4
Combustion
4.1 Overview
The overwhelming majority of both static and mobile power‐generation systems in the world today rely on the burning of fossil fuels, i.e. a chemical reaction between a hydrocarbon compound and air that releases heat.
The energetic gases evolved may be used directly, as in a reciprocating engine or gas turbine, or they may give up their heat in energizing an intermediate working fluid like water/steam. In either case, the gases, which may prove to be environmentally harmful, are discharged to the atmosphere at the end of their usefulness.
This chapter will detail the combustion process from reactant species to product evolution in terms of mass and energy conservation. It will also introduce some of the more practical aspects of combustion and fuel use, for example, excess air, equivalence ratios and calorific values.
Learning Outcomes
- To understand the basic units of combustion chemistry.
- To be able to carry out simple chemical balances on combustion equations.
- To be introduced to basic combustion terminology related to reactant fuel–air mixtures.
- To be able to apply the first law of thermodynamics and conduct energy balances associated with the combustion process.
- To determine the resulting theoretical flame temperatures in a combustion process.
- To be introduced to the generation and removal of sulphur and nitrogen oxide emissions.
4.2 Mass and Matter
At a fundamental level, matter has been classified according to its atomic structure into elements, for example, carbon, hydrogen, argon. Elements are denoted by symbols or abbreviations of one or two letters, for example, C, H and Ar for carbon, hydrogen and argon, respectively.
An element is a single homogenous substance having a clearly identifiable atomic structure that cannot be further subdivided by chemical means.
Matter may comprise a single element or a combination of elements.
Chemical combinations of atoms of the same element or atoms of different elements are called molecules. Molecules of any specific combination have a readily identifiable geometric structure characteristic of the combination and reflecting the inter‐atomic relationship.
The bulk aggregation of a specific molecule is usually termed a compound.
Molecules are represented by formulae that consist of the element symbols and perhaps subscripts, for example, N2 (nitrogen gas), SO2 (sulphur dioxide), H2O (water) etc. The subscripts denote the numbers of atoms of each element present. Note that if only one atom of an element is present, the subscript is omitted; for example, a molecule of carbon dioxide (CO2) comprises one atom of carbon and two atoms of oxygen.
4.2.1 Chemical Quantities
When evaluating the magnitude of matter from a chemical point of view, the unit used is the mole or kilomole. A mole is defined as that amount of substance containing 6.0223 × 1023 molecules or atoms. This number is termed Avogadro's number or Avogadro's constant.
4.2.2 Chemical Reactions
At the most fundamental level, chemical reactions are the result of the migration of electrons and protons.
Chemical reactions describe, on a molecular scale, the combination of simple substances to form new ones and also the breakdown of complex substances into more simple ones.
In chemical reactions, substances are represented by their chemical symbols, with coefficients indicating the number of moles of substance present. For example:
In the above equation, one mole of hydrogen (H2) combines with 0.5 moles of oxygen (O2) to form one mole of water.
