Flashlights are useful for numerous tasks, of which reading under the covers and sending coded messages are only the two most obvious. The common household flashlight can also take center stage in an educational show-and-tell of the magical stuff known as electricity.
Electricity is an amazing phenomenon, managing to be pervasively useful while remaining largely mysterious, even to people who pretend to know how it works. But I'm afraid we must wrestle with electricity anyway. Fortunately, we need to understand only a few basic concepts to comprehend how it's used inside computers.
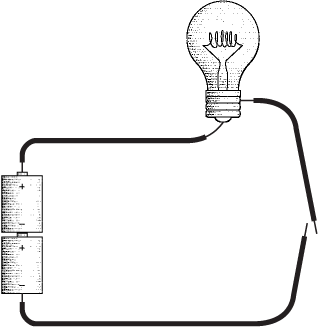
The flashlight is certainly one of the simpler electrical appliances found in most homes. Disassemble a typical flashlight, and you'll find it consists of a couple of batteries, a bulb, a switch, some metal pieces, and a plastic case to hold everything together.
You can make your own no-frills flashlight by disposing of everything except the batteries and the lightbulb. You'll also need some short pieces of insulated wire (with the insulation stripped from the ends) and enough hands to hold everything together.
Notice the two loose ends of the wires at the right of the diagram. That's our switch. Assuming that the batteries are good and the bulb isn't burned out, touching these loose ends together will turn on the light.
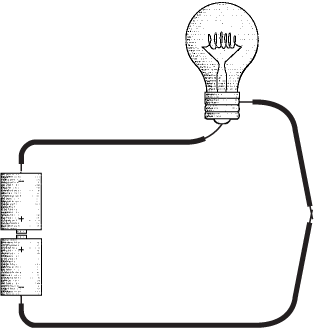
What we've constructed here is a simple electrical circuit, and the first thing to notice is that a circuit is a circle. The lightbulb will be lit only if the path from the batteries to the wire to the bulb to the switch and back to the batteries is continuous. Any break in this circuit will cause the bulb to go out. The purpose of the switch is to control this process.
The circular nature of the electrical circuit suggests that something is moving around the circuit, perhaps like water flowing through pipes. The "water and pipes" analogy is quite common in explanations of how electricity works, but eventually it breaks down, as all analogies must. Electricity is like nothing else in this universe, and we must confront it on its own terms.
The prevailing scientific wisdom regarding the workings of electricity is called the electron theory, which says that electricity derives from the movement of electrons.
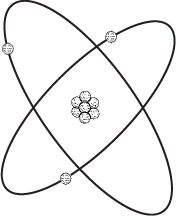
As we know, all matter—the stuff that we can see and feel (usually)—is made up of extremely small things called atoms. Every atom is composed of three types of particles; these are called neutrons, protons, and electrons. You can picture an atom as a little solar system, with the neutrons and protons bound into a nucleus and the electrons spinning around the nucleus like planets around a sun:
I should mention that this isn't exactly what you'd see if you were able to get a microscope powerful enough to see actual atoms, but it works as a convenient model.
The atom shown on the preceding page has 3 electrons, 3 protons, and 4 neutrons, which means that it's an atom of lithium. Lithium is one of 112 known elements, each of which has a particular atomic number ranging from 1 to 112. The atomic number of an element indicates the number of protons in the nucleus of each of the element's atoms and also (usually) the number of electrons in each atom. The atomic number of lithium is 3.
Atoms can chemically combine with other atoms to form molecules. Molecules usually have very different properties from the atoms they comprise. For example, water is composed of molecules that consist of two atoms of hydrogen and one atom of oxygen (hence, H2O). Obviously water is appreciably different from either hydrogen or oxygen. Likewise, the molecules of table salt consist of an atom of sodium and an atom of chlorine, neither of which would be particularly appetizing on French fries.
Hydrogen, oxygen, sodium, and chlorine are all elements. Water and salt are called compounds. Salt water, however, is a mixture rather than a compound because the water and the salt maintain their own properties.
The number of electrons in an atom is usually the same as the number of protons. But in certain circumstances, electrons can be dislodged from atoms. That's how electricity happens.
