Chapter 5
Precision Marketing
Introduction
From consumer trends to regulatory scrutiny, payer power, and portfolios, many factors are leading to a new landscape for biopharma marketing strategy. Drug spending has gained top visibility, triggered in part by Gilead's launches of Sovaldi (sofosbuvir) and Harvoni (ledipasvir/sofosbuvir) and what was perceived as their high price in the large hepatitis C market, and by increased government scrutiny of pricing practices. Aging demographics across countries contribute to pressures on health budgets. Total spending on prescription drugs in the United States rose nearly 6 percent to almost $450 billion in 2016, despite generic utilization rates of nearly 90 percent. Average copays for patients covered by commercial insurers have also grown by more than 25 percent since 2010 [1].
Despite the negotiating power of government payers outside of the United States, the trend is global. According to Quintiles IMS, worldwide medicine spending is forecast to reach nearly $1.5 trillion by 2021, with a compound annual rate of growth declining from recent years but still reaching a 4 percent to 7 percent rate over the next 5 years. In that period, a key spending driver will be oncology with a compound annual growth rate of 9 percent to 12 percent, largely similar to the last 5 years [2]. Consumer pricing concerns have become top issues. According to an online Harris Poll of 2,255 US adults, 69 percent said they would choose a generic more often, if given the choice, and 30 percent said they would always choose a generic. This trend is sharper for older age groups; while 62 percent of Millennials (born 1981 to 1997) preferred generics, 73 percent of Boomers (born 1946 to 1964) and as many as 78 percent of those 70 and older showed that preference [3].
As company portfolios are increasingly dominated by high-priced specialty drugs and biologics, the need is clear for precision marketing (as a counterpart to precision medicine) and specifically for an evidence-based strategy, whereby product positionings are supported by hard clinical and economic data.
This chapter first covers a redefinition of branding models, balancing evidence and experience, targeting new segments such as genotypes, evolving from pills to integrated solutions, and developing dual-targeted and broad branding models. It next analyzes prelaunch and launch strategies, as well as multichannel outreach. Finally, it assesses sustainability strategies beyond the traditional lifecycle, through portfolio management, multiple indications, and successor molecules (Figure 5-1).

Figure 5-1 Precision marketing strategy
Portfolio Shift to Specialty Products
According to a 2016 IMS Health report, spending on specialty drugs doubled in the preceding five years and now accounts for 36 percent of nondiscounted drug spending in the United States, driven by treatments for oncology, hepatitis, and autoimmune diseases. US specialty spending accounted for 42.6 percent of net spending in 2016 [4].
In addition to rising investments in specialty therapies, a key factor has been price inflation. In 2013, more than 100 experts published an article in Blood stressing rapid cost increases, particularly in areas such as oncology and blood disorders such as chronic myeloid leukemia [5]. In response, new metrics for comparative clinical value are being developed, such as the American Society for Clinical Oncology's Value Framework and Memorial Sloan-Kettering's Drug Abacus tool [6].
Orphan diseases show especially sharp growth; their small populations drive the need to recoup R & D costs with high prices, and many have become blockbusters, such as Celgene's Revlimid (lenalidomide) in multiple myeloma, with 2016 global sales of nearly $7.0 billion.
As many as 30 million US patients suffer from a rare disease, and this may be understated, as the average patient visits more than seven physicians during almost five years before receiving an accurate diagnosis. The attention to these drug price tags and regulations has increased, in keeping with that growth. The incentives of the Orphan Drug Act, including marketing exclusivity and tax credits, are now under scrutiny.
Orphan drugs now appear on the payer radar, after being lightly treated because their small populations did not impact strongly the total budget costs [7].
These trends support the need for an evidence-based strategy and also close communication with patient groups. The fact that they are globally linked and acutely aware of new therapies may prove to be a double-edged sword: They can provide access to registries and speed up trial recruitment, but they can also harbor overly high expectations, overlook possible side effects, and exert pressure to include patients who do not fit the trial protocol. In response, some companies have opted for open-label studies, while managing expectations and stating eligibility criteria through multiple channels (advocacy groups, conferences, congresses, and social media). Among others, Vertex communicated well that Kalydeco (ivacaftor) should be used only in the very small segment of cystic fibrosis patients with a specific gene mutation [8].
Balancing Evidence and Experience
While consumer research has shown the importance of understanding the patient journey, a challenge for biopharmas is to balance evidence and experience. Several trends are driving a shift to evidence:
- Regulators worldwide have less tolerance for “me-too” products; France has set up a ranking system based on the innovativeness of new products.