Note that coefficients having a value of 1 are omitted by convention.
The substances initially present in a reaction are termed the reactants. The substances resulting from a reaction are termed the products.
4.2.3 Physical Quantities
On a more macroscopic or everyday scale, the unit of mass is the kilogram.
There are many applications where a conversion between these two units, kilograms and kilomoles, is required and this is facilitated by a substance property called the molar mass (M, kg/kmol).
The approximate elemental molar masses of primary interest in this chapter are shown in Table 4.1.
Table 4.1 Table of common elements associated with combustion.
| Element | Chemical symbol | Approximate molar mass (kg/kmol) |
| Carbon | C | 12 |
| Hydrogen | H | 1 |
| Oxygen | O | 16 |
| Nitrogen | N | 14 |
| Sulphur | S | 32 |
(Note: Units of molar mass are sometimes quoted in a multiple‐free ratio of g/mol).
The molar mass of molecules, or the molecular mass, can be found by simple accountancy. If a molecule contains i different elements, then the resulting molar mass can be calculated by summing the product of the number of atoms of each element with their attendant molar mass:
The mean molar mass of a mixture (![]() ) can be found with knowledge of component molar fractions.
) can be found with knowledge of component molar fractions.
The molar fraction (![]() ) is simply the ratio of the number of moles (ni) of the component of interest (i) to the total number of moles in the mixture (ntot), i.e.:
) is simply the ratio of the number of moles (ni) of the component of interest (i) to the total number of moles in the mixture (ntot), i.e.:
If a mixture contains i different components, then the resulting molar mass of the mixture can be calculated by summing the product of each component molar mass (Mi) with its attendant molar fraction (%) and dividing by a hundred; thus:
The relationship between the number of kilomoles of a substance (n), its mass (m, kg) and molar mass (M, kg/kmol) is given by:
This relationship is extremely useful, as it provides for a conversion from chemical to physical units.
4.3 Balancing Chemical Equations
The principles of mass conservation also have an application to chemical reactions. Chemical reactions must be manipulated or balanced until the law of mass conservation is obeyed. To obtain a balanced equation, identify the products and reactants and set them out as described earlier. The following steps may then be employed:
- Step 1: Set the coefficient (number of moles) of the first reactant to 1 and the coefficients of all the other substances (reactants and products) to unknowns a, b, c etc.
- Step 2: Account for the number of atoms of each element in turn by multiplying the coefficient of that element by its subscript and equate between reactant and product. Solve the balance for each unknown coefficient, a, b, etc.; i.e. the number of moles of each substance.
- Step 3: Rewrite the equation using the coefficient results from the balance.
- Step 4: Check the result by using molar mass data to convert from moles to mass for each substance.
If the equation is balanced correctly, the total mass of reactants will equal the total mass of products.
[Note that numbers of atoms and mass must always balance between reactants and products, however, numbers of moles may not.]
4.3.1 Combustion Equations
Typically, in a combustion process we have:
When the fuel is a hydrocarbon, for example, methane (CH4), ethane (C2H6), octane (C8H18) then:
- For the carbon

