is calculated from either Eq. (4-15) or Eq. (4-5, stage f + 1) or from
(4-20)
![]()
Which can be derived from Eqs. (4-15) and (4-19).
Figure 4-3. Feed-stage balance envelope

EXAMPLE 4-1. Stage-by-stage calculations by the Lewis method
A steady-state countercurrent, staged distillation column is to be used to separate ethanol from water. The feed is a 30 wt % ethanol, 70 wt % water mixture that is a saturated liquid at 1 atm pressure. Flow rate of feed is 10,000 kg/hr. The column operates at a pressure of 1 atm. The reflux is returned as a saturated liquid. A reflux ratio of L/D = 3.0 is being used. We desire a bottoms composition of xB = 0.05 (weight fraction ethanol) and a distillate composition of xD = 0.80 (weight fraction ethanol). The system has a total condenser and a partial reboiler. The column is well insulated.
Use the Lewis method to find the number of equilibrium contacts required if the feed is input on the second stage from the top.
Solution
A. Define. The column and known information are shown in the following figure. Find the number of equilibrium contacts required.

B. Explore. Except for some slight changes in the feed temperature and column pressure, this problem is very similar to Example 3-1. The solution for B and D obtained in that example is still correct. B = 6667 kg/hr, D = 3333 kg/hr. Equilibrium data are available in weight fractions in Figure 2-4 and in mole fraction units in Figure 2-2 and Table 2-1. To use the Lewis method we must have CMO. We can check this by comparing the latent heat per mole of pure ethanol and pure water. (This checks the third and most important criterion for CMO. Since the column is well insulated, the first criterion, adiabatic, will be satisfied.) The latent heats are (Himmelblau, 1974):
λE = 9.22 kcal/g-mole, λW = 9.7171 kcal/g-mole
The difference of roughly 5% is reasonable particularly since we always use the ratio of L/V or ![]() . (Using the ratio causes some of the change in L and V to divide out.) Thus we will assume CMO. Now we must convert flows and compositions to molar units.
. (Using the ratio causes some of the change in L and V to divide out.) Thus we will assume CMO. Now we must convert flows and compositions to molar units.
C. Plan. First, convert to molar units. Carry out preliminary calculations to determine L/V and ![]() . Then start at the top, alternating between equilibrium (Figure 2-2) and the top operating Eq. (4-12b). Since stage 2 is the feed stage, calculate y3 from the bottom operating Eq. (4-14).
. Then start at the top, alternating between equilibrium (Figure 2-2) and the top operating Eq. (4-12b). Since stage 2 is the feed stage, calculate y3 from the bottom operating Eq. (4-14).
D. Do It. Preliminary Calculations: To Convert to Molar Units:
![]()
Average molecular weight of feed is
![]()
Feed rate = (10,000 kg/hr)/(22.03 kg/kmole) = 453.9 kmole/hr
![]()
For distillate, the average molecular weight is
![]()
which is also the average for the reflux liquid and vapor stream V since they are all the same composition.
Then D = (3333 kg/hr)/35.08 = 95.2 kg moles/hr
and
![]()
while V = L + D = 380.9
![]()
Because of CMO, L/V is constant in the rectifying section.
Since the feed is a saturated liquid,
![]()
where we have converted F to kg moles/hr. Since a saturated liquid feed does not affect the vapor, ![]() . Thus,
. Thus,
![]()
An internal check on consistency is L/V < 1 and ![]() .
.
Stage-by-Stage Calculations: At the top of the column, y1 = xD = 0.61. Liquid stream L1 of concentration x1 is in equilibrium with the vapor stream y1. From Figure 2-2, x1 = 0.4. (Note that y1 > x1 since ethanol is the more volatile component.) Vapor stream y2 is a passing stream relative to x1 and can be determined from the operating Eq. (4-12).
![]()
Stream x2 is in equilibrium with y2. From Figure 2-2 we obtain x2 = 0.11.
Since stage 2 is the feed stage, use bottom operating Eq. (4-14) for y3.
![]()
Stream x3 is in equilibrium with y3. From Figure 2-2, this is x3 = 0.02. Since x3 = xB (in mole fraction), we are finished.
The third equilibrium contact would be the partial reboiler. Thus the column has two equilibrium stages plus the partial reboiler.
E. Check. This is a small number of stages. However, not much separation is required, the external reflux ratio is large, and the separation of ethanol from water is easy in this concentration range. Thus the answer is reasonable. We can check the calculation of L/V with mass balances.
Since V1 = L0 + D,

Since L0, V1, and D, are the same composition, L0/D and L0/V1 have the same values in mass and molar units.
F. Generalizations. We should always check that CMO is valid. Then convert all flows and compositions into molar units. The procedure for stepping off stages is easily programmed on a spreadsheet (Burns and Sung, 1996). We could also have started at the bottom and worked our way up the column stage by stage. Going up the column we calculate y values from equilibrium and x values from the operating equations.
Note that L/V < 1 and ![]() . This makes sense, since we must have a net flow of material upwards in the rectifying section (to obtain a distillate product) and a net flow downwards in the stripping section. We must also have a net upward flow of ethanol in the rectifying section (Lxj < Vyj+1) and in the stripping section
. This makes sense, since we must have a net flow of material upwards in the rectifying section (to obtain a distillate product) and a net flow downwards in the stripping section. We must also have a net upward flow of ethanol in the rectifying section (Lxj < Vyj+1) and in the stripping section ![]() . These conditions are satisfied by all pairs of passing streams.
. These conditions are satisfied by all pairs of passing streams.
The Lewis method is obviously much faster and more convenient than the Sorel method. It is also easier to program on a computer or in a spreadsheet. In addition, it is easier to understand the physical reasons why separation occurs instead of becoming lost in the algebraic details. However, remember that the Lewis method is based on the assumption of CMO. If CMO is not valid, the answers will be incorrect.
If the calculation procedure in the Lewis method is confusing to you, continue on to the next section. The graphical McCabe-Thiele procedure explained there is easier for many students to understand. After completing the McCabe-Thiele procedure, return to this section and study the Lewis method again.
4.3 Introduction to the McCabe-Thiele Method
McCabe and Thiele (1925) developed a graphical solution method based on Lewis’s method and the observation that the operating Eqs. (4-12b) and (4-14) plot as straight lines (the operating lines) on a y-x diagram. On this graph the equilibrium relationship can be solved from the y-x equilibrium curve and the mass balances from the operating lines.
To illustrate, consider a typical design problem for a binary distillation column such as the one illustrated in Figure 3-8. We will assume that equilibrium data are available at the operating pressure of the column. These data are plotted as shown in Figure 4-4. At the top of the column is a total condenser. As noted in Chapter 3 in Eq. (3-7), this means that y1 = xD = x0. The vapor leaving the first stage is in equilibrium with the liquid leaving the first stage. This liquid composition, x1, can be determined from the equilibrium curve at y = y1. This is illustrated in Figure 4-4.
Figure 4-4 Equilibrium for top stage on McCabe-Thiele diagram

Liquid stream L1 of composition x1 passes vapor stream V2 of composition y2 inside the column (Figures 3-8 and 4-1A). When the mass balances are written around stage 1 and the top of the column (see balance envelope in Figure 4-1A), the result after assuming CMO and doing some algebraic manipulations is Eq. (4-12) with j = 1. This equation can be plotted as a straight line on the y-x diagram. Suppressing the subscripts j+1 and j, we write Eq. (4-12b) as
(4-21)
![]()
which is understood to apply to passing streams. Eq. (4-21) plots as a straight line (the top operating line) with a slope of L/V and a y intercept (x = 0) of (1 − L/V)xD. Once Eq. (4-12) has been plotted, y2 is easily found from the y value at x = x1. This is illustrated in Figure 4-5. Note that the top operating line goes through the point (y1, xD) since these coordinates satisfy Eq. (4-21).
Figure 4-5 Stage 1 calculation on McCabe-Thiele diagram

With y2 known we can proceed down the column. Since x2 and y2 are in equilibrium, we easily obtain x2 from the equilibrium curve. Then we obtain y3 from the operating line (mass balances), since x2 and y3 are the compositions of passing streams. This procedure of stepping off stages is shown in Figure 4-6. It can be continued as long as we are in the rectifying section. Note that this produces a staircase on the y-x, or McCabe-Thiele, diagram. Instead of memorizing this procedure, you should follow the points on the diagram and compare them to the schematics of a distillation column (Figures 3-8 and 4-1). Note that the horizontal and vertical lines have no physical meaning. The points on the equilibrium curve (squares) represent liquid and vapor streams leaving an equilibrium stage. The points on the operating line (circles) represent the liquid and vapor streams passing each other in the column.
Figure 4-6 Stepping off stages in rectifying section

In the stripping section the top operating line is no longer valid, since different mass balances and, hence, a different operating equation are required. The stripping section operating equation was given in Eq. (4-14). When the subscripts k and k − 1 are suppressed, this equation becomes
(4-22)
![]()
Eq. (4-22) plots as a straight line with slope ![]() and y intercept
and y intercept ![]() , as shown in Figure 4-7. This bottom operating line applies to passing streams in the stripping section. Starting with the liquid leaving the partial reboiler, of mole fraction xB = xN+1, we know that the vapor leaving the partial reboiler is in equilibrium with xB. Thus we can find yN+1 from the equilibrium curve. xN is easily found from the bottom operating line, since liquid of composition xN is a passing stream to vapor of composition yN+1 (compare Figures 4-2 and 4-7). We can continue alternating between the equilibrium curve and the bottom operating line as long as we are in the stripping section.
, as shown in Figure 4-7. This bottom operating line applies to passing streams in the stripping section. Starting with the liquid leaving the partial reboiler, of mole fraction xB = xN+1, we know that the vapor leaving the partial reboiler is in equilibrium with xB. Thus we can find yN+1 from the equilibrium curve. xN is easily found from the bottom operating line, since liquid of composition xN is a passing stream to vapor of composition yN+1 (compare Figures 4-2 and 4-7). We can continue alternating between the equilibrium curve and the bottom operating line as long as we are in the stripping section.
Figure 4-7 Stepping off stages in stripping section

If we are stepping off stages down the column, at the feed stage f we switch from the top operating line to the bottom operating line (refer to Figure 4-3, a schematic of the feed stage). Above the feed stage, we calculate xf−1 from equilibrium and yf from the top operating line. Since liquid and vapor leaving the feed stage are assumed to be in equilibrium, we can determine xf from the equilibrium curve at y = yf and then find yf+1 from the bottom operating line. This procedure is illustrated in Figure 4-8A, where stage 3 is the feed stage. The separation shown in Figure 4-8A would require 5 equilibrium stages plus an equilibrium partial reboiler, or 6 equilibrium contacts, when stage 3 is used as the feed stage. In this problem, stage 3 is the optimum feed stage. That is, a separation will require the fewest total number of stages when feed stage 3 is used. Note in Figure 4-8B and 4-8C that if stage 2 or stage 5 is used, more total stages are required. For binary distillation the optimum feed plate is easy to determine; it will always be the stage where the step in the staircase includes the point of intersection of the two operating lines (compare Figure 4-8A to Figures 4-8B and 4-8C). A mathematical analysis of the optimum feed plate location suitable for computer calculation with the Lewis method is developed later.
Figure 4-8 McCabe-Thiele diagram for entire column; (A) optimum feed stage (stage 3); (B) feed stage too high (stage 2); (C) feed stage too low (stage 5)

When stepping off stages from the top down, the fractional number of stages can be calculated as (see Figure 4-8B and 4-8C),
(4-23)
![]()
where the distances are measured horizontally on the diagram.
Now that we have seen how to do the stage-by-stage calculations on a McCabe-Thiele diagram, let us consider how to start with the design problem given in Figure 3-8 and Tables 3-1 and 3-2. The known variables are F, z, q, xD, xB, L0/D, p, saturated liquid reflux, and we use the optimum feed location. Since the reflux is a saturated liquid, then there will be no change in the liquid or vapor flow rates on stage 1 and L0 = L1 and V1 = V2. This allows us to calculate the internal reflux ratio, L/V, from the external reflux ratio, L0/D, which is specified.
(4-24)
![]()
With L/V and xD known, the top operating line is fully specified and can be plotted.
Since the boilup ratio, ![]() , was not specified, we cannot directly calculate
, was not specified, we cannot directly calculate ![]() , which is the slope of the bottom operating line. Instead, we need to utilize the condition of the feed to determine flow rates in the stripping section. The same procedure used with the Lewis method can be used here. The feed quality, q, is calculated from Eq. (4-17), which is repeated below:
, which is the slope of the bottom operating line. Instead, we need to utilize the condition of the feed to determine flow rates in the stripping section. The same procedure used with the Lewis method can be used here. The feed quality, q, is calculated from Eq. (4-17), which is repeated below:
(4-17)
![]()
Then ![]() is given by Eq. (4-19),
is given by Eq. (4-19), ![]() , and
, and ![]() . We can calculate L as (L/D)D, where D and B are found from mass balances around the entire column. Alternatively, for a simple column Eqs. (3-3) and (3-4) can be substituted into the equations for
. We can calculate L as (L/D)D, where D and B are found from mass balances around the entire column. Alternatively, for a simple column Eqs. (3-3) and (3-4) can be substituted into the equations for ![]() and
and ![]() . When this is done, we obtain
. When this is done, we obtain
(4-25)

With ![]() /
/![]() and xB known, the bottom operating equation is fully specified, and the bottom operating line can be plotted. Eq. (4-25) is convenient for computer calculations but is specific for the simple column shown in Figure 3-8. For graphical calculations the alternative procedure shown in the next section is usually employed.
and xB known, the bottom operating equation is fully specified, and the bottom operating line can be plotted. Eq. (4-25) is convenient for computer calculations but is specific for the simple column shown in Figure 3-8. For graphical calculations the alternative procedure shown in the next section is usually employed.
4.4 Feed Line
In any section of the column between feeds and/or product streams the mass balances are represented by the operating line. In general, the operating line can be derived by drawing a mass balance envelope through an arbitrary stage in the section and around the top or bottom of the column. When material is added or withdrawn from the column the mass balances will change and the operating lines will have different slopes and intercepts. In the previous section the effect of a feed on the operating lines was determined from the feed quality and mass balances around the entire column or from Eq. (4-25). Here we will develop a graphical method for determining the effect of a feed on the operating lines.
Consider the simple single-feed column with a total condenser and a partial reboiler shown in Figure 3-8. The mass balance in the rectifying section for the more volatile component is
(4-26)
![]()
while the balance in the stripping section is
(4-27)
![]()
where we have assumed that CMO is valid. At the feed plate we switch from one mass balance to the other. We wish to find the point at which the top operating line—representing Eq. (4-26)—intersects the bottom operating line—representing Eq. (4-27).
The intersection of these two lines means that
(4-28)
![]()
Equations (4-28) are valid only at the point of intersection. Since the y’s and x’s are equal at the point of intersection, we can subtract Eq. (4-26) from Eq. (4-27) and obtain
(4-29)
![]()
From the overall mass balance around the entire column, Eq. (3-2), we know that the last term is −FzF. Then, solving Eq. (4-29) for y,
(4-30)
![]()
Eq. (4-30) is one form of the feed equation. Since L, ![]() , V,
, V, ![]() , F, and zF are constant, it represents a straight line (the feed line) on a McCabe-Thiele diagram. Every possible intersection point of the two operating lines must occur on the feed line.
, F, and zF are constant, it represents a straight line (the feed line) on a McCabe-Thiele diagram. Every possible intersection point of the two operating lines must occur on the feed line.
For the special case of a feed that flashes in the column to form a vapor and a liquid phase, we can relate Eq. (4-30) to flash distillation. In this case we have the situation shown in Figure 4-9. Part of the feed, VF, vaporizes, while the remainder is liquid, LF. Looking at the terms in Eq. (4-30), we note that ![]() − L is the change in liquid flow rates at the feed stage. In this case,
− L is the change in liquid flow rates at the feed stage. In this case,
(4-31)
![]()
Figure 4-9 Two phase feed