- Payers in most of Europe mandate pharmacoeconomic dossiers, and US groups are developing methods of comparative effectiveness. In July 2015, the nonprofit Institute for Clinical and Economic Review (ICER) launched a program to evaluate new drugs, and it also aims to develop a value-based price benchmark anchored to real benefits for patients [9].
- Physicians have been sensitized to drug recalls, ever since Merck's highly visible recall of its arthritis drug Vioxx (rofecoxib) in 2004, and demand hard clinical data.
- Consumers have online access to scientific data, from search engines to journals.
Given the wide scope of current products, experience-based marketing will continue to coexist with evidence-based approaches. In critical areas such as oncology, evidence will be dominant. In noncritical areas such as allergy, experience will apply, but well-informed consumers will expect it to be supported by evidence as a differentiator, given brand proliferation.
Experience also partly applies in regulator and payer decisions, as randomized trials are complemented by patient-reported outcomes and observational studies. For physicians and patients, delivery mode, dosing convenience and quality of life also play a role in treatment adherence (Figure 5-2).

Figure 5-2 Evidence and experience drivers
A well-known example of the failure to consider stakeholder needs is Pfizer's inhaled insulin, Exubera. For payers, the launch price was perceived as noncompetitive with other insulins. For physicians, there was a significant concern about the long-term impact of insulin on the lungs. For patients, barriers included the requirement of a lung function test before the start of therapy as well as a large and inconvenient device. While Pfizer had bought worldwide rights from Sanofi-Aventis for $1.3 billion, it announced its withdrawal in October 2007, after global sales of only $12 million for that year [10]. MannKind recently introduced its Afrezza inhaler, with a smaller size and breath-activated system increasing reliability, but concerns remain about the systemic impact and acceptance of this delivery method [11].
R & D and Commercial Coordination
A key success factor in this context is the close pairing, early in development, of research and commercial teams. Researchers may not have a clear view of patient needs. For instance, a monthly instead of a daily regimen was used in osteoporosis, to minimize gastric side effects from bisphosphonates such as Roche's Boniva (ibandronate), but a monthly regimen may be a hindrance for older people, who may have difficulty remembering the dosing cycle.
New digital ethnography tools such as video diaries may help uncover drug value in real-life use, but gathering this evidence may face a roadblock with company silos. In particular, target product profiles (TPPs) may be technical documents that do not describe the actual value delivered. In collaboration between scientific and commercial teams, a TPP should include a range of questions:
- Patients: Who are the targets and what are their journeys, including pretreatment information and medical, financial, and lifestyle challenges? Which outcomes are most meaningful to them (such as mobility versus brain lesions in multiple sclerosis)?
- Physicians: What are key decision criteria across specialties? What outcomes are most relevant, and what are treatment challenges, including lack of adherence?
- Regulators: How will the new therapy compare against the standard of care? If it is first in class, what will be the comparator? What is the relative level of innovation and medical benefit?
- Payers: How will the new product impact budget costs? Is there less price sensitivity for smaller populations? Will there be cost-effectiveness studies?
For companies to address all stakeholders, a key factor is to adopt an “outside-in” approach, starting with unmet needs and customer experiences.
An example of a close R & D/commercial coordination is Celgene's focus on science as a key driver of growth and successful product development.
In addition to this focus on science, an understanding of patients' experience is needed to ensure that products and services meet their needs.
Value of Experience: The Consumer Decision Journey
Consumer connections to brands have been researched across industries. While no biopharma brand can match the emotional power of Disney or Apple, a connection pathway may apply in healthcare, as consumers transition from being unconnected to highly satisfied, perceiving brand differentiation and being fully connected. Steps to assess and leverage this emotional connection score include:
- Gathering market research and customer insight data from owned media (websites) to earned media (general platforms and online patient communities)
- Analyzing the best customers—those with the most brand loyalty and advocacy power, such as bloggers and patient community leaders
- Ensuring buy-in from senior leadership, not just brand teams [12].
In addition, a new approach is emerging to address the consumer journey. Instead of the traditional funnel metaphor (awareness, consideration, purchase), starting with many brands and narrowing them down to a final choice, a new journey may progress in four stages—consider, evaluate, buy, and enjoy/advocate/bond:
- The consider stage includes a top-of-mind brand set, from exposure to ads, word-of-mouth or professional recommendations.