- For the hydrogen

For combustion of a hydrocarbon fuel with air, a balanced equation can be obtained by using the method outlined previously, with both reactions being considered simultaneously.
Combustion reactions usually take place with ambient air that consists of 21% O2 and 79% N2 by volume. So every mole of oxygen brings with it (79/21) 3.76 moles of N2. This must be accounted for when balancing combustion equations.
Commonly, N2 is assumed not to undergo any chemical change in simple combustion analysis. In reality, oxides of nitrogen are formed if the temperature is high enough.
Again, in simple analyses, the effects of other atmospheric components present in small concentrations, such as inert gases, water vapour etc., are ignored.
If the products of combustion comprise CO2, H2O, N2, as above (and perhaps O2, as will be shown later) then we have complete combustion.
Incomplete or partial combustion is associated with insufficient air supply or poor mixing and results in products that may contain H2, CO, C, OH and N2.
Any hydrogen or carbon in the products must be regarded as wasted fuel, as it could potentially be reacted with oxygen to release its energy.
4.4 Combustion Terminology
4.4.1 Oxidizer Provision
The minimum amount of air that supplies sufficient O2 for complete combustion in the previous examples is termed the 100% theoretical or stoichiometric (perfectly chemically proportioned) quantity of air.
The term air–fuel ratio (A/F) is defined as the ratio of the mass of air to the mass of fuel in a combustion process, i.e.

Due to imperfect mixing between fuel and air, it is common practice to supply an amount of air in excess of the stoichiometric amount to achieve complete combustion.
To describe this amount, the terms % excess air and % stoichiometric air (or % theoretical air) are in common usage.
The % excess air is commonly quoted in terms of the air–fuel ratio, thus:

and
where n = number of moles of air present.
Note that if excess air is employed, the unused oxygen and its attendant nitrogen will appear in the products. This must be accounted for when carrying out mass and energy balances.
Another term used to describe the state of the fuel–air mixture is the equivalence ratio (φ), defined thus:

 indicates a (fuel) rich mixture.
indicates a (fuel) rich mixture. indicates stoichiometric proportions.
indicates stoichiometric proportions.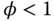 indicates a (fuel) lean mixture.
indicates a (fuel) lean mixture.
4.4.2 Combustion Product Analyses
Sampling and analysis of product gases is commonly employed to determine the state of fuel–air combustion mixtures.
Analyzers are usually dry‐gas based, i.e. the water vapour is removed before exposure to the analyzer's sensors. However, the balancing technique detailed above is still applicable providing that the water is reinstated into the product side of the combustion equation.
4.4.3 Fuel mixtures
The above discussion is limited to the combustion of a pure fuel, i.e. one comprising only a single species of hydrocarbon. In reality, some fuels can be complex mixtures, for example, natural gas is a mixture of methane, ethane and heavier hydrocarbons as well as some nitrogen and carbon dioxide. Coal and biomass‐sourced fuels can be even more complex. Nevertheless, the combustion equation‐balancing technique can still be applied to first approximation.
4.5 Energy Changes During Combustion
The standard enthalpy of formation![]() is the enthalpy of a substance at a specified reference state (usually 298.15 K, 1 atm, denoted by the superscript ‘o’) due to its chemical composition and is associated with the breaking and making of chemical bonds.
is the enthalpy of a substance at a specified reference state (usually 298.15 K, 1 atm, denoted by the superscript ‘o’) due to its chemical composition and is associated with the breaking and making of chemical bonds.
Compounds with a negative enthalpy of formation release energy when they are formed in an exothermic reaction. Compounds with a positive enthalpy of formation require energy for their creation in an endothermic reaction.
Note that, by convention, the enthalpy of formation of some stable substances has been traditionally set to zero, i.e. ![]() .
.
The heat or enthalpy of vaporization![]() is the heat required to completely vaporize a unit quantity of saturated liquid. Combustion calculations are generally performed with fuels in a gaseous (g) state. However, many fuels are supplied in a liquid form. Liquid fuels must be vaporized prior to combustion and the enthalpy of vaporization quantifies this phase change energy requirement. This must be accounted for when performing energy balances associated with combustion involving liquid fuels.
is the heat required to completely vaporize a unit quantity of saturated liquid. Combustion calculations are generally performed with fuels in a gaseous (g) state. However, many fuels are supplied in a liquid form. Liquid fuels must be vaporized prior to combustion and the enthalpy of vaporization quantifies this phase change energy requirement. This must be accounted for when performing energy balances associated with combustion involving liquid fuels.
When reviewing data tables, it will be noted that there are two enthalpy of formation entries for water. The applicable value depends on whether the water exists in a liquid (l) or in a gaseous (g) state. The difference in numerical value is accounted for by the enthalpy of vaporization value for water.
The heat or enthalpy of reaction![]() is the difference between the enthalpy of the combustion products at a specified state (i.e. temperature, pressure) and the enthalpy of the reactants at the same state for a complete reaction.
is the difference between the enthalpy of the combustion products at a specified state (i.e. temperature, pressure) and the enthalpy of the reactants at the same state for a complete reaction.
In some texts and handbooks, the term heat of combustion is used. This parameter is the enthalpy of reaction for a combustion process (and is sometimes quoted with a sign change).
Values of ![]() for some common hydrocarbons and other compounds are presented on a molar‐independent basis in Table B.1 in Appendix B. Note also that thermodynamic properties quoted per kmol are identified by the addition of the bar accent.
for some common hydrocarbons and other compounds are presented on a molar‐independent basis in Table B.1 in Appendix B. Note also that thermodynamic properties quoted per kmol are identified by the addition of the bar accent.
Molar‐independent sensible enthalpy values ![]() for gases at 1 atm and temperatures relative to the standard state are presented in Table B.2 in Appendix B.
for gases at 1 atm and temperatures relative to the standard state are presented in Table B.2 in Appendix B.
At any given state, the total enthalpy![]() of a chemical component is the sum of the enthalpy of formation and sensible enthalpy of the component, i.e.:
of a chemical component is the sum of the enthalpy of formation and sensible enthalpy of the component, i.e.:
Another term commonly used to describe the energetic content of commercial fuel is the heating or calorific value (MJ/kg or MJ/m3). This value will vary according to whether any water vapour in the products is condensed, thus releasing its latent heat of condensation.
If the water vapour is condensed, the total energy release per unit quantity of fuel is known as the higher heating value (HHV) or gross calorific value (GCV). If not, the energy release is known as the lower heating value (LHV) or net calorific value (NCV).
Heating values can be estimated by a simple conversion of the heat of combustion value from molar to mass terms.
The higher heating values of some common hydrocarbons are also given in Table B.1 in Appendix B. Specified pressure and temperature conditions should be associated with any published values.
4.6 First Law of Thermodynamics Applied to Combustion
4.6.1 Steady‐flow Systems (SFEE) [Applicable to Boilers, Furnaces]
Applying the first law of thermodynamics to a combustion control volume and neglecting any potential or kinetic energy changes, the steady flow chemical reaction can be written as:

This form requires knowledge of the molar flow rates ![]() through the volume.
through the volume.
However, it is more common in combustion studies to express this relationship in per mole of fuel terms and to divide the above equation through by the molar flow rate of fuel. (Note that the LHS terms lose their ‘dot’ notation as a consequence.)
Assuming zero work exchange (and zero heat input), this becomes:

If the process is isothermal, the sensible enthalpy terms disappear and this further reduces to:

4.6.2 Closed Systems (NFEE) [Applicable to Engines]
Again, neglecting any kinetic or potential energy changes:

Internal energy tables are available, but to avoid using another property, advantage may be taken of the enthalpy/internal energy/flow work relationship, thus:


4.6.3 Flame Temperature
The adiabatic flame temperature is the ideal maximum temperature of the resulting combustion flame assuming no work, heat loss or changes in kinetic and potential energy, and is calculated from knowledge of the fuel energy content and the heat capacities of the combustion products.
For a constant‐pressure process, such as a gas turbine combustor or the burner of a furnace, these conditions reduce the first law of thermodynamics to:

For a constant‐volume process, such as part of a theoretical engine cycle, the first law becomes:

Since the temperature of the products is unknown at the outset, adiabatic flame temperature calculation involves an iterative process, as follows:
- Perform a balance for the combustion equation accounting for any prevailing conditions, for example, excess air etc.
- Guess a temperature for the products.
- Using enthalpy tables, perform a reactant–product energy balance at the estimated temperature.
For example, in the case of a constant‐pressure process:
If ![]() then the guess is correct.
then the guess is correct.
In the absence of equality, guess a new product temperature using the magnitude and sign of the result of the previous guess as a guide. Re‐attempt a balance.
If still unlucky, interpolate over a small interval, say 200 K, to solve for a reactant–product balance and the resulting adiabatic flame temperature.
(As an alternative, plot the results on an enthalpy–temperature basis and read off.)
Actual flame temperatures are always less than this theoretical value due to heat loss from the flame by convection/radiation and unwanted dissociation (reverse chemical reactions) of the combustion products that consume energy.
4.7 Oxidation of Nitrogen and Sulphur
Hydrocarbon fossil fuels are commonly more than just hydrogen and carbon in composition and can comprise a range of other elements and compounds, albeit in relatively small amounts. For example, sulphur can be found in solid and liquid fuels and, in association with hydrogen, in gaseous fuels. Sulphur will burn with oxygen in a combustion process but, unfortunately, the resulting products of combustion are unwelcome, being both poisonous and able to go on to become corrosive.
Fuels may also contain nitrogen that can, under certain circumstances, also play an unwanted part in a combustion process. This occurrence is greatly exacerbated by the presence of nitrogen in the combustion oxidant gas that is usually atmospheric air. Again, the resulting products of combustion are able to go on to become a noxious emission.
4.7.1 Nitrogen and Sulphur
Elemental nitrogen is a relatively inert and harmless non‐metal. It was first identified as an element in 1772 by British chemist Daniel Rutherford. It has a relative atomic mass of approximately 14 and boils at −196 °C, so at normal temperature and pressure it is a gas.
Air in the lower atmosphere comprises approximately 79% nitrogen by volume and 76% by mass. Nitrogen makes up about 48% of all dissolved oceanic gas.
In combination with other elements, it is essential for life and it is to be found in many bio‐compounds, including DNA. It can be fixed directly from the air by some soil bacteria. When converted into nitrates it can be made available to plants.
It is not truly inert, as, under the right conditions, it will react with hydrogen, oxygen and some metals.
Nitrogen reacts with hydrogen to form ammonia (NH3). Its compounds are essential in the manufacture of many products including fertilizer and explosives.
It is also used in processes requiring an operating environment with a low reactivity, and in its liquid form it is used as a coolant.
Elemental sulphur is a bright yellow non‐metal that can exist in three forms – as a powder, in crystalline form or as a soft solid. It was known to the ancient Greeks and Egyptians. French scientists Louis‐Josef Gay‐Lussac and Louis‐Jacques Thénard first identified it as an element in 1809.
It has a relative atomic mass of approximately 32. It melts at 115 °C and boils at 445 °C.
Its natural occurrence is closely associated with volcanic activity and some fossil fuel reserves. It is malodorous and hazardous to health when in combination with hydrogen or when burnt.
Again, it is important in biochemistry as a component of some amino acids.
Its compounds are commonly used in bleaches and preservatives and are used in the production of sulphuric acid (H2SO4), which is a component that underpins a myriad of manufacturing processes.
4.7.2 Formation of Nitrogen Oxides (NOx)
The reduction (i.e. the addition of hydrogen) of nitrogen produces ammonia (NH3):
Oxidation of nitrogen produces, progressively, nitric oxide (NO) then nitrogen dioxide (NO2).
In short:
At high levels of concentration, NO2 is a lung irritant, causing inflammation of the airways and bronchitis‐like symptoms. The World Health Organization's time‐based concentration limits for exposure to the gas (2016) are:
NOx in combination with hydrocarbon compounds (HC) also plays a part in the formation of low‐level ozone (O3) which, again, is a lung irritant.
There are three main combustion‐sourced mechanisms of NOx formation:
- Thermal NOx formed by the combination of nitrogen and oxygen in high‐temperature (>1800 K) combustion systems over a range of equivalence ratios. The commonly used model of thermal NOx formation is called the (extended) Zeldovich mechanism.
This assumes that O radicals attack N2 molecules, thus:

The resulting N radicals can form NO by the following reaction:

Nitrogen can also combine with hydroxyl radicals (OH), thus:

Its formation is slow compared to the attendant fuel–oxygen combustion reactions and so its generation is generally limited to the exhaust gas environment.
- Prompt NOx from reaction of fuel‐derived radicals with N2, ultimately leading to NO. The commonly used model of NOx formation, in this case, is called the Fenimore mechanism and is more prevalent in fuel‐rich mixtures. As suggested by its denomination, its formation is faster than thermal NOx reactions and its generation can take place in the flame zone environment.
The mechanism assumes the presence of hydrocarbon radicals (CH, C, H) to initiate the process and generate nitrogen (N), thus:


Alternative routes to NOx generation are possible.
One of the most commonly quoted paths utilizing hydrogen cyanide (HCN) is:




- N2O intermediate NOx is generated in combustion systems with equivalence ratios of less than 1, i.e. fuel‐lean mixtures, and requires the presence of an unchanged, facilitating chemical species (M) and hydrogen and oxygen radicals, thus:



N2O and prompt NOx are only weakly dependent on temperature.
4.7.3 NOx Control
Two approaches to NOx control are possible: combustion process modification or post flame/exhaust gas treatment.
4.7.3.1 Modify the Combustion Process
Several possibilities present themselves:
- Eliminate the atmospheric nitrogen by the use of pure oxygen for combustion. This is a very expensive solution.
- Manipulate the air–fuel ratio.
- Operating on a fuel‐rich basis reduces the amount of available oxygen and related nitrogen. Operating on a fuel‐lean basis, i.e. providing high excess air, increases the amount of nitrogen but reduces the resulting flame temperature.
- Recirculation of the flue gases to the combustion chamber to reduce peak flame temperature. NOx reductions of 70–80% are possible.
- Water injection into the combustion chamber, hence reducing the temperature due to the use of energy in vaporizing the water.
- Air‐staged (rich–lean) combustion or low‐NOx burners producing a twin‐peaked temperature–time characteristic.
- In the first stage, the air–fuel ratio is such that all the O2 is used up, leaving none for NOx formation.
- In the second stage, due to the heat released in the first stage, the temperature is limited below that for large‐scale NOx formation.
- Highly effective mixing and/or inter‐stage cooling is required. With this method, NOx reduction of 40–70% is possible; however, HC and CO emissions may increase in the exhaust gases.
- Re‐burning, which is another staged combustion strategy employing the staging of fuel and air.
4.7.3.2 Post‐flame Treatment
Most post‐flame treatments either add a reducing agent to the combustion gas stream to take oxygen away from NO or pass the NOx over a catalyst.
For power plants and large furnaces, ammonia (or urea) is commonly added as a reducing agent.
and
Note the above reducing reactions are only effective at temperatures of between 850 and 1000 °C. Above 1000 °C the following reaction may occur, increasing NO formation:
Below 850 °C the conversion to N2 and water vapour is unacceptably slow.
NOx reductions of 60–80% are possible.
In a modern car engine, post‐flame treatment is carried out using a catalytic converter:
However, control over the NO/CO ratio is important for good conversion.
4.7.4 Formation of Sulphur Oxides (SOx)
Oxides of sulphur are air pollutants.
Elemental sulphur will react with oxygen in the air to produce sulphur dioxide (SO2):
The rate of the reaction increases with increasing temperature.
Sulphur dioxide (SO2) is an air pollutant causing irritation and inflammation to the respiratory tract and eyes at high levels of concentration. The World Health Organization's time‐based concentration limits for exposure to the gas (2016) are:
- 20 µg/m3 – 24‐hour mean
- 500 µg/m3 – 10‐minute mean.
Sulphur in contact with hydrogen can be reduced to hydrogen sulphide (H2S), also regarded as an air pollutant.
4.7.5 SOx Control
Two fuel‐related sources of sulphur emissions will be considered here.
4.7.5.1 Flue Gas Sulphur Compounds from Fossil‐fuel Consumption
Sulphur is commonly found in coal and oil, so SO2 in low (<0.5%) concentrations will be a minor component of their combustion gases. The total volume of the emission on a global scale is, however, very large.
Scrubbing systems for this problem are called flue gas desulphurization (FGD) units.
For this application, three possible removal arrangements are commonly in use: gas bubblers, spray chambers and packed chambers.
In a simple bubbler, the waste gas is forced, under pressure, through a perforated pipe submerged in the scrubbing liquid. With small bubbles, good contact will result. However, large gas‐side pressure drops may occur in tall towers.
In a spray chamber, the gas flows up an open chamber through a shower of descending scrubbing liquid released from spray nozzles. Gas–liquid contact is inferior to a bubbler but lower pressure drops prevail.
A packed column bed (see Figure 4.1) is a modified spray chamber with the column interior filled with some kind of solid medium on which the liquid can form a thin film, giving a large surface area to facilitate mass transfer. The medium or bed can comprise engineered ceramic, plastic or metal shapes or even natural materials such as rocks/gravel. This type of arrangement provides the best mass transfer per unit pressure drop performance.

Figure 4.1 Packed column SO2 scrubbing tower arrangement.
In this application, the SO2 is ultimately converted to calcium sulphate (CaSO4) by using limestone (CaCO3) slurry as the liquid in a wet scrubber. This facilitates its capture and removal. The basic chemical reaction is:
A typical arrangement for calcium sulphate removal and scrubbing liquid reuse is shown in Figure 4.2. The principal components of the system are the scrubber, holding tank and settling chamber.