The change in vapor flow rates is
(4-32)
![]()
Equation (4-30) then becomes
(4-33)
![]()
which is essentially the same as Eq. (2-11), the operating equation for flash distillation. Thus the feed line represents the flashing of the feed into the column. Equation (4-33) can also be written in terms of the fraction vaporized, f = VF/F, as [see Eqs. (2-12) and (2-13)]
(4-34)
![]()
In terms of the fraction remaining liquid, q = LF/F [see Eqs. (2-14) and (2-15)], Eq. (4-33) is
(4-35)
![]()
Equations (4-33) to (4-35) were all derived for the special case where the feed is a two-phase mixture, but they can be used for any type of feed. For example, if we want to derive Eq. (4-35) for the general case we can start with Eq. (4-30). An overall mass balance around the feed stage (balance envelope shown in Figure 4-9) is
![]()
which can be rearranged to
![]()
Substituting this result into Eq. (4-30) gives
![]()
and dividing numerator and denominator of each term by the feed rate F, we get
![]()
which becomes Eq. (4-35), since q is defined to be (![]() )/F. Equation (4-34) can be derived in a similar fashion (obviously, another homework problem).
)/F. Equation (4-34) can be derived in a similar fashion (obviously, another homework problem).
Previously, we solved the mass and energy balances and found that
(4-17)
![]()
From Eq. (4-17) we can determine the value of q and hence the slope, q/(q − 1), of the feed line. For example, if the feed enters as a saturated liquid (that is, at the liquid boiling temperature at the column pressure), then hF = h and the numerator of Eq. (4-17) equals the denominator. Thus q = 1.0 and the slope of the feed line, q/(q − 1) = ∞. The feed line is vertical.
The various types of feeds and the slopes of the feed line are illustrated in Table 4-1 and Figure 4-10. Note that all the feed lines intersect at one point, which is at y = x. If we set y = x in Eq. (4-35), we obtain
(4-36)
![]()
as the point of intersection (try this derivation yourself). The feed line is easy to plot from the points y = x = zF or y intercept (x = 0) = zF/(1 − q) or x intercept (y = 0) = zF/q, and the slope, which is q/(q − 1). (This entire process of plotting the feed line should remind you of graphical binary flash distillation.)
Table 4-1 Feed conditions

Figure 4-10 Feed lines
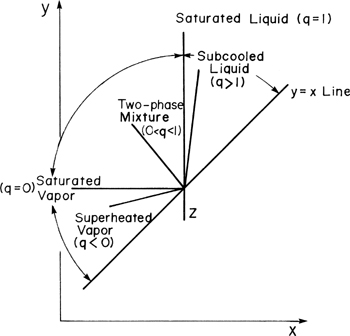
The feed line was derived from the intersection of the top and bottom operating lines. It thus represents all possible locations at which the two operating lines can intersect for a given feed (zF, q). Thus if we change the reflux ratio we change the points of intersection, but they all lie on the feed line. This is illustrated in Figure 4-11A. If the reflux ratio is fixed (the top operating line is fixed) but q varies, the intersection point varies as shown in Figure 4-11B. The slope of the bottom operating line, L/V, depends upon L0/D, xD, xB, and q as was shown in Eq. (4-25).
Figure 4-11 Operating line intersection; (A) changing reflux ratio with constant q; (B) changing q with fixed reflux ratio

In Figure 4-8 we illustrated how to determine the optimum feed stage graphically. For computer applications an explicit test is easier to use. If the point of intersection of the two operating lines (yI, xI), is determined, then the optimum feed plate, f, is the one for which
(4-37a)
![]()
(4-37b)
![]()
This is illustrated in Figure 4-12. The intersection point can be determined by straightforward but tedious algebraic manipulation as
(4-38)

for the simple column shown in Figure 3-8.
Figure 4-12 Optimum feed plate calculation

The feed equations were developed for this simple column; however, Eqs. (4-30), and (4-33) through (4-35) are valid for any column configuration if we generalize the definitions of f and q. In general,
(4-39a)
![]()
(4-39b)
![]()
EXAMPLE 4-2 Feed line calculations
Calculate the feed line slope for the following cases.
a. A two-phase feed where 80% of the feed is vaporized under column conditions.
The slope is q/(q − 1), where q = (Lbelow feed − Labove feed)/F (other expressions could also be used). With a two-phase feed we have the situation shown.

![]() . Since 80% of the feed is vapor, 20% is liquid and LF = 0.2F.
. Since 80% of the feed is vapor, 20% is liquid and LF = 0.2F.
Then

This agrees with Figure 4-10.
b. A superheated vapor feed where 1 mole of liquid will vaporize on the feed stage for each 9 moles of feed input.
Solution
Now the situation is shown in the following figure.

When the feed enters, some liquid must be boiled to cool the feed. Thus,
(4-40)
![]()
and the amount vaporized is v = (1/9) F.
Thus,

which agrees with Figure 4-10.
c. A liquid feed subcooled by 35 ° F. Average liquid heat capacity is 30 Btu/lb-mole- ° F and λ = 15,000 Btu/lb-mole.
Solution
Here some vapor must be condensed by the entering feed. Thus the situation can be depicted as shown.

and ![]() , where c is the amount condensed.
, where c is the amount condensed.
Since the column is insulated, the source of energy to heat the feed to its boiling point is the condensing vapor.
(4-41a)
![]()
where ΔT = TBP − TF = 35°
or
(4-41b)

This agrees with Figure 4-10. Despite the large amount of subcooling, the feed line is fairly close to vertical, and the results will be similar to a saturated liquid feed. If TF is given instead of ΔT, we need to estimate TBP. This can be done with a temperature composition graph (Figure 2-3), an enthalpy-composition graph (Figure 2-4), or a bubble point calculation (Section 6.4).
d. A mixture of ethanol and water that is 40 mole % ethanol. Feed is at 40 ° C. Pressure is kg/cm2.
Solution
We can now use Eq. (4-17):
![]()
The enthalpy data are available in Figure 2-4. To use that figure we must convert to weight fraction. 0.4 mole fraction is 0.63 wt frac. Then from Figure 2-4 we have
hF(0.63, 40 ° C) = 20 kcal/kg
The vapor (represented by H) and liquid (represented by h) will be in equilibrium at the feed stage, but the concentrations of the feed stage are not known. Comparing the feed stage locations in Figures 4-8A, 4-8B, and 4-8C, we see that liquid and vapor concentrations on the feed stage can be very different and are usually not equal to the feed concentration z or to the concentrations of the intersection point of the operating line, yI and xI (Figure 4-12). However, since CMO is valid, H and h in molal units will be constant. We can calculate all enthalpies at a weight fraction of 0.63, convert the enthalpies to enthalpies per kilogram mole, and estimate q. From Figure 2-4 H (0.63, satd vapor) = 395, h (0.63, satd liquid) = 65 kcal/kg, and
![]()
Since all the molecular weights are at the same concentration, they divide out.

This agrees with Figure 4-10. Despite considerable subcooling, this feed line is also steep. Note that feed rate was not needed to calculate q or the slope for any of these calculations.
4.5 Complete McCabe-Thiele Method
We are now ready to put all the pieces together and solve a design distillation problem by the McCabe-Thiele method. We will do this in the following example.
EXAMPLE 4-3 McCabe-Thiele method
A distillation column with a total condenser and a partial reboiler is separating an ethanol-water mixture. The feed is 20 mole % ethanol, feed rate is 1000 kg moles/hr, and feed temperature is 80 ° F. A distillate composition of 80 mole % ethanol and a bottoms composition of 2 mole % ethanol are desired. The external reflux ratio is 5/3. The reflux is returned as a saturated liquid and CMO can be assumed. Find the optimum feed plate location and the total number of equilibrium stages required. Pressure is 1 atm.
A. Define. The column is sketched in the figure

Find the optimum feed plate location and the total number of equilibrium stages.
B. Explore. Equilibrium data at 1 atm is given in Figure 2-2. An enthalpy-composition diagram at 1 atm will be helpful to estimate q. These are available in other sources (e.g., Brown et al. (1950) or Foust et al. (1980) p. 36), or a good estimate of q can be made from Figure 2-4 despite the pressure difference. In Example 4-1 we showed that CMO is valid. Thus we can apply the McCabe-Thiele method.
C. Plan. Determine q from Eq. (4-17) and the enthalpy-composition diagram at 1 atm. Plot the feed line. Calculate L/V. Plot the top operating line; then plot the bottom operating line and step off stages.
D. Do It. Feed Line: To find q, first convert feed concentration, 20 mole %, to wt % ethanol = 39 wt %. Two calculations in different units with different data are shown.
Exact Calculation |
Approx Calc. |
Data at p = 1 atm from Brown et al. (1950) |
(p = 1 kg/cm2) using Figure 2-4 |
hF = 25 Btu/lb (80 ° F) |
hF = 15 kcal/kg (30 ° C) |
H = 880 Btu/lb (sat’d vapor) |
H = 485 kcal/kg (sat’d vapor) |
h = 125 Btu/lb (sat’d liquid) |
h = 70 kcal/kg (sat’d liquid) |
|
|
Thus small differences caused by pressure differences in the diagrams do not change the value of q. Note that molecular weight terms divide out as in Example 4-2d. Then
Feed line intersects y=x line at feed concentration z=0.2. Feed line is plotted in Figure 4-13.
Top Operating Line:

Alternative solution: Intersection of top operating line and y = x (solve top operating line and y = x simultaneously) is at y = x = xD. The top operating line is plotted in Figure 4-13.
Figure 4-13Solution for Example 4-3

![]()
We know that the bottom operating line intersects the top operating line at the feed line; this is one point. We could calculate ![]() from mass balances or from Eq. (4-25), but it easier to find another point. The intersection of the bottom operating line and the y=x line is at y=x=xB (see Problem 4-C9). This gives a second point.
from mass balances or from Eq. (4-25), but it easier to find another point. The intersection of the bottom operating line and the y=x line is at y=x=xB (see Problem 4-C9). This gives a second point.
The feed line, top operating line, and bottom operating line are shown in Figure 4-13. We stepped off stages from the bottom up (this is an arbitrary choice). The optimum feed stage is the second above the partial reboiler. 12 equilibrium stages plus a partial reboiler are required.
E. Check. We have a built-in check on the top operating line, since a slope and two points are calculated. The bottom operating line can be checked by calculating ![]() from mass balances and comparing it to the slope. The numbers are reasonable, since L/V < 1,
from mass balances and comparing it to the slope. The numbers are reasonable, since L/V < 1, ![]() > 1, and q > 1 as expected. The most likely cause of error in Figure 4-13 (and the hardest to check) is the equilibrium data.
> 1, and q > 1 as expected. The most likely cause of error in Figure 4-13 (and the hardest to check) is the equilibrium data.
F. Generalization. If constructed carefully, the McCabe-Thiele diagram is quite accurate. Note that there is no need to plot parts of the equilibrium diagram that are greater than xD or less than xB. Specified parts of the diagram can be expanded to increase the accuracy.
We did not have to use external balances in this example, while in Example 4-1 we did. This is because we used the feed line as an aid in finding the bottom operating line. The y=x intersection points are useful, but when the column configuration is changed their location may change.
4.6 Profiles for Binary Distillation
Figure 4-13 essentially shows the complete solution of Example 4-3; however, it is useful to plot compositions, temperatures, and flow rates leaving each stage (these are known as profiles). From Figure 4-13 we can easily find the ethanol mole fractions in the liquid and vapor leaving each stage. Then xW = 1 − xE and yW = 1 − yE. The temperature of each stage can be found from equilibrium data (Figure 2-3) because the stages are equilibrium stages. Since we assumed CMO, the flow rates of liquid and vapor will be constant in the enriching and stripping sections, and we can determine the changes in the flow rates at the feed stage from the calculated value of q.
The profiles are shown in Figure 4-14. As expected, the water concentration in both liquid and vapor streams decreases monotonically as we go up the column, while the ethanol concentration increases. Since the stages are discrete, the profiles are not smooth curves. Compare Figures 4-13 and 4-14. Note that the concentration and temperature and equilibrium curves are close together. When these two lines almost touch, we have a pinch point. Then the composition profiles will become almost horizontal and there will be very little change in composition from stage to stage. The location of a pinch point within the column depends on the system and the operating conditions.
Figure 4-14 Profiles for Example 4-3