- In the evaluate stage, consumers seek input from peers, online reviewers, and competitor brand communications.
- In the buy stage, point-of-purchase factors such as pharmacist input (e.g., switching to a generic) also play a role.
- In the enjoy/advocate/bond stage, consumers continue online research; in case of a strong bond, this phase may skip earlier stages and lead to long-term loyalty.
In this context, marketers have three major roles:
- Orchestrator of communications across functions (product development, marketing, customer service, sales, and IT) and channels.
- Publisher and content manager across business units. For instance, Apple has aligned product descriptions and created a library of demonstration videos.
- Market intelligence leader, as market and competitive data are often collected by different functions and business units [13].
In healthcare, the decision flow is especially complex, including “opt-out” steps:
- Pre-diagnosis information—as some symptoms may emerge
- Search for professional help—this may be standard or alternative medicine
- Medical diagnosis—seek to understand treatment or opt for alternative course
- Prescription filling—from benefits versus side effects, continue or abandon treatment
- Condition changes—new decision cycle if no stabilization or cure is achieved.
Companies often do not engage patients at the earliest stage. A Google or YouTube search may include in its top results external sources rather than company information. Similarly, the time a prescription is filled is only one of many touch points, and others may be more influential, such as the pain of a first biologic injection or the difficulty in using an asthma inhaler, which may block adherence.
Consumer insights, in early stages, may come from search engine trends and patient communities and later from pharmacy claims and electronic medical records [14].
Marketing Beyond the Pill
This type of precision marketing through the decision journey changes the biopharma model from detection and treatment to prediction and prevention.
At the prediction stage, genomic databases and tools such as Watson Health allow the early identification of gene mutations and support the joint development of drugs and companion diagnostics (CDx). When Genentech pioneered this model in 1998 with Herceptin (trastuzumab), designed to block HER2 overexpression in metastatic breast cancer, it partnered with Dako to produce the CDx HercepTest.
This requires the coordination of two different processes. Discovery timelines, approval criteria, channels, customers, and margins are different for diagnostics and therapeutics. Dual salesforces for labs and physicians must be coordinated, and consumer and physician education is needed to communicate the importance of CDx. Postlaunch, monitoring is necessary through biosensor tracking and biomarkers, to support long-term outcomes and Phase IV studies and publications [15]. This dual process is illustrated in Figure 5-3.

Figure 5-3 Marketing beyond the pill
Targeting New Consumer Segments
In precision marketing, the first segmentation base is the genotype, followed by disease state (early to late stage). For biologics, beyond standard demographics such as age and income, location matters. Rural patients far from infusion centers may need to opt for subcutaneous injections, if they have a choice. Ethnicity and gender also matter, as trials increasingly include gender comparisons and ethnic subtypes.
Consumer attitudes are also key, differentiating between a vocal subgroup of activists versus more passive individuals, and mainstream versus alternative medicine–oriented patients. In the HIV area, activists were instrumental in accelerating approvals of antivirals and accessing them prelaunch through compassionate use programs [16]. Technographics are now also an influential segmentation base.
Especially in rare diseases and critical areas, Internet leaders exert a powerful diffusion or criticism role, and can even trigger an observational trial, as was the case for a lithium study in ALS (amyotrophic lateral sclerosis) by members of PatientsLikeMe. The study disproved lithium's effectiveness, and this was later confirmed by a randomized trial.
New Physician Segments
These segmentation bases have counterparts among physicians. In rare diseases or those requiring a double specialty, such as hematology/oncology in leukemia, there may be as few as 5,000 relevant specialists worldwide. A prelaunch success factor is the capture of key opinion leaders (KOLs) as trial investigators.
Practice type is also key; many group practices ban sales visits but may remain open to on-demand virtual details or medical science liaisons (MSLs).
Attitudinal segments also matter, as companies must identify early adopters and circumvent the concerns of conservative or cost-conscious physicians.
Technographics follow the same pattern for physicians as for consumers, ranging from web opinion leaders (bloggers or frequent webinar speakers) to passive information seekers and users of medical communities such as Sermo or Doximity. These segmentation bases are shown in Figure 5-4.