The words electron and electricity both derive from the ancient Greek word ηλεκτρον (elektron), which you might expect means something like "little tiny invisible thing." But no—ηλεκτρον is actually the Greek word for "amber," which is the glasslike hardened sap of trees. The reason for this unlikely derivation is that the ancient Greeks experimented with rubbing amber with wool, which produces something we now call static electricity. Rubbing wool on amber causes the wool to pick up electrons from the amber. The wool winds up with more electrons than protons, and the amber ends up with fewer electrons than protons. In more modern experiments, carpeting picks up electrons from the soles of our shoes.
Protons and electrons have a characteristic called charge. Protons are said to have a positive (+) charge and electrons are said to have a negative (–) charge. Neutrons are neutral and have no charge. But even though we use plus and minus signs to denote protons and electrons, the symbols don't really mean plus and minus in the arithmetical sense or that protons have something that electrons don't. The use of these symbols just means that protons and electrons are opposite in some way. This opposite characteristic manifests itself in how protons and electrons relate to each other.
Protons and electrons are happiest and most stable when they exist together in equal numbers. An imbalance of protons and electrons will attempt to correct itself. When the carpet picks up electrons from your shoes, eventually everything gets evened out when you touch something and feel a spark. That spark of static electricity is the movement of electrons by a rather circuitous route from the carpet through your body back to your shoes.
Another way to describe the relationship between protons and electrons is to note that opposite charges attract and like charges repel. But this isn't what we might assume by looking at the diagram of the atom. It looks like the protons huddled together in the nucleus are attracting each other. The protons are held together by something stronger than the repulsion of like charges, and that something is called the strong force. Messing around with the strong force involves splitting the nucleus, which produces nuclear energy. In this chapter, we're merely fooling around with the electrons to get electricity.
Static electricity isn't limited to the little sparks produced by fingers touching doorknobs. During storms, the bottoms of clouds accumulate electrons while the tops of clouds lose electrons; eventually, the imbalance is evened out with a stroke of lightning. Lightning is a lot of electrons moving very quickly from one spot to another.
The electricity in the flashlight circuit is obviously much better mannered than a spark or a lightning bolt. The light burns steadily and continuously because the electrons aren't just jumping from one place to another. As one atom in the circuit loses an electron to another atom nearby, it grabs another electron from an adjacent atom, which grabs an electron from another adjacent atom, and so on. The electricity in the circuit is the passage of electrons from atom to atom.
This doesn't happen all by itself. We can't just wire up any old bunch of stuff and expect some electricity to happen. We need something to precipitate the movement of electrons around the circuit. Looking back at our diagram of the no-frills flashlight, we can safely assume that the thing that begins the movement of electricity is not the wires and not the lightbulb, so it's probably the batteries.
Almost everybody knows a few things about the types of batteries used in flashlights:
They're tubular in shape and come in different sizes, such as D, C, A, AA, and AAA.
Regardless of the battery's size, they're all labeled "1.5 volts."
One end of the battery is flat and is labeled with a minus sign (–); the other end has a little protrusion and is labeled with a plus sign (+).
If you want your appliance to work right, it's a good idea to install the batteries correctly with the plus signs facing the right way.
Batteries wear out eventually. Sometimes they can be recharged, sometimes not.
And finally, we suspect that in some weird way, batteries produce electricity.
In all batteries, chemical reactions take place, which means that some molecules break down into other molecules, or molecules combine to form new molecules. The chemicals in batteries are chosen so that the reactions between them generate spare electrons on the side of the battery marked with a minus sign (called the negative terminal, or anode) and demand extra electrons on the other side of the battery (the positive terminal, or cathode). In this way, chemical energy is converted to electrical energy.
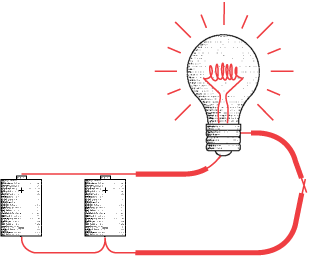
The chemical reaction can't proceed unless there's some way that the extra electrons can be taken away from the negative terminal of the battery and delivered back to the positive terminal. So if the battery isn't connected to anything, nothing much happens. (Actually the chemical reactions still take place, but very slowly.) The reactions take place only if an electrical circuit is present to take electrons away from the negative side and supply electrons to the positive side. The electrons travel around this circuit in a counterclockwise direction:
In this book, the color red is used to indicate that electricity is flowing through the wires.