In this ethanol-water column the temperature decreases rapidly for the first few contacts above the reboiler but is almost constant for the last eight stages. This occurs mainly because of the shape of the temperature-composition diagram for ethanol-water (see Figure 2-3).
Since we assumed CMO, the flow profiles are flat in each section of the column. As expected, ![]() >
> ![]() and V > L (a convenient check to use). Since stage 2 is the feed stage, L2 is in the stripping section while V2 is in the enriching section (draw a sketch of the feed stage if this isn’t clear). Different quality feeds will have different changes at the feed stage. Liquid and vapor flow rates can increase, decrease, or remain unchanged in passing from the stripping to the enriching section.
and V > L (a convenient check to use). Since stage 2 is the feed stage, L2 is in the stripping section while V2 is in the enriching section (draw a sketch of the feed stage if this isn’t clear). Different quality feeds will have different changes at the feed stage. Liquid and vapor flow rates can increase, decrease, or remain unchanged in passing from the stripping to the enriching section.
Figure 4-13 illustrates the main advantage of McCabe-Thiele diagrams. They allow us to visualize the separation. Before the common use of digital computers, large (sometimes covering a wall) McCabe-Thiele diagrams were used to design distillation columns. McCabe-Thiele diagrams cannot compete with the speed and accuracy of process simulators (see this chapter’s appendix) or for binary separations with spreadsheets; however, McCabe-Thiele diagrams still provide superior visualization of the separation (Kister, 1995). Ideally, McCabe-Thiele diagrams will be used in conjunction with process simulator results for both analysis and troubleshooting.
4.7 Open Steam Heating
We now have all the tools required to solve any binary distillation problem with the graphical McCabe-Thiele procedure. As a specific example, consider the separation of methanol from water in a staged distillation column.
EXAMPLE 4-4. McCabe-Thiele analysis of open steam heating
The feed is 60 mole % methanol and 40 mole % water and is input as a two-phase mixture that flashes so that VF/F = 0.3. Feed flow rate is 350 kg moles/hr. The column is well insulated and has a total condenser. The reflux is returned to the column as a saturated liquid. An external reflux ratio of L0/D = 3.0 is used. We desire a distillate concentration of 95 mole % methanol and a bottoms concentration of 8 mole % methanol. Instead of using a reboiler, saturated steam at 1 atm is sparged directly into the bottom of the column to provide boilup. (This is called direct or open steam.) Column pressure is 1 atm. Calculate the number of equilibrium stages and the optimum feed plate location.
Solution
A. Define. It helps to draw a schematic diagram of the apparatus, particularly since a new type of distillation is involved. This is shown in Figure 4-15. We wish to find the optimum feed plate location, NF, and the total number of equilibrium stages, N, required for this separation. We could also calculate Qc, D, B, and the steam rate S, but these were not asked for. We assume that the column is adiabatic since it is well insulated.
B. Explore. The first thing we need is equilibrium data. Fortunately, these are readily available (see Table 2-7 in Problem 2.D1).
Second, we would like to assume CMO so that we can use the McCabe-Thiele analysis procedure. An easy way to check this assumption is to compare the latent heats of vaporization per mole (Himmelblau, 1974).
ΔHvap methanol (at bp) = 8.43 kcal/g-mole
ΔHvap water (at bp) = 9.72 kcal/g-mole
These values are not equal, and in fact, water’s latent heat is 15.3% higher than methanol’s. Thus, CMO is not strictly valid; however, we will solve this problem assuming CMO and will check our results with a process simulator.
Figure 4-15 Distillation with direct steam heating, Example 4-4
A look at Figure 4-15 shows that the configuration at the bottom of the column is different than when a reboiler is present. Thus we should expect that the bottom operating equations will be different from those derived previously.
C. Plan. We will use a McCabe-Thiele analysis. Plot the equilibrium data on a y-x graph.
Top Operating Line: Mass balances in the rectifying section (see Fig. 4-15) are
Vj+1 = Lj + D
yj+1Vj+1 = Lj xj +DxD
Assume CMO and solve for yj+1.
| yj+1 = (L/V)xj + (1 − L/V)xD | |
| Slope = L/V, | y intercept (x = 0) = (1 − L/V) xD |
| Intersection y = x = xD |
Since the reflux is returned as a saturated liquid,
Enough information is available to plot the top operating line.
Feed Line:

Once we substitute in values, we can plot the feed line.
Bottom Operating Line: The mass balances are
![]()
Solve for y:
![]()
Simplifications: Since the steam is pure water vapor, ys = 0.0 (contains no methanol). Since steam is saturated, S = V and B = L (constant molal overflow). Then
(4-42)
![]()
Note this is different from the operating equation for the bottom section when a reboiler is present. slope = ![]() /
/![]() (unknow), y intercept = -(
(unknow), y intercept = -(![]() )xB(unknow),
)xB(unknow),
![]()
One known point is the intercept of the top operating line with the feed line. We still need a second point, and we can find it at the x intercept. When y is set to zero, x = xB (this is left as Problem 4-C1).
D. Do It. Equilibrium data are plotted on Figure 4-16.
Top Operating Line:
![]()
Figure 4-16 Solution for Example 4-4

y = x = xD = 0.95
y intercept = (1 − L/V)xD = 0.2375
We can plot this straight line as shown in Figure 4-16.
Feed Line: Slope = q/(q − 1) = 0.7/(0.7 − 1) = − 7/3.
Intersects at y = x = z = 0.6. Plotted in Figure 4-16.
Bottom Operating Line: We can plot this line between two points, the intercept of top operating line and feed line, and
x intercept (y = 0) = xB = 0.08
This is also shown in Figure 4-16.
Step off stages, starting at the top. x1 is in equilibrium with y1 at xD. Drawing a horizontal line to the equilibrium curve gives value x1. y2 and x1 are related by the operating line. At constant y2 (horizontal line), go to the equilibrium curve to find x2. Continue this stage-by-stage procedure.
Optimum feed stage is determined as in Figure 4-8A. Optimum feed in Figure 4-16 is on stage 3 or 4 (since by accident x3 is at intersection point of feed and operating lines). Since the feed is a two-phase feed, we would introduce it above stage 4 in this case.
Number of stages: Five is more than enough. We can calculate a fractional number of stages.
![]()
In Figure 4-16,
![]()
We need 4 + 0.9 = 4.9 equilibrium contacts.
E. Check. There are a series of internal consistency checks that can be made. Equilibrium should be a smooth curve. This will pick up misplotted points. L/V < 1 (otherwise no distillate product), and ![]() (otherwise no bottoms product). The feed line’s slope is in the correct direction for a two-phase feed. A final check on the assumption of CMO would be advisable since the latent heats vary by 15%.
(otherwise no bottoms product). The feed line’s slope is in the correct direction for a two-phase feed. A final check on the assumption of CMO would be advisable since the latent heats vary by 15%.
This problem was also run on the Aspen Plus process simulator (see problem 4.G1 and chapter appendix). Aspen Plus does not assume CMO and with an appropriate vapor-liquid equilibrium (VLE) correlation (the nonrandom two-liquid model was used) should be more accurate than the McCabe-Thiele diagram, which assumes CMO. With 5 equilibrium stages and feed on stage 4 (the optimum location), xD = 0.9335 and xB = 0.08365, which doesn’t meet the specifications. With 6 equilibrium stages and feed on stage 5 (the optimum), xD = 0.9646 and xB = 0.0768, which is slightly better than the specifications. The differences in the McCabe-Thiele and process simulation results are due to the error involved in assuming CMO and, to a lesser extent, differences in equilibrium. Note that the McCabe-Thiele diagram is useful since it visually shows the effect of using open steam heating.
F. Generalize. Note that the y = x line is not always useful. Don’t memorize locations of points. Learn to derive what is needed. The total condenser does not change compositions and is not counted as an equilibrium stage. The total condenser appears in Figure 4-16 as the single point y = x = xD. Think about why this is true. In general, all inputs to the column can change flow rates and hence slopes inside the column. The purpose of the feed line is to help determine this effect. The reflux stream and open steam are also inputs to the column. If they are not saturated streams the flow rates are calculated differently; this is discussed later.
Note that the open steam can be treated as a feed with q = (Lbelow – Labove)/S = ![]() . Thus,
. Thus, ![]() . The slope of this feed line is q/(q – 1) = 0 and it intersects the y = x line at y = x = z = 0, which means the feed line for saturated steam is the x-axis.
. The slope of this feed line is q/(q – 1) = 0 and it intersects the y = x line at y = x = z = 0, which means the feed line for saturated steam is the x-axis.
Ludwig (1997) states that one tray is used to replace the reboiler and 1/3 to one and possibly more trays to offset the water dilution; however, since reboilers are typically much more expensive than trays (see 11) this practice is economical. Open steam heating can be used even if water is not one of the original components.
4.8 General McCabe-Thiele Analysis Procedure
The open steam example illustrated one specific case. It is useful to generalize this analysis procedure. A section of the column is the segment of stages between two input or exit streams. Thus in Figure 4-15 there are two sections: top and bottom. Figure 4-17 illustrates a column with four sections. Each section’s operating equation can be derived independently. Thus, the secret (if that’s what it is) is to treat each section as an independent subproblem connected to the other subproblems by the feed lines (which are also independent).
Figure 4-17 Distillation column with four sections

An algorithm for any problem is the following:
1. Draw a figure of the column and label all known variables (e.g., as in Figure 4-15). Check to see if CMO is valid.
2. For each section:
a . Draw a mass balance envelope. We desire this envelope to cut the unknown liquid and vapor streams in the section and known streams (feeds, specified products or specified side-streams). The fewer streams involved, the simpler the mass balances will be. This step is important, since it controls how easy the following steps will be.
b . Write the overall and most volatile component mass balances.
c . Derive the operating equation.
d . Simplify.
e . Calculate all known slopes, intercepts, and intersections.
3. Develop feed line equations. Calculate q values, slopes, and y = x intersections.
4. For operating and feed lines:
a . Plot as many of the operating lines and feed lines as you can.
b . If all operating lines cannot be plotted, step off stages if the stage location of any feed or side stream is specified.
c . If needed, do external mass and energy balances (see Example 4-5). Use the values of D and B in step 2.
5. When all operating lines have been plotted, step off stages, determine optimum feed plate locations and the total number of stages. If desired, calculate a fractional number of stages.
Not all of these general steps were illustrated in the previous examples, but they will be illustrated in the examples that follow.
This problem-solving algorithm should be used as a guide, not as a computer code to be followed exactly. The wide variety of possible configurations for distillation columns will allow a lot of practice in using the McCabe-Thiele method for solving problems.
EXAMPLE 4-5. Distillation with two feeds
We wish to separate ethanol from water in a distillation column with a total condenser and a partial reboiler. We have 200 kg moles/hr of feed 1, which is 30 mole % ethanol and is saturated vapor. We also have 300 kg moles/hr of feed 2, which is 40 mole % ethanol. Feed 2 is a subcooled liquid. One mole of vapor must condense inside the column to heat up 4 moles of feed 2 to its boiling point. We desire a bottoms product that is 2 mole % ethanol and a distillate product that is 72 mole % ethanol. External reflux ratio is L0/D = 1.0. The reflux is a saturated liquid. Column pressure is 101.3 kPa, and the column is well insulated. The feeds are to be input at their optimum feed locations. Find the optimum feed locations (reported as stages above the reboiler) and the total number of equilibrium stages required.
Figure 4-18 Two-feed distillation column for Example 4-5

A . Define. Again a sketch will be helpful; see Figure 4-18. Since the two feed streams are already partially separated, it makes sense to input them separately to maintain the separation that already exists. We have made an inherent assumption in Figure 4-18. That is, feed 2 of higher mole fraction ethanol should enter the column higher up than feed 1. This assumption will be checked when the optimum feed plate locations are calculated, but it will affect the way we do the preliminary calculations. Since the feed plate locations were asked for as stages above the reboiler, the stages have been numbered from the bottom up.
B . Explore. Obviously, equilibrium data are required, and they are available from Figure 2-2. We already checked (Example 4-1) that CMO is a reasonable assumption. A look at Figure 4-18 shows that the top section is the same as top sections used previously. The bottom section is also familiar. Thus the new part of this problem is the middle section. There will be two feed lines and three operating lines.
C . Plan. We will look at the two feed lines, top operating line, bottom operating line, and middle operating line. The simple numerical calculations will also be done here.
![]()
Feed 1: Saturated vapor, q1 = 0, slope = 0, y intercept = z1/(q1 − 1) = 0.3, intersection at y =x=z1=0.3.
Feed 2:
![]()
The feed stage for feed 2 looks schematically as shown in the figure:

Then, Lbelow feed = L′ = L + F2 + c
Amount condensed = c = (1/4) F2,

Intersection: y = x = z2 = 0.4
Top Operating Line: We can derive the top operating equation:
![]()
This is the usual top operating line. With saturated liquid reflux the slope is,
![]()
Intersection: y = x = xD = 0.72
Bottom Operating Line: We can derive the bottom operating equation:
![]()
This is the usual bottom operating line. Then, slope = ![]() /
/![]() is unknown.
is unknown.
y = x intersection is at y = x = xB = 0.02. One other point is the intersection of the F1 feed line and the middle operating line, if we can find the middle operating line.
Middle Operating Line: To derive an operating equation for the middle section, we can write a mass balance around the top or the bottom of the column. The resulting equations will look different, but they are equivalent. We arbitrarily use the mass balance envelope around the top of the column as shown in Figure 4-18. These mass balances are
F2 + V′ = L′ + D
F2z2 + V′ y = L′ x + DxD (MVC)
Solve this equation for y to develop the middle operating equation,
(4-44)
![]()
One known point is the intersection of the F2 feed line and the top operating line. A second point is needed. We can try y intercept =
![]()
but this is unknown.
The intersection of the middle operating line with the y = x line is found by setting y = x in Eq. (4-44) and solving,
![]()
From the overall balance equation,
V′ − L′ = D − F2
Thus,
(4-45)
![]()
This point is not known, but it can be calculated once D is known.
Slope = L′/V′ is unknown, but it can be calculated from the feed-stage calculation. From the definition of q2,
(4-46)
![]()
where L = (L/D)D. Once D is determined, L and then L′ can be calculated. Then the mass balance gives
V′ = L′ + D − F2
and the slope L′/V′ can be calculated.
The conclusion from these calculations is, we have to calculate D. To do this we need external mass balances:
(4-47a)
![]()
(4-47b)
![]()
These two equations have two unknowns, D and B, and we can solve for them. Rearranging the first, we have B = F1 + F2 − D. Then, we substitute this into the more volatile component (MVC) mass balance,
F1z1 + F2z2 = DxD + (F1 + F2)xB −DxB
and we solve for D:
(4-48)
![]()
D . Do It. The two feed lines and the top operating line can immediately be plotted on a y-x diagram. This is shown in Figure 4-19. Before plotting the middle operating line we must find D from Eq. (4-48).