Figure 5-4 Segmentation bases
Leveraging these bases entails specific planning steps:
- For targeted therapies, investing in predictive tools and early diagnosis of genotypes
- Working with both physician and patient KOLs for medical and observational studies
- Developing prelaunch awareness of genomic screening and CDx
- During and post-treatment, monitoring behavior, adherence, and outcomes via biosensors and biomarkers to support Phase IV studies with real-world evidence
Dual Branding Models
These evolving segmentation bases are leading to the coexistence of two different branding models for targeted versus mass-marketed products. While many biotechs focus on targeted drugs, this poses a challenge for large pharmas with broad portfolios, such as Sanofi, with insulins addressing more than 350 million diabetics worldwide, versus its Genzyme drugs such as Myozyme (alglucosidase alfa) for Pompe disease, with as few as 5,000 to 10,000 patients worldwide. While their populations are limited, targeted therapies may garner top revenues due to high prices. Roche's Rituxan (rituximab) and Herceptin (trastuzumab) reached, respectively, $8.6 billion and $6.7 billion in 2016 global sales [17].
The superior return on investment for these drugs is supported by several components of their business model: specialists requiring very small salesforces and more reliance on MSLs and patient counselors, no or little use of mass media, and strong links with patient communities supporting product diffusion. Rapid global expansion is also achieved thanks to tightly linked specialist communities and standardized treatment protocols. By contrast, a mass market product such as Crestor (rosuvastatin) faces challenges such as salesforce costs, multiple specialties, and regional treatment variance.
For osteoporosis drugs such as Actonel (risedronate), medical targets may include generalists, endocrinologists, or orthopedists (post-fracture stage), and treatments may vary by country, from biologics such as Amgen's Prolia (denosumab) to bisphosphonates, calcitonin, hormones, or calcium (Figure 5-5).

Figure 5-5 Scope of branding models
Despite these differences, a success factor for large pharmas is knowledge transfer across these models, in particular, the application of evidence-based marketing to mass-market products.
New Launch Strategies
From early development, launch success relies on a cocreation process, with physician and patient KOL input in R & D and product formulation. For instance, Novo Nordisk has maintained its position against larger competitors such as Sanofi and Lilly with its focus on diabetes and patient needs. Early on, it understood that the differentiator was not insulin, perceived as a quasi-commodity, but the delivery mode; it developed the first easy-to-use autoinjector and branded it as NovoPen.
The launch model itself is evolving. The traditional formula was to be first-in-class with a novel mechanism of action that triggered rapid prescribing from early adopters. This was the case for Gilead's Sovaldi (sofosbuvir), launched as a breakthrough in hepatitis C. While it took months for payers to set up coverage policies in the United States, physicians prescribed it rapidly, and it reached peak worldwide sales of nearly $5.2 billion by 2015. Its successor, Harvoni (ledipasvir/sofosbuvir), then reached peak global sales of almost $13.9 billion by 2015. Both products' sales decreased in 2016, to $4 billion for Sovaldi and $9 billion for Harvoni, as a competitor entered the market, providing more leverage to payers [19].
Once payers started to access restrictions, physicians and patients filed coverage reviews, appeals, and exceptions, and there was a court case for a patient denied Harvoni due to not being symptomatic enough. In future cases, payers may draft some restrictive policies before launch, such as label-only coverage, step therapy, and prior authorizations.
A new standard for market success may then be “best value in class,” as defined by comparisons of efficacy, safety, economics, and ease of use. This may allow a dominant position as a category definer such as Xerox or FedEx once were, and is a strong protection against competitive entrants. Physicians are unwilling to switch stabilized patients, and switching to cheaper products may also go against the incumbent's rebates to payers [20].
Companion Diagnostics
In addition to these trade-offs between “first-in-class” and “best-value-in-class,” another controversy has emerged about the relative potential of genotype-targeted drugs with or without companion diagnostics.
An example of an oncology drug with a CDx is Pfizer's Xalkori (crizotinib) in 2011. Its challenge, ensuring that drug marketing was directly linked to the diagnostic, remains an issue today. Xalkori addressed a subset of patients with non-small cell lung cancer (NSCLC) with a defect in the ALK gene (anaplastic lymphoma kinase). Pfizer had to coordinate its messaging to multiple stakeholders—from pulmonologists to interventional radiologists, pathologists, and nurses—all of whom had to be convinced to adopt a new hospital treatment system. For instance, this entailed taking more biopsy tissue than normal to ensure patient prequalification.
Abbott Molecular, maker of the Vysis ALK probe test, also aimed to present the drug and test as a single package. This included joint sales calls with Pfizer to “double-team” doctors, as well as rigorous training to ensure science-based detailing.