Figure 4.2 Typical flue gas desulphurization (FGD) waste recovery system.
The liquid exiting the scrubber is transferred to a holding tank where finely ground limestone and oxygen are added.
The resulting slurry is recirculated from the holding tank to the scrubber liquid inlet.
A fraction of the holding tank recirculation is transferred to a settling chamber to facilitate solid removal.
The outflow of the settling chamber has its water content reduced by vacuum filtration and is then often mixed with dry flyash (particulate matter from the combustion process) for easier handling before going to landfill. The liquid fraction overflow of the settling chamber is returned to the holding tank.
Liquid circulation rates are very large in these types of scrubbers, with the slurry spending only a few seconds in the tower and up to ten minutes in the holding tank, where most of the reactions take place.
In modern plant, it is becoming less common for an FGD's output to end up in landfill. Many plants are able to sell their calcium sulphate waste on to the construction supply industry for the manufacture of gypsum‐based plasters.
Limestone scrubbers have associated with them many operational considerations. For example:
- Scrubbing cools the flue gas and so there is a requirement to reheat the cleaned flue gas to maintain plume buoyancy for later dispersal. Reheating is also required to raise the temperature to above the acid dew point, preventing any acid condensation and corrosion downstream of the scrubber.
- As indicated earlier, the basic removal of limestone reaction generates carbon dioxide.
- Solid deposition either via entrainment of slurry droplets in the exhaust system or plugging and scaling of FGD‐associated fluid flow systems, e.g. pumps, valves etc., is a problem.
This list is not exhaustive.
4.7.5.2 Sulphur Compounds from Petroleum and Natural Gas Streams
Some natural gas streams contain greater than 1% by weight of hydrogen sulphide (H2S), which must be removed to make the gas acceptable for domestic use. Gases with high H2S concentrations are often described as sour and their removal as sweetening.
Absorber/stripper arrangements utilizing amine solutions are commonly employed for its removal. Sulphur recovered by this process has a market value, making a significant contribution to global supply. This technique is also used in some proposed CO2 removal systems. More detail on gas absorption is supplied in Chapter 6.
4.7.6 Acid Rain
The natural acidity of freshwater sources such as lakes, rivers and streams is dependent on several factors including rainfall as well as the local geology and ecosystem.
For example, as a result of a reaction with atmospheric carbon dioxide, carbonic acid (H2CO3) can be formed in naturally occurring rainwater.
Rainfall pH values of around 5.6 are possible.
The pH of freshwater can also be altered by the composition of run‐off and leachate entering its volume after passage through/over surrounding rocks and soils.
However, as a consequence of nitrogen and sulphur oxide reactions with atmospheric water, more extreme acid deposition, more commonly referred to as acid rain, can result.
In the presence of water vapour in the atmosphere, NO2 can react to form nitric acid (HNO3):
Oxidation of sulphur dioxide produces sulphur trioxide (SO3):
Upon contact of SO3 with atmospheric water, sulphuric acid (H2SO4) will be formed:
As a result of the above reactions, localized and trans‐national freshwater lake pH levels can be as low as 3.5 in some areas. The impact is exacerbated by the fact that some elemental metals become soluble at elevated pH values and so can be dissolved out of rocks and soils then washed into feeder streams and rivers, where they can affect the developmental biology of a number of species.
Sulphur‐based acid rain can be particularly damaging to buildings constructed from carbonate‐based materials that react with the pollutant, replacing the carbonates with less‐cohesive sulphate compounds (gypsum) that have a tendency to blacken and flake from the building surface.
The conversion process from air pollutant gas to water pollutant is not instantaneous and, in some cases, oxide gases can be carried by the prevailing wind far from their source before acid deposition occurs. For example, much of the historical acid deposition experienced in Scandinavian forests is thought to have originated in other, predominantly coal‐burning areas of northern Europe such as Germany and the UK. A similar set of circumstances prevails between the coal‐burning plants of the north‐eastern states of the USA and Canada.
4.8 Worked Examples
4.9 Tutorial Problems
Note: When attempting the questions below, a reasonable approach should be made with respect to rounding of values to achieve the final result. This is especially applicable to enthalpy‐based problems.
- 4.1
Determine the molar mass (kg/kmol) of the following molecules:
- Carbon monoxide (CO).