Electrons from the chemicals in the batteries might not so freely mingle with the electrons in the copper wires if not for a simple fact: All electrons, wherever they're found, are identical. There's nothing that distinguishes a copper electron from any other electron.
Notice that both batteries are facing the same direction. The positive end of the bottom battery takes electrons from the negative end of the top battery. It's as if the two batteries have been combined into one bigger battery with a positive terminal at one end and a negative terminal at the other end. The combined battery is 3 volts rather than 1.5 volts.
If we turn one of the batteries upside down, the circuit won't work:
The two positive ends of the battery need electrons for the chemical reactions, but there's no way electrons can get to them because they're attached to each other. If the two positive ends of the battery are connected, the two negative ends should be also:
This works. The batteries are said to be connected in parallel rather than in series as shown earlier. The combined voltage is 1.5 volts, which is the same as the voltage of each of the batteries. The light will probably still glow, but not as brightly as with two batteries in series. But the batteries will last twice as long.
We normally like to think of a battery as providing electricity to a circuit. But we've seen that we can also think of a circuit as providing a way for a battery's chemical reactions to take place. The circuit takes electrons away from the negative end of the battery and delivers them to the positive end of the battery. The reactions in the battery proceed until all the chemicals are exhausted, at which time you throw away the battery or recharge it.
From the negative end of the battery to the positive end of the battery, the electrons flow through the wires and the lightbulb. But why do we need the wires? Can't the electricity just flow through the air? Well, yes and no. Yes, electricity can flow through air (particularly wet air), or else we wouldn't see lightning. But electricity doesn't flow through air very readily.
Some substances are significantly better than others for carrying electricity. The ability of an element to carry electricity is related to its subatomic structure. Electrons orbit the nucleus in various levels, called shells. An atom that has just one electron in its outer shell can readily give up that electron, which is what's necessary to carry electricity. These substances are conducive to carrying electricity and thus are said to be conductors. The best conductors are copper, silver, and gold. It's no coincidence that these three elements are found in the same column of the periodic table. Copper is the most common substance for making wires.
The opposite of conductance is resistance. Some substances are more resistant to the passage of electricity than others, and these are known as resistors. If a substance has a very high resistance—meaning that it doesn't conduct electricity much at all—it's known as an insulator. Rubber and plastic are good insulators, which is why these substances are often used to coat wires. Cloth and wood are also good insulators as is dry air. Just about anything will conduct electricity, however, if the voltage is high enough.
Copper has a very low resistance, but it still has some resistance. The longer a wire, the higher the resistance it has. If you tried wiring a flashlight with wires that were miles long, the resistance in the wires would be so high that the flashlight wouldn't work.
The thicker a wire, the lower the resistance it has. This may be somewhat counterintuitive. You might imagine that a thick wire requires much more electricity to "fill it up." But actually the thickness of the wire makes available many more electrons to move through the wire.
I've mentioned voltage but haven't defined it. What does it mean when a battery has 1.5 volts? Actually, voltage—named after Count Alessandro Volta (1745–1827), who invented the first battery in 1800—is one of the more difficult concepts of elementary electricity. Voltage refers to a potential for doing work. Voltage exists whether or not something is hooked up to a battery.
Consider a brick. Sitting on the floor, the brick has very little potential. Held in your hand four feet above the floor, the brick has more potential. All you need do to realize this potential is drop the brick. Held in your hand at the top of a tall building, the brick has much more potential. In all three cases, you're holding the brick and it's not doing anything, but the potential is different.
A much easier concept in electricity is the notion of current. Current is related to the number of electrons actually zipping around the circuit. Current is measured in amperes, named after André Marie Ampère (1775–1836), but everybody calls them amps, as in "a 10-amp fuse." To get one amp of current, you need 6,240,000,000,000,000,000 electrons flowing past a particular point per second.
The water-and-pipes analogy helps out here: Current is similar to the amount of water flowing through a pipe. Voltage is similar to the water pressure. Resistance is similar to the width of a pipe—the smaller the pipe, the larger the resistance. So the more water pressure you have, the more water that flows through the pipe. The smaller the pipe, the less water that flows through it. The amount of water flowing through a pipe (the current) is directly proportional to the water pressure (the voltage) and inversely proportional to the skinniness of the pipe (the resistance).