Now the middle operating line y=x intercept can be determined from Eq. (4-45)
This intersection point could be used, but it is off of the graph in Figure 4-19. Instead of using a larger sheet of paper, we will calculate the slope L′/V′.
![]()
L′ = 300(5/4) + (1.0)(242.9) = 617.9 kg moles/hr
V′ = L′ + D − F2 = 617.9 + 242.9 − 300 = 560.8
L′/V′ = 1.10
Now the middle operating line is plotted in Figure 4-19 from the intersection of feed line 2 and the top operating line, with a slope of 1.10. The bottom operating line then goes from y=x=xB to the intersection of the middle operating line and feed line 1 (see Figure 4-19).
Since the feed locations were desired as stages above the reboiler, we step off stages from the bottom up, starting with the partial reboiler as the first equilibrium contact. The optimum feed stage for feed 1 is the first stage, while the optimum feed stage for feed 2 is the second stage. 6 stages + partial reboiler are more than sufficient.
Figure 4-19 Solution for Example 4-5


We need 5 ½ stages + partial reboiler.
E . Check. The internal consistency checks all make sense. Note that L′/V′ can be greater than or less than 1.0. Since the latent heats of vaporization per mole are close, CMO is probably a good assumption. Our initial assumption that feed 2 enters the column higher up than feed 1 is shown to be valid by the McCabe-Thiele diagram. We could also calculate ![]() and
and ![]() and check that the slope of the bottom operating line is correct.
and check that the slope of the bottom operating line is correct.
F . Generalize. The method of inserting the overall mass balance to simplify the intersection of the y = x line and middle operating line to derive Eq. (4-45) can be used in other cases. The method for calculating L′/V′ can also be generalized to other situations. That is, we can calculate D (or B), find flow rate in section above (or below), and use feed conditions to find flow rates in the desired section. Since we stepped off stages from the bottom up, the fractional stage is calculated from the difference in y values (that is, vertical distances) in Eq. (4-49). Industrial problems use lower reflux ratios and have more stages. A relatively large reflux ratio is used in this example to keep the graph simple.
4.9 Other Distillation Column Situations
A variety of modifications of the basic columns are often used. In this section we will briefly consider the unique aspects of several of these. CMO will be assumed. Detailed examples will not be given but will be left to serve as homework problems.
4.9.1: Partial Condensers
A partial condenser condenses only part of the overhead stream and returns this as reflux. This distillate product is removed as vapor as shown in Figure 4-20. If a vapor distillate is desired, then a partial condenser will be very convenient. The partial condenser acts as one equilibrium contact.
Figure 4-20 Partial condenser; (A) balance envelope, (B) top operating line

If a mass balance is done on the more volatile component using the mass balance envelope shown in Figure 4-20, we obtain
Vy = Lx + DyD
Removing D and solving for y, we obtain the operating equation
(4-50)
![]()
This is essentially the same as the equation for a top operating line with a total condenser except that yD has replaced xD. The top operating line will intersect the y = x line at y = x= yD. The top operating line is shown in Figure 4-20. The major difference between this case and that for a total condenser is that the partial condenser serves as the first equilibrium contact.
4.9.2: Total Reboilers
A total reboiler vaporizes the entire stream sent to it; thus, the vapor composition is the same as the liquid composition. This is illustrated in Figure 4-21. The mass balance and the bottom operating equation with a total reboiler are exactly the same as with a partial reboiler. The only difference is that a partial reboiler is an equilibrium contact and is labeled as such on the McCabe-Thiele diagram. The total reboiler is not an equilibrium contact and appears on the McCabe-Thiele diagram as the single point y = x = xB.
Figure 4-21 Total reboiler

Some types of partial reboilers may act as more or less than one equilibrium contact. In these cases, exact details of the reboiler construction are required.
4.9.3: Side Streams or Withdrawal Lines
If a product of intermediate composition is required, a vapor or liquid side stream may be withdrawn. This is commonly done in petroleum refineries and is illustrated in Figure 4-22A for a liquid side stream. Three additional variables such as flow rate, S, type of side draw (liquid or vapor), and location or composition xS or yS, must be specified. The operating equation for the middle section can be derived from mass balances around the top or bottom of the column. For the situation shown in Figure 4-22A, the middle operating equation is
(4-51)
![]()
Figure 4-22 Liquid side stream (A) column, (B) McCabe-Thiele diagram

(4-52)
![]()
This point can be plotted if S, xS, D, and xD are known. Derivation of Eqs. (4-51) and (4-52) is left as Problem 4-C3.
A second point can be found where the side stream is withdrawn. A saturated liquid withdrawal is equivalent to a negative feed of concentration xS. Thus there must be a vertical feed line at x = xS. The top and middle operating lines must intersect at this feed line.
Side-stream calculations have one difference that sets them apart from feed calculations. The stage must hit exactly at the point of intersection of the two operating lines. This is illustrated in Figure 4-22B. Since the liquid side stream is withdrawn from tray 2, we must have xS = x2. If the stage location is given, xS can be found by stepping off the required number of stages.
For a liquid withdrawal, a balance on the liquid gives
(4-53)
![]()
while vapor flow rates are unchanged, V = V′. Thus slope, L′/V′, of the middle operating line can be determined if L and V are known. L and V can be determined from L/D and D, where D can be found from external balances once xS is known.
For a vapor side stream, the feed line is horizontal at y = yS. A balance on vapor flow rates gives
(4-54)
![]()
while liquid flow rates are unchanged. Again L′/V′ can be calculated if L and V are known.
If a specified value of xS (or yS) is desired, the problem is trial and error. The top operating line is adjusted (change L/D) until a stage ends exactly at xS or yS.
Calculations for side streams below the feed can be developed using similar principles (see Problem 4.C4).
4.9.4: Intermediate Reboilers and Intermediate Condensers
Another modification that is used occasionally is to have an intermediate reboiler or an intermediate condenser. The intermediate reboiler removes a liquid side stream from the column, vaporizes it, and reinjects the vapor into the column. An intermediate condenser removes a vapor side stream, condenses it, and reinjects it into the column. Figure 4-23A illustrates an intermediate reboiler.
Figure 4-23 Intermediate reboiler; (A) balance envelopes, (B) McCabe-Thiele diagram

An energy balance around the column will show that QR without an intermediate reboiler is equal to QR + QI with the intermediate reboiler (F, z, q, xD, xB, p, L/D constant). Thus the amount of energy required is unchanged; what changes is the temperature at which it is required. Since xS > xB, the temperature of the intermediate reboiler is lower than that of the reboiler, and a cheaper heat source can be used. (Check this out with equilibrium data.)
Since the column shown in Figure 4-23A has four sections, there will be four operating lines. This is illustrated in the McCabe-Thiele diagram of Figure 4-23B. One would specify that the liquid be withdrawn at flow rate S at either a specified concentration xS or a given stage location. The saturated vapor is at concentration yS = xS. Thus there is a horizontal feed line at yS. If the optimum location for inputting the vapor is immediately below the stage where the liquid is withdrawn, the L”/V” line will be present, but no stages will be stepped off on it, as shown in Figure 4-23B. (The optimum location for vapor feed may be several stages below the liquid withdrawal point.) Development of the two middle operating lines is left as Problem 4.C5. Use the mass balance envelopes shown in Figure 4-23A.
Intermediate condensers are useful since the coolant can be at a higher temperature. See Problem 4.C6.
4.9.5: Stripping and Enriching Columns
Up to this point we have considered complete distillation columns with at least two sections. Columns with only a stripping section or only an enriching section are also commonly used. These are illustrated in Figures 4-24A and B. When only a stripping section is used, the feed must be a subcooled or saturated liquid. No reflux is used. A very pure bottoms product can be obtained but the vapor distillate will not be pure. In the enriching or rectifying column, on the other hand, the feed is a superheated vapor or a saturated vapor, and the distillate can be very pure but the bottoms will not be very pure. Striping columns and enriching columns are used when a pure distillate or a pure bottoms, respectively, is not needed.
Figure 4-24. Stripping and enriching columns; (A) stripping, (B) enriching, (C) McCabe-Thiele diagram for stripping column

Analysis of stripping and enriching columns is similar. We will analyze the stripping column here and leave the analysis of the enriching column as a homework assignment (Problem 4.C8). The stripping column shown in Figure 4-24A can be thought of as a complete distillation column with zero liquid flow rate in the enriching section. Then the top operating line is y = yD. The bottom operating line can be derived as
![]()
which is the usual equation for a bottom operating equation with a partial reboiler. Top and bottom operating lines intersect at the feed line. If the specified variables are F, q, z, p, xB, and yD, the feed line can be plotted and then the bottom operating line can be obtained from its intersection at y = x = xB and its intersection with the feed line at yD. (Proof is left as Problem 4.C7.) If the boilup rate, ![]() /B, is specified, then yD will not be specified and can be solved for. The McCabe-Thiele diagram for a stripping column is shown in Figure 4-24C.
/B, is specified, then yD will not be specified and can be solved for. The McCabe-Thiele diagram for a stripping column is shown in Figure 4-24C.
4.10: Limiting Operating Conditions
It is always useful to look at limiting conditions. For distillation, two limiting conditions are total reflux and minimum reflux. In total reflux, all of the overhead vapor is returned to the column as reflux, and all of the underflow liquid is returned as boilup. Thus distillate and bottoms flow rates are zero. At steady state the feed rate must also be zero. Total reflux is used for starting up columns, for keeping a column operating when another part of the plant is shut down, and for testing column efficiency.
The analysis of total reflux is simple. Since all of the vapor is refluxed, L = V and L/V = 1.0. Also, ![]() =
= ![]() and
and ![]() = 1.0. Thus both operating lines become the y = x line. This is illustrated in Figure 4-25B. Total reflux represents the maximum separation that can be obtained with a given number of stages but zero throughput. Total reflux also gives the minimum number of stages required for a given separation. Although simple, total reflux can cause safety problems. Leakage near the top of the column can cause concentration of high boilers with a corresponding increase in temperature. This can result in polymerization, fires, or explosions (Kister, 1990). Thus, the temperature at the top of the column should be monitored, and an alarm should sound if this temperature becomes too high.
= 1.0. Thus both operating lines become the y = x line. This is illustrated in Figure 4-25B. Total reflux represents the maximum separation that can be obtained with a given number of stages but zero throughput. Total reflux also gives the minimum number of stages required for a given separation. Although simple, total reflux can cause safety problems. Leakage near the top of the column can cause concentration of high boilers with a corresponding increase in temperature. This can result in polymerization, fires, or explosions (Kister, 1990). Thus, the temperature at the top of the column should be monitored, and an alarm should sound if this temperature becomes too high.
Figure 4-25 Total reflux; (A) column, (B) McCabe-Thiele diagram

Minimum reflux, (L/D)min, is defined as the external reflux ratio at which the desired separation could just be obtained with an infinite number of stages. This is obviously not a real condition, but it is a useful hypothetical construct. To have an infinite number of stages, the operating and equilibrium lines must touch. In general, this can happen either at the feed or at a point tangent to the equilibrium curve. These two points are illustrated in Figures 4-26A and 4-26B. The point where the operating line touches the equilibrium curve is called the pinch point. At the pinch point the concentrations of liquid and vapor do not change from stage to stage. This is illustrated in Figure 4-26C for a pinch at the feed stage. If the reflux ratio is increased slightly, then the desired separation can be achieved with a finite number of stages.
Figure 4-26 Minimum reflux; (A) pinch at feed stage, (B) tangent pinch, (C) concentration profile for L/D ~ (L/D)min

For binary systems the minimum reflux ratio is easily determined. The top operating line is drawn to a pinch point as in Figure 4-26A or 4-26B. Then (L/V)min is equal to the slope of this top operating line (which cannot be used for an actual column, since an infinite number of stages are needed), and
(4-55)
![]()
Note that the minimum reflux ratio depends on xD, z, and q and can depend on xB. The calculation of minimum reflux may be more complex when there are two feeds or a sidestream. This is explored in the homework problems.
The minimum reflux ratio is commonly used in specifying operating conditions. For example, we may specify the reflux ratio as L/D = 1.2(L/D)min. Minimum reflux would use the minimum amount of reflux liquid and hence the minimum amount of heat in the reboiler, but the maximum (infinite) number of stages and a maximum (infinite) diameter for a given separation. Obviously, the best operating conditions lies somewhere between minimum and total reflux. As a rule of thumb, the optimum external reflux ratio is between 1.05 and 1.25 times (L/D)min. (See 11 for more details.)
A maximum ![]() /
/![]() and hence a minimum boilup ratio
and hence a minimum boilup ratio ![]() /B can also be defined. The pinch points will look the same as in Figure 4-26A or 4-26B. Problem 4.C12 looks at this situation further.
/B can also be defined. The pinch points will look the same as in Figure 4-26A or 4-26B. Problem 4.C12 looks at this situation further.
4.11: Efficiencies
Up until now we have always assumed that the stages are equilibrium stages. Stages that are very close to equilibrium can be constructed, but they are only used for special purposes, such as determining equilibrium concentrations. To compare the performance of an actual stage to an equilibrium stage, we use a measure of efficiency.
Many different measures of efficiency have been defined. Two that are in common use are the overall efficiency and the Murphree efficiency. The overall efficiency, Eo, is defined as the number of equilibrium stages required for the separation divided by the actual number of stages required:
(4-56)
![]()
Partial condensers and partial reboilers are not included in either the actual or equilibrium number of stages, since they will not have the same efficiency as the stages in the column.
The overall efficiency lumps together everything that happens in the columns. What variables would we expect to affect column efficiency? The hydrodynamic flow properties such as viscosity and gas flow rate would affect the flow regime. The mass transfer rate, which is affected by the diffusivity, will in turn affect efficiency. Overall efficiency is usually smaller as the separation becomes easier (αAB increases). The column size can also have an effect. Correlations for determining the overall efficiency will be discussed in Chapter 10. For now, we will consider that the overall efficiency is determined from operating experience with similar distillation columns.
The overall efficiency has the advantage of being easy to use but the disadvantage that it is very difficult to calculate from first principles. Stage efficiencies are defined for each stage and may vary from stage to stage. The stage efficiencies are easier to estimate from first principles or to correlate with operating data. The most commonly used stage efficiencies for binary distillation are the Murphree vapor and liquid efficiencies (Murphree, 1925). The Murphree vapor efficiency is defined as
(4-57)
![]()
Murphree postulated that the vapor between trays is well mixed, that the liquid in the downcomers is well mixed, and that the liquid on the tray is well mixed and is of the same composition as the liquid in the downcomer leaving the tray. For the nomenclature illustrated in Figure 4-27A, the Murphree vapor efficiency is
(4-58)
![]()
![]()