- Sulphur dioxide (SO2).
- Benzene (C6H6).
- Ethyl alcohol (C2H5OH).
- 4.2 A biogas resulting from a process known as anaerobic digestion (a little like composting without air) comprises 60% methane (CH4) and 40% carbon dioxide (CO2) per kmol of fuel. Determine the resulting molar mass (kg/kmol) of the gas mixture.
[Answer: 27.2 kg/kmol]
- 4.3 Dodecane (C12H26) is often used as an approximate model for commercial diesel fuel. If the density of the fuel is 755 kg/m3, determine the number of moles per litre of fuel. [Answer: 4.44 moles]
- 4.4 Produce balanced stoichiometric combustion equations for the following fuels with air:
- Pentane (C5H12).
- Ethyl alcohol (C2H5OH).
- Nitromethane (C2H3NO2).
[Answers: 15.34, 9.0, 3.31]
- 4.5 A kilomole of methane (CH4) is burnt with dry air that contains 3 kmol of O2. Calculate the % excess air, the air–fuel ratio and the molar fraction (%) of oxygen in the products. [Answers: 50%, 25.88, 6.5%]
- 4.6 A butane (C4H10)–air mixture has an A/F ratio of 21. Determine:
- The stoichiometric A/F ratio.
- The % excess air and the % stoichiometric air.
- Whether the mixture is rich or lean (justify your answer).
- The volume percentage of CO2 in the products.
- The CO2/fuel mass ratio.
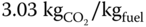 ]
] - 4.7 Propane (C3H8) is burned with dry air. A volumetric analysis of the products on a dry basis gives 11% CO2, 4.5% O2 and 84.5% N2. Calculate the air–fuel ratio and the % excess air.
[Answers: 19.2, 22%]
- 4.8 An unknown hydrocarbon fuel combusts with dry air. The resulting products have the following dry volumetric analysis: 7.7% CO2, 9.8% O2 and 82.5% N2. Estimate the fuel composition in terms of its carbon/hydrogen atomic ratio. Calculate the % excess air.
[Answers: 0.43, 81%]
- 4.9 Carbon is burnt with pure oxygen to form carbon dioxide in a steady‐flow reaction. Assuming isothermal and isobaric standard state conditions, calculate the energy evolved. [Answer: −393 520 kJ/kmol of fuel]
- 4.10 Assuming steady flow, isothermal conditions (25 °C, 1 atm), verify that the enthalpy of combustion for a stoichiometric mixture of gaseous propane (C3H8) and air is −2 220 000 kJ/kmol. What is the effect on the enthalpy of combustion (kJ/kmol) if the propane enters the combustion process in a liquid state?
Take the enthalpy of vaporization of propane to be 15 060 kJ/kmol.
Assume product water to be in a liquid state in both cases.
[Answer: −15060 kJ/kmol of fuel]
- 4.11 Gaseous methyl alcohol or methanol (CH3OH) and air in stoichiometric proportions at 25 °C, 1 atm are burnt in a steady‐flow combustion chamber. If the products leave the chamber at 600 K and 1 atm, calculate the heat release.
[Answer: Approximately −542 000 kJ/kmol of fuel]
- 4.12 Liquid benzene (C6H6) at 25 °C, 1 atm is burnt with 95% stoichiometric air in a steady‐flow combustor. The products contain the stoichiometric amount of water. The products also contain both carbon dioxide (CO2) and carbon monoxide (CO). If the enthalpy of vaporization of benzene is 33 830 kJ/kmol, determine:
- The carbon/hydrogen mass ratio for the fuel.
- The molar fraction of CO in the products (%).
- The heat released (kJ/kmol of fuel) if the products leave the combustion chamber at 1000 K.
- 4.13 Butane (C4H10) at 25 °C is burnt in a steady‐flow combustion chamber with 50% excess air. The air supply is preheated to 400 K. However, only 97% of the carbon is converted to CO2 with the remaining 3% of carbon appearing as carbon monoxide (CO) in the products. If the products leave the chamber at 1000 K, calculate the heat transfer. Assume the pressure remains constant at 1 atm.
[Answer: approximately −1 638 000 kJ/kmol of fuel]
- 4.14 Nitromethane (CH3NO2) is a liquid fuel with a structure based on the simple methane molecule. Assuming stoichiometric proportions with air, a steady‐flow analysis and isothermal conditions, using the data below, determine for the methane derivative:
- The standard enthalpy of combustion (kJ/kmol of fuel), assuming the product water is in a liquid state.
- The product/reactant molar ratio for the reaction.
Nitromethane (liquid) data:
- Enthalpy of formation (hof) at 25 °C, 1 atm: −110 000 kJ/kmol.
[Answers: −712 265 kJ/kmol, 1.27 K]
- 4.15 Ethane (C2H6) at 25 °C undergoes complete combustion with air at 400 K, 1 atm in a steady‐flow, insulated combustor. Determine the adiabatic flame temperature for 50% excess air.
[Answer: 1894 K]
- 4.16 An attempt to reduce nitric oxide (NO) emissions in a flue gas uses ammonia in the following reaction:
Determine the mass (kg) of ammonia required per tonne of NO.

[Answer: 378 kg]
- 4.17 A large coal‐fired power plant emits 30 kt of sulphur dioxide (SO2) per annum. It is proposed to capture the emission using calcium carbonate according to the following basic reaction:
Determine how much carbon dioxide (CO2) is generated per annum as a consequence of the SO2 clean‐up.

[Answer: 20 625 tonnes per annum]