In electricity, you can calculate how much current is flowing through a circuit if you know the voltage and the resistance. Resistance—the tendency of a substance to impede the flow of electrons—is measured in ohms, named after Georg Simon Ohm (1789–1854), who also proposed the famous Ohm's Law. The law states
| I = E / R |
where I is traditionally used to represent current in amperes, E is used to represent voltage (it stands for electromotive force), and R is resistance.
For example, let's look at a battery that's just sitting around not connected to anything:
The voltage E is 1.5. That's a potential for doing work. But because the positive and negative terminals are connected solely by air, the resistance (the symbol R) is very, very, very high, which means the current (I) equals 1.5 volts divided by a large number. This means that the current is just about zero.
Now let's connect the positive and negative terminals with a short piece of copper wire (and from here on, the insulation on the wires won't be shown):
This is known as a short circuit. The voltage is still 1.5, but the resistance is now very, very low. The current is 1.5 volts divided by a very small number. This means that the current will be very, very high. Lots and lots of electrons will be flowing through the wire. In reality, the actual current will be limited by the physical size of the battery. The battery will probably not be able to deliver such a high current, and the voltage will drop below 1.5 volts. If the battery is big enough, the wire will get hot because the electrical energy is being converted to heat. If the wire gets very hot, it will actually glow and might even melt.
Most circuits are somewhere between these two extremes. We can symbolize them like so:
The squiggly line is recognizable to electrical engineers as the symbol for a resistor. Here it means that the circuit has a resistance that is neither very low nor very high.
If a wire has a low resistance, it can get hot and start to glow. This is how an incandescent lightbulb works. The lightbulb is commonly credited to America's most famous inventor, Thomas Alva Edison (1847–1931), but the concepts were well known at the time he patented the lightbulb (1879) and many other inventors also worked on the problem.
Inside a lightbulb is a thin wire called a filament, which is commonly made of tungsten. One end of the filament is connected to the tip at the bottom of the base; the other end of the filament is connected to the side of the metal base, separated from the tip by an insulator. The resistance of the wire causes it to heat up. In open air, the tungsten would get hot enough to burn, but in the vacuum of the lightbulb, the tungsten glows and gives off light.
Most common flashlights have two batteries connected in series. The total voltage is 3.0 volts. A lightbulb of the type commonly used in a flashlight has a resistance of about 4 ohms. Thus, the current is 3 volts divided by 4 ohms, or 0.75 ampere, which can also be expressed as 750 milliamperes. This means that 4,680,000,000,000,000,000 electrons are flowing through the lightbulb every second.
(A brief reality check: If you actually try to measure the resistance of a flashlight lightbulb with an ohmmeter, you'll get a reading much lower than 4 ohms. The resistance of tungsten is dependent upon its temperature, and the resistance gets higher as the bulb heats up.)
As you may know, lightbulbs you buy for your home are labeled with a certain wattage. The watt is named after James Watt (1736–1819), who is best known for his work on the steam engine. The watt is a measurement of power (P) and can be calculated as
| P = E x I |
The 3 volts and 0.75 amp of our flashlight indicate that we're dealing with a 2.25-watt lightbulb.
Your home might be lit by 100-watt lightbulbs. These are designed for the 120 volts of your home. Thus, the current that flows through them is equal to 100 watts divided by 120 volts, or about 0.83 ampere. Hence, the resistance of a 100-watt lightbulb is 120 volts divided by 0.83 ampere, or 144 ohms.
So we've seemingly analyzed everything about the flashlight—the batteries, the wires, and the lightbulb. But we've forgotten the most important part!
Yes, the switch. The switch controls whether electricity is flowing in the circuit or not. When a switch allows electricity to flow, it is said to be on, or closed. An off, or open, switch doesn't allow electricity to flow. (The way we use the words closed and open for switches is opposite to the way we use them for a door. A closed door prevents anything from passing through it; a closed switch allows electricity to flow.)
Either the switched is closed or it's open. Either current flows or it doesn't. Either the lightbulb lights up or it doesn't. Like the binary codes invented by Morse and Braille, this simple flashlight is either on or off. There's no in-between. This similarity between binary codes and simple electrical circuits is going to prove very useful in the chapters ahead.