Vwhere y∗j is vapor mole fraction in equilibrium with actual liquid mole fraction xj.
Once the Murphree vapor efficiency is known for every stage, it can easily be used on a McCabe-Thiele diagram. (In fact, Murphree adjusted his paper to use the newly developed McCabe-Thiele diagram.) The denominator in Eq. (4-58) represents the vertical distance from the operating line to the equilibrium line. The numerator is the vertical distance from the operating line to the actual outlet concentration. Thus the Murphree vapor efficiency is the fractional amount of the total vertical distance to move from the operating line. If we step off stages from the bottom up, we get the result shown in Figure 4-27B. Note that the partial reboiler is treated separately since it will have a different efficiency than the remainder of the column.
The Murphree efficiency can be used as a ratio of distances as shown in Figure 4-27B. If the Murphree efficiencies are accurate, the locations labeled by the stage numbers represent the actual vapor and liquid compositions leaving a stage. These points can be connected to form a pseudo-equilibrium curve, but this curve depends on the operating lines used and thus has to be redrawn for each new set of operating lines. Figure 4-27B allows us to calculate the real optimum feed plate location and the real total number of stages.
A Murphree liquid efficiency can be defined as
(4-59)
![]()
which is the actual change in mole fraction divided by the change for an equilibrium stage. The Murphree liquid efficiency is similar to the Murphree vapor efficiency except that it uses horizontal distances. Note that EML ≠ EMV.
For binary mixtures the Murphree efficiencies are the same whether they are written in terms of the more volatile or least volatile component. For multicomponent mixtures they can be different for different components and can even be negative.
4.12: Simulation Problems
In a simulation problem the column has already been built, and we want to know how much separation can be obtained. As noted in Tables 3-1 and 3-3, the real number of stages, the real feed location, the column diameter, and the types and sizes of reboiler and condenser are known. The engineer does the detailed stage-by-stage calculation and the detailed diameter calculation, and finally he or she checks that the operation is feasible.
To be specific, consider the situation where the known variables are F, z, q, xD, Treflux (saturated liquid), p, Qcol = 0, Nactual, Nfeed actual, diameter, L0/D, the overall efficiency Eo, and CMO. This column is illustrated in Figure 4-28A. The engineer wishes to determine the bottoms composition, xB.
Figure 4-28 Simulation problems; (A) existing column, (B) McCabe-Thiele diagram

We start by deriving the top and bottom operating equations. These are the familiar forms,
![]()
and
![]()
The feed line equation is also unchanged
Since the reflux is a saturated liquid and the external reflux ratio, L0/D, is known, we calculate L /V and plot the top operating line (Figure 4-28B). The feed line can also be plotted. The intersection of the top operating line and the feed line gives one point on the bottom operating line. Unfortunately, the bottom operating line cannot be plotted since neither ![]() nor xB is known (why won’t external balances give one of these variables?).
nor xB is known (why won’t external balances give one of these variables?).
To proceed we must use the three items of information that have not been used yet. These are Nactual, Nfeed actual, and Eo. We can estimate the equilibrium number of stages as
(4-60)
![]()
The feed location in equilibrium stages, NF, must be estimated as an integer, but the total number of equilibrium stages, N, could be a fractional number. Now we can step off equilibrium stages on the top operating line until we reach the feed stage NF. At this point we need to switch to the bottom operating line, which is not known. To use the final bit of information, the value N, we must guess xB, plot the bottom operating line, and check to see if the separation is achieved with N + 1 (the +1 includes the partial reboiler) equilibrium contacts. Thus, the simulation problem is one of trial and error when a stage-by-stage computation procedure is used. This procedure is illustrated in Figure 4-28B. Note that the actual feed stage may not be (and probably is not) optimum.
Once xB has been determined the external balances can be completed, and we can determine B, D, Qc, and QR. Now L, V, ![]() , and
, and ![]() can be calculated and we can proceed to an exact check that V and
can be calculated and we can proceed to an exact check that V and ![]() are less than Vmax. This is done by calculating a permissible vapor velocity. This calculation is similar to calculating uperm for a flash drum and is shown in Chapter 10. The condenser and reboiler sizes can also be checked. If the flow rates are too large or the condenser and reboiler are too small, the existing column will not satisfactorily do the desired separation. Either the feed rate can be decreased or L /D can be decreased. This latter change obviously requires that the entire solution be repeated.
are less than Vmax. This is done by calculating a permissible vapor velocity. This calculation is similar to calculating uperm for a flash drum and is shown in Chapter 10. The condenser and reboiler sizes can also be checked. If the flow rates are too large or the condenser and reboiler are too small, the existing column will not satisfactorily do the desired separation. Either the feed rate can be decreased or L /D can be decreased. This latter change obviously requires that the entire solution be repeated.
When other variables are specified, the stage-by-stage calculation is still trial and error. The basic procedure remains the same. That is, calculate and plot everything you can first, guess the needed variable, and then check whether the separation can be obtained with the existing number of stages. Murphree stage efficiencies are easily employed in these calculations.
Simulation problems are probably easier to do using the matrix approach developed in Chapter 6, which is easily adapted to computer use.
4.13: New Uses for Old Columns
Closely related to simulation is the use of existing or used distillation systems for new separations. The new use may be debottlenecking—that is, increasing capacity for the same separation. With increasing turnover of products, the problem of using equipment for new separations is becoming much more common.
Why would we want to use an existing column for a problem it wasn’t designed for? First, it is usually cheaper to modify a column that has already been paid for than to buy a new one. Second, it is usually quicker to do minor modifications than to wait for construction of a new column. Finally, for many engineers solving the often knotty problems involved in adapting a column to a new separation is an interesting challenge.
The first thing to do when new chemicals are to be separated is clean the entire system and inspect it thoroughly. Is the system in good shape? If not, will minor maintenance and parts replacement put the equipment in working order? If there are major structural problems such as major corrosion, it may be cheaper and less of a long-term headache to buy new equipment.
Do simulation calculations to determine how close the column will come to meeting the new separation specifications. Rarely will the column provide a perfect answer to the new problem. Difficulties can be classified as problems with the separation required and problems with capacity.
What can be done if the existing column cannot produce the desired product purities? The following steps can be explored (they are listed roughly in the order of increasing cost).
1. Find out whether the product specifications can be relaxed. A purity of 99.5% is much easier to obtain than 99.99%.
2. See if a higher reflux ratio will do the separation. Remember to check if column vapor capacity and the reboiler and condenser are large enough. If they are, changes in L/D affect only operating costs.
3. Change the feed temperature. This may make a nonoptimum feed stage optimum.
4. Will a new feed stage at the optimum location (the existing feed stage is probably nonoptimum) allow you to meet product specifications?
5. Consider replacing the existing trays (or packing) with more efficient or more closely spaced trays (or new packing). This is relatively expensive but is cheaper than buying a completely new system.
6. Check to see if two existing columns can be hooked together in series to achieve the desired separation. Feed can be introduced at the feed tray of either column or in between the two columns. Since vapor loading requirements are different in different sections of the column (see 10), the columns do not have to be the same diameter.
What if the column produces product much purer than specifications? This problem is pleasant. Usually the reflux ratio can be decreased, which will decrease operating expenses.
Problems with vapor capacity are discussed in more detail in Chapter 10. Briefly, if the column diameter is not large enough, the engineer can consider:
1. Operating at a reduced L/D, which reduces V. This may make it difficult to meet the product specifications.
2. Operating at a higher pressure, which increases the vapor density. Note that the column must have been designed for these higher pressures and the chemicals being separated must be thermally stable.
3. Using two columns in parallel.
4. Replacing the downcomers with large downcomers (see Chapter 10).
5. Replacing the trays or packing with trays or packing with a higher capacity. Major increases in capacity are unlikely.
If the column diameter is too large, vapor velocities will be low. The trays will operate at tray efficiencies lower than designed, and in severe cases they may not operate at all since liquid may dump through the holes. Possible solutions include:
1. Decrease column pressure to decrease vapor density. This increases the linear vapor velocity.
2. If the column has sieve trays, cover some of the holes. This increases the vapor velocity in the open holes reducing weeping.
3. Increase L/D to increase V.
4. Recycle some distillate and bottoms product to effectively increase F.
Using existing columns for new uses often requires a creative solution. Thus these problems can be both challenging and fun; they are also often assigned to engineers just out of school.
4.14: Subcooled Reflux and Superheated Boilup
What happens if the reflux liquid is subcooled or the boilup vapor is superheated? We have already looked at two similar cases where we have a subcooled liquid or a superheated vapor feed. In those cases we found that a subcooled liquid would condense some vapor in the column, while a superheated vapor would vaporize some liquid. Since reflux and boilup are inputs to the column, we should expect exactly the same behavior if these streams are subcooled or superheated.
Subcooled reflux often occurs if the condenser is at ground level. Then a pump is required to return the reflux to the top of the column. A saturated liquid will cause cavitation and destroy the pump; thus, the liquid must be subcooled if it is to be pumped. To analyze the effect of subcooled reflux, consider the top of the column shown in Figure 4-29. The cold liquid stream, L0, must be heated up to its boiling point. This energy must come from condensing vapor on the top stage, stream c in Figure 4-29. Thus, the flow rates on the first stage are different from those in the rest of the rectifying section. CMO is valid in the remainder of the column. The internal reflux ratio in the rectifying column is L1/V2 = L/V, and the top operating line is
![]()
Figure 4-29 Balance envelope for subcooled reflux

Now, L/V cannot be directly calculated from the external reflux ratio L0/D, since L and V change on the top stage.
Balances on vapor and liquid streams give
(4-61)
![]()
(4-62)
![]()
An energy balance using the balance envelope in Figure 4-29 is
(4-63)
![]()
With CMO, H1 ~ H2. Then Eq. (4-63) becomes
(V2 − V1)H ~ L1h1 − L0hreflux
Using Eqs. (4-61) and (4-62), this becomes
cH ~ (L0 + c)h1 − L0hreflux
Solving for amount condensed, c, we get
(4-64a)
![]()
which can also be written as
(4-64b)
![]()
or
(4-64c)
![]()
where fc is the fraction condensed per mole of reflux. We can now calculate the internal reflux ratio, L/V = L1/V2. To do this, we start with the ratio we desire and use Eqs. (4-61), (4-62), (4-64), and (4-65).
(4-65)
![]()
The ratio L0/V1 is easily found from L0/D as
![]()
Using this expression in Eq. (4-65) we obtain
(4-66)
![]()
Note that when fc = 0, Eqs. (4-65) and (4-66) both say L1/V2 = L0/V1. As the fraction condensed increases (reflux is subcooled more), the internal reflux ratio, L1/V2, becomes larger. Thus the net result of subcooled reflux is equivalent to increasing the reflux ratio. Numerical calculations (such as Problems 4.A9 or 4.D5) show that a large amount of subcooling is required to have a significant effect on L/V. With highly subcooled reflux, an extra tray should be added for heating the reflux (Kister, 1990).
A superheated direct steam input or a superheated boilup from a total reboiler will cause vaporization of liquid inside the column. This is equivalent to a net increase in the boilup ratio, ![]() /B, and makes the slope of the stripping section operating line approach 1.0. Since superheated vapor inputs can be analyzed in the same fashion as the subcooled liquid reflux, it will be left as homework assignment 4.C14.
/B, and makes the slope of the stripping section operating line approach 1.0. Since superheated vapor inputs can be analyzed in the same fashion as the subcooled liquid reflux, it will be left as homework assignment 4.C14.
4.15 Comparisons between Analytical and Graphical Methods
Both the Lewis and McCabe-Thiele methods are based on the CMO assumption. When the CMO assumption is valid, the energy balances are automatically satisfied, which greatly simplifies the stage-by-stage calculations. The reboiler and condenser duties, QR and Qc, are determined from the balances around the entire column. If CMO is not valid, QR and Qc will be unaffected if the same external reflux ratio can be used, but this may not be possible. The exact calculation, which can be done using the methods developed in Chapter 6, may show that a higher or lower L0/D is required if CMO is invalid.
When a programmable calculator or a computer is to be used, the analytical method is more convenient, particularly if a spreadsheet is used (Burns and Sung, 1996). The computer calculations are obviously more convenient if a very large number of stages are required, if a trial-and-error solution is required, or if many cases are to be run to explore the effect of many variables (e.g., for economic analysis). However, since accuracy is limited by the equilibrium data, the computer calculations are not more accurate than the graphical method.
If calculations are to be done by hand, the graphical method is faster than alternating between the analytical forms of the equilibrium relationship and the operating equations. In the McCabe-Thiele method, solution of the equilibrium relationship is simply a matter of drawing a line to the equilibrium curve. In the analytical solution we must first either fit the equilibrium data to an analytical expression or develop an interpolation routine. Then we must solve this equation, which may be nonlinear, each time we do an equilibrium calculation. Since the operating equation is linear, it is easy to solve analytically. With a sharp pencil, large graph paper, and care, the McCabe-Thiele technique can easily be as accurate as the equilibrium data (two significant figures).
When doing the stage-by-stage calculations from the top down, we solve for x values from the equilibrium relationship and for y values from the operating equation. If we step off stages from the bottom up, then we calculate x values from the operating equation and y values from equilibrium. Check this statement out on a McCabe-Thiele diagram. When going from the bottom up, we want to solve all the operating equations for x. As noted previously, the optimum feed stage can be determined from the test in Eq. (4-37), and the point of intersection (Eq. 4-38) is more convenient to use than the feed line. For more complex situations, the point of intersection of a feed line and an operating equation can be found by simultaneously solving the equations. The biggest problem in utilizing the Lewis method on the computer is obtaining a good fit for the equilibrium data. The data can be fit to curves, or an interpolation routine can be used to interpolate between data points. Burns and Sung (1996) report that linear interpolation is accurate if enough data points are entered for the equilibrium data.
Figure 4-30 Computer flowchart for simple distillation column

One of the most convenient ways to discuss computer and calculator methods is by reference to a flowchart. The flowchart is fairly general while computer code is very specific to the language used. Consider the specific design problem we solved in Example 4-3. Assume that we decide to step off stages from the top down. Now the computer or calculator program can proceed as shown in Figure 4-30. Other flowcharts are possible. If we step off stages from the bottom up, we will calculate yi from equilibrium and xi from the operating equation. Note that a McCabe-Thiele diagram is very useful for following the logic of flowcharts. Try this with Figure 4-30.
For a more complex column the flowchart in Figure 4-30 would be modified by:
1. Including equations for intermediate operating lines.
2. Including additional tests for optimum feeds [change Eqs. (4-37a,b) or use alternative test discussed in Problem 4.C18]
3. Including side streams
The principles again follow McCabe-Thiele calculations step by step.
The development of digital computers has made the graphical McCabe-Thiele technique obsolete for detailed design calculations. Engineers used to cover an entire wall with graph paper to do a McCabe-Thiele diagram when a very large number of stages were required. The method is still useful for one or two calculations, but its major uses are as a teaching tool and as a visualization tool. The graphical procedure presents a very clear visual picture of the calculation that is easier to understand than the interactions of the equations. The graphs are also extremely useful as a tool to help determine what the effect of changing variables will be, as a diagnostic when the computer program appears to be malfunctioning, and as a diagnostic when the column appears to be malfunctioning (Kister, 1995). Because of its visual impact, we have used the McCabe-Thiele diagram extensively to explore a variety of distillation systems.
4.16 Summary—Objectives
In this long chapter we have developed the stage-by-stage balances for distillation columns and showed how to solve these equations when CMO is valid. You should now be able to satisfy the following objectives:
1. Write the mass and energy balances and equilibrium expressions for any stage in a column.
2. Explain what CMO is, and determine if CMO is valid in a given situation.
3. Derive the operating equations for CMO systems.
4. Calculate the feed quality and determine its effect on flow rates. Plot the feed line on a y-x diagram.
5. Determine the number of stages required, using the Lewis method and the McCabe-Thiele method.
6. Develop and explain composition, temperature, and flow profiles.
7. Solve any binary distillation problem where CMO is valid. This includes:
a . Open steam
b . Multiple feeds
c . Partial condensers and total reboiler
e . Intermediate reboilers and condensers
f . Stripping and enriching columns
g . Total and minimum reflux
h . Overall and Murphree efficiencies
i . Simulation problems
j . Any combination of the above
8. Include the effects of subcooled reflux or superheated boilup in your McCabe-Thiele and Lewis analyses.
9. Develop flowcharts for any binary distillation problem.
References
Brown, G. G. and Associates, Unit Operations, Wiley, New York, 1950.
Burns, M. A. and J. C. Sung, “Design of Separation Units Using Spreadsheets,” Chem. Engr. Educ., 30 (1), 62 (Winter 1996).
Foust, A. S., L. A. Wenzel, C. W. Clump, L. Maus and L. B. Andersen, Principles of Unit Operations, 2nd ed., Wiley, New York, 1980.
Himmelblau, D. M., Basic Principles and Calculations in Chemical Engineering, 3rd ed., Prentice Hall, Upper Saddle River, New Jersey, 1974.
Horwitz, B. A., “Hardware, Software, Nowhere,” Chem. Engr. Progress, 94 (9), 69 (Sept. 1998).
Kister, H. Z., Distillation Operation, McGraw-Hill, New York, 1990.
Kister, H. Z., “Troubleshoot Distillation Simulations,” Chem. Engr. Progress, 91 (6), 63 (June 1995).
Lewis, W. K., “The Efficiency and Design of Rectifying Columns for Binary Mixtures,” Ind. Engr. Chem., 14, 492 (1922).
Ludwig, E. E., Applied Process Design, 3rd ed., Vol. 2, Gulf Publishing Co., Houston, 1997.
McCabe, W. L. and E. W. Thiele, “Graphical Design of Fractionating Columns,” Ind. Engr. Chem., 17, 602 (1925).
Murphree, E. V., “Graphical Rectifying Column Calculations,” Ind. Engr. Chem., 17, 960 (1925).
Perry, R. H., C. H. Chilton and S. D. Kirkpatrick (Eds.), Chemical Engineer’s Handbook, 4th ed., McGraw-Hill, New York, 1963.
Ponchon, M., Tech. Moderne, 13, 20 and 53 (1921).
Savarit, R., Arts et Metiers, 65, 142, 178, 241, 266, and 307 (1922).
Sorel, E., La Retification de l’Alcohol, Gauthier-Villars, Paris, 1893.
Homework
A . Discussion Problems
A1. In the figure shown, what streams are represented by point A? By point B? How would you determine the temperature of stage 2? How about the temperature in the reboiler? If feed composition is as shown, how can the liquid composition on the optimum feed stage be so much less than z?

A2. For this McCabe-Thiele diagram answer the following questions.
a. (1)The actual feed tray is?
(2)The mole fraction MVC in the feed is?
(3)The vapor composition on the feed tray is?
(4)The liquid composition on the feed tray is?
b. Is the feed a superheated vapor feed, saturated vapor feed, two-phase feed, saturated liquid feed, or subcooled liquid feed?
c. Is the temperature at stage 7 higher, lower, or the same as at stage 1?
A3. Suppose that constant mass overflow is valid instead of CMO. Explain how to carry out the Lewis and McCabe-Thiele procedures in this case.
A4. Drawing the McCabe-Thiele graph as yMVC vs. xMVC is traditional but not necessary. Repeat Example 4-3, but plot yw vs. xw. Note the differences in the diagram. Do you expect to get the same answer?
A5. For distillation at CMO, show the flow profiles schematically (plot Lj and Vj vs. stage location) for
Subcooled liquid feed
Two-phase feed
Superheated vapor feed
A6. If L0/V with saturated reflux is the same as L0/V with subcooled reflux, is |Qc| greater, the same, or less for the saturated reflux case?
A7. What is the effect of column pressure on distillation? To explore this consider pressure’s effect on the reboiler and condenser temperatures, the volumetric flow rates inside the column, and the relative volatility (which can be estimated for hydrocarbons from the DePriester charts).
A8. What happens if we try to step off stages from the top down and EMV is given? Determine how to do this calculation.
A9. When would it be safe to ignore subcooling of the reflux liquid and treat the reflux as a saturated liquid? Do a few numerical calculations for either methanol and water or ethanol and water to illustrate.
A10. Eqs. (4-53) and (4-54) are mass balances on particular phases. When will these equations be valid?
A11. When might you use an intermediate condenser on a column? What are the possible advantages?
A12. When would just stripping or just an enriching column be used?
A13. What is the usefulness of calculating a fractional number of equilibrium stages?
A14. Explain with a McCabe-Thiele diagram how changing feed temperature (or equivalently, q) may help an existing column achieve the desired product specifications.
A15. Develop a key relations chart for binary McCabe-Thiele distillation. That is, on one sheet of paper summarize everything you need to know about binary distillation. You will probably want to include information about operating lines, feed lines, efficiencies, subcooled reflux, and so forth.
B . Generation of Alternatives
B1. Invent your own problem that is distinctly different from those discussed in this chapter. Show how to solve this problem.
B2. Several ways of adapting existing columns to new uses were listed. Generate new methods that might allow existing systems to meet product specifications that could not be met without modification. Note that you can postulate a complex existing column such as one with an intermediate reboiler.
C . Derivations
C1. For Example 4-4 (open steam), show that the x intercept (y = 0) is at x = xB.
C2. Derive the bottom operating line for a column with a total reboiler. Show that this is the same result as is obtained with a partial reboiler.
C3. Derive Eqs. (4-51) and (4-52).
C4. For a side stream below the feed:
a. Draw a sketch corresponding to Figure 4-22A.
b. Derive the operating equation and y = x intercept.
c. Sketch the McCabe-Thiele diagram.
C5. Derive the operating equations for the two middle operating sections when an intermediate reboiler is used (see Figures 4-23A and 4-23B). Show that the operating line with slope of L′/V′ goes through the point y = x = xB.
C6. Show that the total amount of cooling needed is the same for a column with one total condenser (Qc) as for a column with a total condenser and an intermediate total condenser (Qc + QI). F, z, q, xD, xB, and QR are constant for the two cases. Sketch a system with an intermediate condenser. Derive the operating equations for the two middle operating lines, and sketch the McCabe-Thiele diagram.
C7. For the stripping column shown in Figures 4-24A and 4-24C, show formally that the intersection of the bottom operating line and the feed line is at yD. In other words, solve for the intersection of these two lines.
C8. Develop the McCabe-Thiele procedure for the enriching column shown in Figure 4-24B.
C9. For Example 4-3 prove that:
a. The top operating line and the y=x line intersect at y=x=xD.
b. The bottom operating line and the y=x line intersect at y=x=xB.
C10. Derive Eq. (4-25).
![]()
C11. The boilup ratio ![]() may be specified. Derive an expression for
may be specified. Derive an expression for ![]() as a function of
as a function of ![]() for a partial reboiler.
for a partial reboiler.
C12. Show how to determine (![]() )min. Derive an equation for calculation of (
)min. Derive an equation for calculation of (![]() )min from (
)min from (![]() )max.
)max.
C13. Sketch the McCabe-Thiele diagram if the Murphree liquid efficiency is constant and EML = 0.75.
C14. Derive the equations to calculate ![]() when a superheated boilup is used.
when a superheated boilup is used.
C15. Derive the equations to calculate ![]() when direct superheated steam is used.
when direct superheated steam is used.
C16. Show how fc in Eqs. (4-64) and (4-65) is related to q.
C17. Derive the operating equation for section 2 of Figure 4-17. Show that the equations are identical whether the mass balance envelope is drawn around the top of the column or the bottom of the column.
C18. Eqs. (4-37) are one way to test for the optimum feed location. An alternative method is to determine at each equilibrium value which operating line is lower.
a. Develop this test for a two-feed column. Do it both going down and going up the column.
b. Does this test work for a column with a side stream? (See Figure 4-22)
D . Problems
*Answers to problems with an asterisk are at the back of the book.
D1. A continuous, steady-state distillation column with a total condenser and a partial reboiler is separating methanol from water at one atmosphere (See Table 2-7 for data). The feed rate is 100 kg mole per hour. The feed is 55 mole % methanol and 45 mole % water. We desire a distillate product that is 90 mole % methanol and a bottoms product that is 5 mole % methanol. Assume CMO.
a. If the external reflux ratio L/D = 1.25 plot the top operating line.
b. If the boilup ratio ![]() = 2.0 plot the bottom operating line.
= 2.0 plot the bottom operating line.
c. Step off stages starting at the bottom with the partial reboiler. Use the optimum feed stage. Report the optimum feed stage and the total number of stages.
d. Plot the feed line. Calculate its slope. Calculate q. What type of feed is this?
D2. Practice finding the feed line. We are feeding methanol and water to a distillation column at one atmosphere total pressure. Equilibrium data are available in Table 2-7. Heat capacity and latent heat data are available in problem 3.D8. Assume CMO. Calculate q and plot the feed line for each part below.
a. Feed is 20 mole % methanol and is a saturated vapor.
b. Feed is 20 mole % methanol and is a saturated liquid.
c. Feed is 40 mole % methanol and is a two-phase mixture that is 30% vapor.
d. Feed is 40 mole % methanol and is a two-phase mixture that is 30% liquid.
e. Feed is 45 mole % methanol and is a sub cooled liquid. 1 mole of vapor must condense to heat 5 moles of feed to boiling point.
f. Feed is 45 mole % methanol and is a superheated vapor. 1 mole of liquid must be vaporized to cool 8 moles of feed to boiling point.
g. Feed is 60 mole % methanol at 40°C (subcooled liquid).
h. Feed is 60 mole % methanol at 100°C (superheated vapor).
Do an approximate calculation for parts g & h by estimating latent heat as
λfeed = zM λM + zw λw
D3. *a. A feed mixture of ethanol and water is 40 mole % ethanol. The feed is at 200 °C and is at a high enough pressure that it is a liquid. It is input into the column through a valve, where it flashes. Column pressure is 1 kg/cm2. Find the slope of the feed line.
b. We are separating ethanol and water in a distillation column at a pressure of 1 kg/cm2. Feed is 50 wt % ethanol, and feed rate is 1 kg/hr. The feed is initially a liquid at 250 °C and then flashes when the pressure is dropped as it enters the column. Find q. Data are in Problem 3-D5 and in Figure 2-4. You may assume that CMO is valid.
D4. *a. We have a superheated vapor feed of 60 mole % more volatile component at 350 °C. Feed flow rate is 1000 kg moles/hr. On the feed plate the temperature is 50 °C. For this mixture the average heat capacities are
CPL = 50 cal/g-mole- °C, CPV = 25 cal/g-mole- °C
while the latent heat of vaporization is λ = 5000 cal/g-mole. Plot the feed line for this feed.
b. If a feed to a column is a two-phase feed that is 40 mole % vapor, find the value of q and the slope of the feed line.
c. If the feed to a column is a superheated vapor and 1 mole of liquid is vaporized on the feed plate to cool 5 moles of feed to a saturated vapor, what is the value of q? What is the slope of the feed line?
D5. *A distillation column is operating with a subcooled reflux. The vapor streams have an enthalpy of H1 = H2 = 17,500 Btu/lb-mole, while the saturated liquid h1 = 3100 Btu/lb-mole. Enthalpy of the reflux stream is h0 = 1500 Btu/lb-mole. The external reflux ratio is set at L0/D = 1.1. Calculate the internal reflux ratio inside the column, L1/V2.
D6. A distillation column at 1 kg/cm2 pressure is separating a feed that is 5.0 mole % ethanol and 95.0 mole % water. The feed is a two-phase mixture at 97 °C. Find q and the slope of the feed line. Use data from Figure 2-4. Watch your units.
D7a. *A distillation column with a total condenser is separating acetone from ethanol. A distillate concentration of xD = 0.90 mole fraction acetone is desired. Since CMO is valid, L/V = constant. If L/V is equal to 0.8, find the composition of the liquid leaving the fifth stage below the total condenser.
b. A distillation column separating acetone and ethanol has a partial reboiler that acts as an equilibrium contact. If the bottoms composition is xB = 0.13 mole fraction acetone and the boilup ratio ![]() = 1.0, find the vapor composition leaving the second stage above the partial reboiler.
= 1.0, find the vapor composition leaving the second stage above the partial reboiler.
c. The distillation column in parts a and b is separating acetone from ethanol and has xD = 0.9, xB = 0.13, L/V = 0.8, and ![]() = 1.0. If the feed composition is z = 0.3 (all concentrations are mole fraction of more volatile component), find the optimum feed plate location, total number of stages, and required q value of the feed. Equilibrium data for acetone and ethanol at 1 atm (Perry et al., 1963, p. 13-4) are
= 1.0. If the feed composition is z = 0.3 (all concentrations are mole fraction of more volatile component), find the optimum feed plate location, total number of stages, and required q value of the feed. Equilibrium data for acetone and ethanol at 1 atm (Perry et al., 1963, p. 13-4) are
| xA | .10 | .15 | .20 | .25 | .30 | .35 | .40 | .50 | .60 | .70 | .80 | .90 |
| yA | .262 | .348 | .417 | .478 | .524 | .566 | .605 | .674 | .739 | .802 | .865 | .929 |
D8. *For Problem 4-D7c for separation of acetone from ethanol, determine:
a. How many stages are required at total reflux?
b. What is (L/V)min? What is (L/D)min?
c. The L/D used is how much larger than (L/D)min?
d. If EMV = 0.75, how many real stages are required for L/V = 0.8?
D9. A distillation column with a total condenser and a partial reboiler is separating acetone from ethanol. The feed is 10.0 kgmoles/minute. Feed is 60.0 mole % acetone and is a saturated liquid. The distillate is 95.0 mole % acetone and leaves as a saturated liquid. The bottoms is 5.0 mole % acetone. Column pressure is 1.0 atmosphere. Reflux is returned as a saturated liquid. Column is adiabatic. External reflux ratio is L/D = 4.7. Assume CMO. Step off stages from top down. Equilibrium data is in problem 4.D7.
a. Find the optimum feed stage and total number of equilibrium stages. (Step off stages from the top down).
b. Calculate (L/D)min.
c. Calculate Nmin at total reflux.
D10. *A distillation column is separating phenol from p-cresol at 1 atm pressure. The distillate composition desired is 0.96 mole fraction phenol. An external reflux ratio of L/D = 4 is used, and the reflux is returned to the column as a saturated liquid. The equilibrium data can be represented by a constant relative volatility, αphenol–cresol = 1.76 (Perry et al., 1963, p. 13-3). CMO can be assumed.
a. What is the vapor composition leaving the third equilibrium stage below the total condenser? Solve this by an analytical stage-by-stage calculation alternating between the operating equation and the equilibrium equation.
b. What is the liquid composition leaving the sixth equilibrium stage below the total condenser? Solve this problem graphically using a McCabe-Thiele diagram plotted for αp–c = 1.76.
D11. A mixture of methanol and water is being separated in a distillation column with open steam. The feed is 100.0 kmoles/hr. Feed is 60.0 mole % methanol and is at 40°C. The column is at 1.0 atm. The steam is pure steam (yM = 0) and is a saturated vapor. The distillate product is 99.0 mole % methanol and leaves as a saturated liquid. The bottoms is 2.0 mole % methanol and since it leaves an equilibrium stage must be a saturated liquid. The column is adiabatic. The column has a total condenser. External reflux ratio is L/D = 2.3. Assume CMO is valid. Equilibrium data is in Table 2-7. Data for water and methanol is available in problem 3.D8.
a. Estimate q.
b. Find optimum feed stage and total number of equilibrium stages (step off stages from top down). Use a McCabe-Thiele diagram.
c. Find (L/D)min. Use a McCabe-Thiele diagram.
D12. Solve problem 3.D9 for the optimum feed location and the total number of stages. Assume CMO and use a McCabe-Thiele diagram. Expand the portions of the diagram near the distillate and bottoms to be accurate.
D13. A saturated liquid feed that is 40.0 mole % acetone and 60.0 mole % ethanol (acetone is more volatile) is being processed in a stripping column. Feed flow rate is 10.0 kmoles/hr. Operation is at 1.0 atm. pressure. The system has a partial reboiler. We desire a bottoms product that is 5.0 mole % acetone. Equilibrium data are in problem 4.D7.
a. a. If the boilup ratio is ![]() = 3.0, find the value of the distillate mole fraction, yd, and the number of equilibrium stages needed for the separation.
= 3.0, find the value of the distillate mole fraction, yd, and the number of equilibrium stages needed for the separation.
b. What is the minimum boilup ratio (![]() )min?
)min?
D14. A distillation column with open steam heating is separating a feed that is 80.0 mole % methanol and 20.0 mole % water in a steady state operation. The column has 10 stages, a total condenser, and the feed is on stage 5. Operation is at 1.0 atm. The steam is pure water and is a saturated vapor. CMO can be assumed to be valid. At 2:16 a.m. 25 days ago the feed and distillate flows were shut off (D = F = 0), but the steam rate was unchanged and the total condenser is still condensing the vapor to a saturated liquid. The column has now reached a new steady state operation.
a. What is the current methanol mole fraction in the bottoms?
b. At the new steady state estimate the methanol mole fraction in the liquid leaving the total condenser.
D15. A distillation column is separating ethylene dichloride from trichloroethane. Equilibrium data can be represented by α = 2.4. The column has a total condenser and a partial reboiler. Reflux is returned as a saturated liquid. The feed is 60.0 mole % ethylene dichloride, the MVC. Feed rate is 100.0 kg moles/hr. Distillate mole fraction is 0.92 ethylene dichloride and bottoms mole fraction is 0.08 ethylene dichloride. Assume CMO. Pressure is 1.0 atm.
a. Find B and D.
a. b. If the external reflux ratio L/D = 2.0, and the boilup ratio ![]() = 1.2 find the optimum feed stage, and the total number of equilibrium stages.
= 1.2 find the optimum feed stage, and the total number of equilibrium stages.
c. What is the value of q? What type of feed is this?
D16. *∗Estimate q for Problem 3-D6. Estimate that the feed stage is at same composition as the feed.
D17. A mixture of acetone and ethanol (acetone is more volatile) is fed to an enriching column that has a liquid side stream withdrawn. The feed flow rate is 100.0 gmole/minute. Feed is 60.0 mole % acetone and is a saturated vapor. The liquid side product is withdrawn from the second stage below the total condenser at a flow rate of S = 15.0 gmole/minute. The reflux is returned as a saturated liquid. The distillate should be 90.0 mole % acetone. The external reflux ratio is L/D = 7/2. Column pressure is 1.0 atm. Column is adiabatic and CMO is valid. Equilibrium data is in problem 4.D7.
Note: Trial and error is not required.
Find the mole fraction of acetone in the sidestream xS, the mole fraction of acetone in the bottoms xB, and the number of equilibrium stages required.
D18. A distillation column with a total condenser and a partial reboiler is separating acetone from ethanol. The feed is 10.0 kgmoles/minute. Feed is 60.0 mole % acetone and is a saturated liquid. The distillate is 95.0 mole & acetone and leaves as a saturated liquid. The bottoms is 5.0 mole % acetone. Column pressure is 1.0 atmosphere. Reflux is returned as a saturated liquid. Column is adiabatic. External reflux ratio is L/D = 4.7. Assume CMO. Step off stages from top down. Use McCabe-Thiele diagram with VLE data from problem 4.D7.
a. Find optimum feed stage and total number of equilibrium stages. (Step off stages from top down.)
b. Calculate (L/D)min.
c. Calculate Nmin at total reflux.
D19. *A distillation column is separating acetone and ethanol. The column effectively has six equilibrium stages plus a partial reboiler. Feed is a two-phase feed that is 40% liquid and 75 mole % acetone. Feed rate is 1000 kg moles/hr, and the feed stage is fourth from the top. The column is now operating at a steady state with the bottoms flow valve shut off. However, a distillate product is drawn off, and the vapor is boiled up in the reboiler. L0/D = 2. Reflux is a saturated liquid. CMO can be assumed. p = 1 atm. Equilibrium data are in Problem 5-D7. Find the distillate composition. If one drop of liquid in the reboiler is withdrawn and analyzed, predict xB.
D20. *A distillation column with a total condenser and a partial reboiler is separating ethanol and water at 1 kg/cm2 pressure. Feed is 0.32 mole fraction ethanol and is at 30° C. Feed flow rate is 100 kg moles/hr. The distillate product is a saturated liquid, and the distillate is 80 mole % ethanol. The condenser removes 2,065,113 kcal/hr. The bottoms is 0.04 mole fraction ethanol. Assume that CMO is valid. Find the number of stages and the optimum feed stage location.
Data: CPL EtOH = 24.65 cal/g-mole- °C at 0 °C
Other data are in Problem 3.D5. The enthalpy-composition diagram is given in Figure 2-4, and the y-x diagram is in Figure 2-2. Note: Watch your units.
D21. An enricher column has two feeds. Feed F1 (input at the bottom) is a saturated vapor. Flow rate F1 = 100.0 kg moles/hr. This feed is 20.0 mole % methanol and 80.0 mole % water. Feed F2 (input part way up the column) is a two-phase mixture that is 90 % liquid. Flow rate F2 = 80.0 kgmoles/hr. Feed F2 is 45.0 moles % methanol and 55.0 mole % water. We desire a distillate that is 95.0 mole % methanol. Reflux is returned as a saturated liquid. Pressure is one atmosphere. L/D = 1.375. Assume CMO. Data are available in Table 2-7.
Find: D, B, xB, optimum feed location and number of equilibrium stages required.
D22. *When water is the more volatile component we do not need a condenser but can use direct cooling with boiling water. This was shown in Problem 3-D3. We set yD = 0.92, xB = 0.04, z = 0.4 (all mole fractions water), feed is a saturated vapor, feed rate is 1000 kg moles/hr, p = 1 atm, CMO is valid, the entering cooling water (W) is a saturated liquid and is pure water, and W/D = 3/4. Derive and plot the top operating line. Note that external balances (that is, balances around the entire column) are not required.
D23. *When water is the more volatile component we do not need a condenser but can use direct cooling. This was illustrated in Problem 3-D3. We set yD = 0.999, xB = 0.04, z = 0.4 (all mole fractions water), feed is a saturated liquid, feed rate is 1000 kg moles/hr, p = 1 atm, CMO is valid, the entering cooling water (W) is pure water and W/D = 3/4. The entering cooling water is at 100 °F while its boiling temperature is 212°F.
![]()
Find the slope of the top operating line, L/V.
Note: Equilibrium data are not needed.
D24. We have a distillation column with a partial condenser and a total reboiler separating a feed of 200.0 kmoles/hr. The feed is 40.0 mole % acetone and 60.0 mole % ethanol. The feed is a 2 phase mixture that is 80 % liquid. We desire a distillate vapor that is 85.0 mole % acetone and a bottoms that is 5.0 mole % acetone. Column pressure is one atmosphere. Reflux is returned as a saturated liquid and L/D = 3.25. Assume CMO. Equilibrium data are in problem 4.D7.
a. Find the optimum feed location and the total number of equilibrium stages.
b. What is the minimum external reflux ratio?
c. What is the minimum number of stages (total reflux)?
D25. We are separating methanol and water. Calculate the internal reflux ratio inside the column, L1/V2, for the following cases. The column is at 101.3 kPa. Data are available in Table 2-7 and in problem 3.D8.
a. Distillate product is 99.9 mole % methanol. External reflux ratio is L0/D = 1.2. Reflux is cooled to 40.0° C. (i.e., it is subcooled).
b. Repeat part a except for a saturated liquid reflux. Compare with Part a.
D26. A distillation column with a partial condenser and a total reboiler is separating acetone and ethanol. There are two feeds. One feed is 50.0 mole % acetone, flows at 100.0 moles/minute, and is a superheated vapor where approximately 1 mole of liquid will vaporize on the feed stage for each 20 moles of feed. The other feed is a saturated liquid, flows at 150.0 moles/minute and is 35.0 mole % acetone. We desire a distillate product that is yD = 0.85 mole fraction acetone and a bottoms product that is xB = 0.10 mole fraction acetone. The column has a partial condenser and a total reboiler. Boilup is returned as a saturated vapor. Column operates at a pressure of 1.0 atm. Assume CMO and use a McCabe-Thiele diagram. VLE data is given in problem 4.D7.
a. Find ![]() .(Plot both feed lines to decide which one to use.)
.(Plot both feed lines to decide which one to use.)
b. Operate at ![]() = 3 x
= 3 x ![]() The partial condenser is an equilibrium contact. If the stages each have a Murphree liquid efficiency of 0.75, find optimum feed locations for each feed and total number of real stages. (It is easiest to start at top and step down.)
The partial condenser is an equilibrium contact. If the stages each have a Murphree liquid efficiency of 0.75, find optimum feed locations for each feed and total number of real stages. (It is easiest to start at top and step down.)
D27. *A distillation column is separating methanol from water at 1 atm pressure. The column has a total condenser and a partial reboiler. In addition, a saturated vapor stream of pure steam is input on the second stage above the partial reboiler (see figure). The feed flow rate is 2000 kg moles/day. Feed is 48 mole % methanol and 52 mole % water and is a subcooled liquid. For every 4 moles of feed, 1 mole of vapor must condense inside the column. Distillate composition is 92 mole % methanol. Reflux is a saturated liquid, and L0/D = 1.0. Bottoms composition is 8 mole % methanol. Boilup ratio is ![]() = 0.5. Equilibrium data are given in Table 2-7. Assume that CMO is valid. Find the optimum feed plate location and the total number of equilibrium stages required.
= 0.5. Equilibrium data are given in Table 2-7. Assume that CMO is valid. Find the optimum feed plate location and the total number of equilibrium stages required.

D28. We wish to process 100.0 kg moles per hour of a feed that is 35.0 mole % methanol and 65.0 mole % water in a stripping column. The column has a partial reboiler and 2 equilibrium stages (total 3 equilibrium contacts). We want a bottoms mole fraction that is 0.05 methanol. The feed is a saturated liquid. Assume CMO. Operation is at 1.0 atm. Equilibrium data is available in Table 2-7. Find the required boilup ratio, the resulting distillate mole fraction yD, and flow rates D and B.
D29. A distillation column will use the optimum feed stage. A liquid side stream is withdrawn on the third stage below the total condenser at a rate of 15.0 kg moles/hr. The feed is a two phase mixture that is 20 % vapor. Feed to the column is 100.0 kg moles/hr. The feed is 60.0 mole % acetone and 40.0 mole % ethanol. We desire a distillate composition that is 90.0 mole % ethanol. We operate with an external reflux ratio of L/D = 3. The bottoms product is 10.0 mole % acetone. A partial reboiler is used. Find the mole fraction ethanol in the side stream xs, the optimum feed location, and the total number of equilibrium contacts needed. Equilibrium data are available in problem 4.D7.
D30. *A distillation column is separating methanol from water. The column has a total condenser that subcools the reflux so that 1 mole of vapor is condensed in the column for each 3 moles of reflux. L0/D = 3. A liquid side stream is withdrawn from the second stage below the condenser. This side stream is vaporized to a saturated vapor and then mixed with the feed and input on stage 4. The side withdrawal rate is S = 500 kg moles/hr. The feed is a saturated vapor that is 48 mole % methanol. Feed rate is F = 1000 kg moles/hr. A total reboiler is used, which produces a saturated vapor boilup. We desire a distillate 92 mole % methanol and a bottoms 4 mole % methanol. Assume CMO. Equilibrium data are given in Table 2-7. Find:
a. The total number of equilibrium stages required.
b. The value of ![]() /B.
/B.

D31. We wish to separate feed that is 48.0 mole % acetone and 52.0 mole % ethanol. The feed rate is 100.0 kg moles/hr. We desire a distillate composition that is 80.0 mole % acetone and a bottoms that is 8.0 mole % acetone. Find the minimum external reflux ratio, the minimum value of the boilup ratio, the approximate (based on CMO) minimum amount of energy that would be required for the reboiler, and the approximate (based on CMO) minimum amount of energy required for the total condenser for the following feeds:
a. If the feed is a saturated vapor.
b. If the feed is a two-phase mixture that is 50 % liquid.
c. If the feed is a saturated vapor.
d. From the point of view of the energy required in the reboiler is it advantageous to vaporize all or part of the feed? How about in the condenser? Since each kJ for heating is five to ten times as expensive as for cooling (when cooling water can be used), which feed is likely to result in the lowest operating costs?
VLE data are in Problem 4.D7. The latent heat of pure ethanol = 38,580 kJ/kg mole. The latent heat of pure acetone = 30,200 kJ/kg mole.
e. How many equilibrium contacts are required if the separation is done at total reflux? Note that the answer is the same for parts a, b and c.
f. If we do part b at L/D = 1.5 x (L/D)minimum, how many real stages with a Murphree vapor efficiency of 0.75 are needed and what is the real optimum feed location? Remember that the partial reboiler acts as an equilibrium contact.
D32.∗ A distillation column is separating acetone from ethanol. Feed is a saturated liquid that is 40 mole % acetone. Feed rate is 50 kg moles/hr. Operation is at 1 atm and CMO can be assumed. The column has a total condenser and a partial reboiler. There are eight equilibrium stages in the column, and the feed is on the third stage above the reboiler. Three months ago the distillate flow was shut off (D = 0), but the column kept running. The boilup ratio was set at the value of ![]() = 1.0. Equilibrium data are given in Problem 4.D7.
= 1.0. Equilibrium data are given in Problem 4.D7.
a. What is xB?
b. If a drop of distillate were collected, what would xD be?
D33. *A distillation column is separating 1000 moles/hr of a 32 mole % ethanol, 68 mole % water mixture. The feed enters as a subcooled liquid that will condense 1 mole of vapor on the feed plate for every 4 moles of feed. The column has a partial condenser and uses open steam heating. We desire a distillate product yD = 0.75 and a bottoms product xB = 0.10. CMO is valid. The steam used is pure saturated water vapor. Data are in Table 2-1 and Figure 2-2.
a. Find the minimum external reflux ratio.
b. Use L/D = 2.0(L/D)min, and find the number of real stages and the real optimum feed location if the Murphree vapor efficiency is 2/3 for all stages.
c. Find the steam flow rate used.
D34. We have a distillation column separating acetone and ethanol at one atmosphere pressure. The feed is 44.0 mole % acetone and is a saturated liquid. Feed rate is 1000.0 kg moles/hr. We desire a bottoms stream that is 4.0 % acetone and a distillate that is 96.0 % acetone. The column has a total reboiler. Instead of a condenser we use pure boiling ethanol (stream C) as the reflux liquid (the column looks like the drawing in problem 3.D3). We want to operate at a reflux rate that is 1.3 times (L/D)min. Find the minimum L/D, plot the feed line and the operating lines, step off stages using the optimum feed plate location, and determine the total number of equilibrium stages needed. Note that this problem requires deriving a top operating line that is different than top operating lines when there is a condenser. The minimum reflux line will also look different. Also L/V is not (L/D)/(1+L/D).
D35. A stripping column is separating 10,000.0 kg mole/day of a saturated liquid feed. The column has a partial reboiler. The feed is 60.0 mole % acetone and 40.0 mole % ethanol. We desire a bottoms that is 2.0 mole % acetone. Operate at a boilup rate ![]() = 1.5 (
= 1.5 (![]() )min. Find yD, N, D and B. Equilibrium data are in problem 4.D7.
)min. Find yD, N, D and B. Equilibrium data are in problem 4.D7.
D36. *A distillation column with a total condenser and a partial reboiler is separating ethanol from water. Feed is a saturated liquid that is 25 mole % ethanol. Feed flow rate is 150 moles/hr. Reflux is a saturated liquid and CMO is valid. The column has three equilibrium stages (i.e., four equilibrium contacts), and the feed stage is second from the condenser. We desire a bottoms composition that is 5 mole % ethanol and a distillate composition that is 63 mole % ethanol. Find the required external reflux ratio. Data are in Table 2-1 and Figure 2-2.
D37. *A distillation column with two equilibrium stages and a partial reboiler (three equilibrium contacts) is separating methanol and water. The column has a total condenser. Feed, a 45 mole % methanol mixture, enters the column on the second stage below the condenser. Feed rate is 150 moles/hr. The feed is a subcooled liquid. To heat 2 moles of feed to the saturated liquid temperature, 1 mole of vapor must condense at the feed stage. A distillate concentration of 80 mole % methanol is desired. Reflux is a saturated liquid, and CMO can be assumed. An external reflux ratio of L/D = 2.0 is used. Find the resulting bottoms concentration xB. Data are in Table 2-7.
E . More Complex Problems
E1. A system known as a pump-around is shown below. Saturated liquid is withdrawn from stage 2 above the partial reboiler, and the liquid is returned to stage 3 (assume it is still a saturated liquid). Pump-around rate is P = 40.0 kgmoles/h. The column is separating methanol and water at 101.3 kPa. The feed flow rate is 100.0 kgmoles/hr. The feed is 60.0 mole % methanol and 40.0 mole % water. The feed is saturated liquid. We desire a bottoms product that is 2.5 mole % methanol. The distillate product should be 95.0 mole % methanol. The column has a total condenser and the reflux is a saturated liquid. Assume CMO. Use (L/D) = 2.0 x (L/D)min. Data are given in Table 2-7. Find xp, the optimum feed stage and total number of equilibrium stages required.

E2. *A distillation column is separating methanol and water. The column has open (direct) steam heating and a total of five stages. A liquid side stream is withdrawn from the second plate above the bottom of the column. The feed is 30 mole % methanol and is a subcooled liquid. One mole of vapor is condensed to heat 2 moles of feed to the saturated liquid temperature on the feed plate. Feed rate is 1000 moles/hr. A bottoms concentration of 1.5 mole % is desired. The steam used is pure saturated water vapor and the steam flow rate is adjusted so that
Steam flow rate/Bottoms flow rate = 0.833
The side stream is removed as a saturated liquid. The side-stream flow rate is adjusted so that Side-stream flow rate/Bottoms flow rate = 0.4
A total condenser is used. Reflux is a saturated liquid, and CMO can be assumed. Find the side-stream concentration and the distillate concentration. Data are given in Table 2-7.
E3. *A distillation column with at total condenser and a total reboiler is separating ethanol from water. Reflux is returned as a saturated liquid, and boilup is returned as a saturated vapor. CMO can be assumed. Assume that the stages are equilibrium stages. Column pressure is 1 atm. A saturated liquid feed that is 32 mole % ethanol is fed to the column at 1000 kg moles/hr. The feed is to be input on the optimum feed stage. We desire a distillate composition of 80 mole % ethanol and a bottoms composition that is 2 mole % ethanol. A liquid side stream is removed on the eighth stage from the top of the column at a flow rate of S = 457.3 kg moles/hr. This liquid is sent to an intermediate reboiler and vaporized to a saturated vapor, which is returned to the column at its optimum feed location. The external reflux ratio is L0/D = 1.86. Find the optimum feed locations of the feed and of the vapor from the intermediate reboiler. Find the total number of equilibrium stages required. Be very neat! Data are in Table 2-1.
F . Problems Requiring Other Resources
F1. A distillation column separating ethanol from water uses open steam heating. The bottoms composition is 0.00001 mole fraction ethanol. The inlet steam is pure water vapor and is superheated to 700 °F. The pressure is 1.0 atm. The ratio of bottoms to steam flow rates is B/S = 2.0. Find the slope of the bottom operating line.
F2. The paper by McCabe and Thiele (1925) is a classic paper in chemical engineering. Read it.
a. Write a one-page critique of the paper.
b. McCabe and Thiele (1925) show a method for finding the feed lines and middle operating lines for a column with two feeds that is not illustrated here. Generalize this approach when q ≠ 1.0.
F3. *If we wish to separate the following systems by distillation, is CMO valid?
a. Methanol and water
b. Isopropanol and water
c. Acetic acid and water
d. n-Butane from n-pentane
e. Benzene from toluene
G . Computer Problems
G1. *a. Solve Example 4-4 with a process simulator.
b. Repeat this problem but with L/D = 1.5.
G2. For problem 4.D9 find the optimum feed stage and total number of stages using a process simulator. Report the VLE package used. Compare answers with problem 4.D9.
G3. *Write a computer, spreadsheet, or calculator program to find the number of equilibrium stages and the optimum feed plate location for a binary distillation with a constant relative volatility. System will have CMO, saturated liquid reflux, total condenser, and a partial reboiler. The given variables will be F, zF, q xB, xD, α, and L0/D. Test your program by solving the following problems:
a. Separation of phenol from p-cresol. F = 100, zF = 0.6, q = 0.4, xB = 0.04, xD = 0.98, α = 1.76, and L/D = 4.00.
b. Separation of benzene from toluene. F = 200, zF = 0.4, q = 1.3, xB = 0.0005, xD = 0.98, α = 2.5, and L/D = 2.00.
c. Since the relative volatility of benzene and toluene can vary from 2.61 for pure benzene to 2.315 for pure toluene, repeat part b for α = 2.315, 2.4, and 2.61.
Write your program so that it will calculate the fractional number of stages required. Check your program by doing a hand calculation.
G4. Use a process simulator and find the optimum feed stage and total number of equilibrium stages for problem 3.D6. Report the VLE correlation used. Record the values of Qcondenser and Qreboiler (in Btu/hr).
Chapter 4 Appendix Computer Simulations for Binary Distillation
Although binary distillation problems can be done conveniently on a McCabe-Thiele diagram, 6 will show that multicomponent distillation problems are easiest to solve as matrix solutions for simulation problems (the number of stages and feed locations are known). Commercial simulators typically solve all problems this way. Lab 3 in this appendix provides an opportunity to use a process simulator for binary distillation. Although the instructions discuss Aspen Plus, other simulators will be similar.
Prerequisite : This appendix assumes you are familiar with the Appendix to Chapter 2, which included Labs 1 and 2, and that you are able to do basic steps with your simulator. If you need to, use the instructions in Lab 1 as a refresher on how to use Aspen Plus. If problems persist while trying to run the simulations, see the Appendix: Aspen Plus Separations Trouble Shooting Guide, that follows Chapter 17.
Lab 3 : The goals of this lab are: 1) To become familiar with Aspen Plus simulations using RADFRAC for binary distillation systems, and 2) To explore the effect of changing operating variables on the results of the binary distillation. There is no assignment to hand in. However, understanding this material should help you understand the textbook and will help you do later labs.
Start a blank simulation with general metric units. At the bottom of the flowsheet page click on the tab labeled “columns.” Use the column simulator RADFRAC. Draw a flowsheet of a simple column with one feed, a liquid distillate product (red arrow), and a liquid bottoms product. (This is the most common configuration for distillation columns.) Click on the arrow to the right of the RADFRAC button to see alternate sketches of the column. Do not use a packed bed system. The screen should look similar to Figure 4-A1.
Figure 4-A1. Aspen Plus screen shot for simple distillation

In specifying columns, Aspen Plus calls the total condenser stage 1 and the partial reboiler stage N. Thus, if you specify N = 40 there are 38 equilibrium stages, a partial reboiler (which is an equilibrium contact) and a total condenser (which does not separate). Feed and side stream locations are thus counted down from the top (condenser is 1). This notation agrees with Figure 6-2.
In the runs that follow, if RADFRAC is completed with errors, try running a similar separation that converges, and then run the desired simulation again. Aspen Plus uses the previous converged run to initialize the new run. A column that will converge one time may not converge when run with different initial conditions. If the run is close to converged, the values may still be quite reasonable. You can try changing the conditions and run again. Runs with critical errors should be ignored (don’t look at the results since they have no meaning). If Aspen Plus has a critical error, such as the column drying up, reinitialize before you do another run. The systems for this lab were chosen so that they should converge.
When you do runs, record L/D, feed rate, feed condition (fraction vaporized or temperature), the feed stage location and the number of stages (you set these values), and the results: distillate and bottoms compositions, the temperatures of distillate and bottoms, the maximum and minimum flow rates of liquid and vapor, and the heat loads in condenser and reboiler. Do not print out the entire Aspen Plus report.
1. Binary Distillation. Separate 1,2-dichloroethane from 1,1,2-trichloroethane. Feed is 100 kg moles/hr of 60% dichloroethane. Operation is at 1.0 atm. Column has a total condenser and a kettle type reboiler. Peng-Robinson is a reasonable VLE package. Be sure to select the correct components from the AspenPlus menu. Note that 1,1,1-trichloroethane should not be used since it has very different properties.
a. Plot the x-y diagram from Aspen Plus and compare to an approximate plot with a constant a relative volatility of 2.24 (an over-simplification, but close). Note: Input the data for Step 1b first.
b. The feed is 100% vapor. (Set the vapor fraction in the feed to 1.0.) We want a distillate product that is 92 mole % dichloroethane and a bottoms that is 8 mole % dichloroethane. Since Aspen Plus is a simulator program, it will not allow you to specify these concentrations directly. Thus, you need to try different columns (change number of stages and feed location) to find some that work. Use L/D =2.0. Set Distillate flow rate that will satisfy the mass balance. (Find D and B from mass balances. Do this calculation accurately or you may never get the solution you want.) Pick a reasonable number of stages and a reasonable feed stage (Try N=21 and feed at 9 [above stage] to start). Simulate the column and check the distillate and bottoms mole fractions. This is easiest to do with the liquid composition profiles for stage 1 and N. They should be purer than you want. Also, check the K values and calculate the relative volatility at the top, feed stage, and bottom of the column to see how much it varies.
c. Continue part 1b: Find the optimum feed stage by trying different feed stages to see which one gives the greatest separation (highest dichloroethane mole fraction in distillate and lowest in bottoms). Then decrease the total number of stages (using optimum feed stage each time) until you have the lowest total number of stages that still satisfies the specifications for distillate and bottoms. Note that since the ratio (optimum feed stage)/(total number) is approximately constant you probably need to check only three or four feed stages for each new value of the total number of stages. If your group works together and have different members check different runs, this should not take too long.
d. Triple the pressure, and repeat steps a and b. Determine the effect of higher pressure on the relative volatility.
e. Return to p = 1 atm and your answer for part c. Now increase the temperature of the feed instead of specifying a vapor fraction of 1. What happens?
f. Return to the simulation in part c. Determine the boilup ratio (Vreboiler/B). Now, run the simulation with this boilup ratio instead of specifying Distillate rate. Then start decreasing the boilup ratio in the simulation. What happens?
g. Return to the simulation in part e. Instead of setting fraction vaporized in the feed, set the feed temperature. (Specify L/D = 2 and boilup ratio, N and feed location.) Raise the feed temperature and see what happens.
h. Return to part b, but aim for a distillate product that is 99% dichloro and a bottoms that is 2% dichloro. Do the mass balances (accurately) and determine settings for Distillate flow rate. Try to find the N and feed location that just achieves this separation with an L/D = 2. Note that this separation should not be possible since the external reflux ratio (L/D) is too low. See what happens as you increase L/D (try 4 and 8).
In the appendix to Chapter 6 we will use process simulators for multicomponent distillation calculations.
One caveat applies to these and all other simulations. The program can only solve the problem that you give it. If you make a mistake in the input (e.g., by not including a minor component that appears in the plant) the simulator cannot predict what will actually happen in the plant. A simulation that is correct for the problem given it is not helpful if that problem does not match plant conditions. Chemical engineering judgment must always be applied when using the process simulator (Horwitz, 1998).