For patient education, Pfizer also supported the LungCancerProfiles.com website, including patient profiles and interactive tools for questions during physician visits.
While these efforts helped communicate the need for molecular testing, the test may have remained a partial barrier, as Xalkori sales reached only $546 million by 2015 [21].
The controversy has now extended to the new category of checkpoint inhibitor therapies in immuno-oncology.
In order for a development model that includes rapid multiple indications to succeed, launch plans must include early-stage milestones and accountabilities. While clinical and commercial teams have traditionally been evaluated with separate criteria, a new scorecard needs to assess their integration, and their incentives need to be aligned in order to gather real-world evidence. There should be coordination to shape the market, company, and product. The product benefits most from early-stage multiple indications and formulations. The market includes physicians, patients, and payers. For physicians, KOL capture as investigators is essential. For consumers, KOLS are equally important as co-creators and can be drawn from advocacy groups. For payers, reimbursement depends on pharmacoeconomics dossiers [24]. These launch success factors are summarized in Figure 5-6.

Figure 5-6 Launch success drivers
Global Organization
Finally, a key success factor is a well-coordinated global launch, given the need to recover rapidly development costs. The following should be kept in mind:
- As early as in the preclinical stage, a global advisory panel can provide input about unmet needs, including in emerging markets. For instance, China has the world's largest hepatitis C market, but only a small part of its population has reimbursement. The same pattern applies to India across many diseases.
- At later stages, regional KOLs may co-lead and communicate trials.
- Throughout development, close links with global patient communities can speed recruitment, set up multi-country registries and gather evidence worldwide.
A primary challenge remains the trade-off between central efficiency and local responsiveness, and between economies of scale and market focus. Although biologics benefit from pan-European Union registration, biosimilar policies differ by country. Pricing is vastly different, with reference pricing in Europe and elsewhere.
Companies must therefore balance global branding consistency and local adaptation. For instance, Pfizer and AstraZeneca both positioned Lipitor and Crestor worldwide on their potency. However, flexibility is needed to respect pricing and channel variance (the same products sold as prescription or over-the-counter), brand name, and delivery mode variations (e.g., effervescent vitamins versus pills) 25. These drivers and barriers are shown in Figure 5-7.
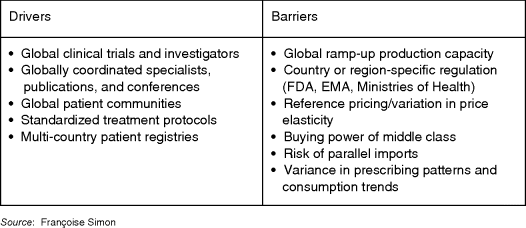
Figure 5-7 Global drivers and barriers
Multichannel Communications
Communication strategies are moving from mass media to a multichannel ecosystem, but this expansion is questioned, given the anti-pharma trend among consumers. This is exacerbated by the massive scale and visibility of direct-to-consumer (DTC) promotion in the United States (outside of the United States, branded DTC is only allowed in New Zealand; it was rejected in Europe, where only unbranded DTC is allowed). Total DTC spend in the United States reached $5.8 billion in 2016, with Pfizer, Bristol-Myers Squibb, AbbVie, and Lilly as the top spenders; traditional media led spending, with more than $4 billion going to television versus only $515 million for digital promotion.
Despite the controversy surrounding its pricing policy in hepatitis C, Gilead still spent nearly $102 million on Harvoni, which may risk adding fuel to public protests. While in past years, targeted products such as Novartis' Gleevec were not advertised in the mass media, Bristol-Myers Squibb spent more than $170 million on its campaign for Opdivo [26].
This trend runs counter to a sharp outcry from many stakeholders. Consumers are avoiding print ads, cutting cable television subscriptions, and skipping video ads, and some 10 percent of US desktop Internet users already have ad blockers installed, with a growing trend in mobile. Across sectors, almost half of the 24,000 consumers surveyed in 2015 by GfK/MRI agreed that “much of advertising is too annoying.” In a 2016 Accenture survey of 28 countries, 84 percent of respondents said that digital ads are too frequent. Millennials are leading the exodus; they are spending nearly 30 percent less time per week watching television than they were in 2012, according to Nielsen, and the drop is 18 percent for the 24 to 35 age group [27].
For the first time, physicians are officially protesting DTC promotion. On November 17, 2015, the American Medical Association (AMA) called for a ban on DTC advertising of prescription drugs and medical devices out of concern that “a growing proliferation of ads is driving demand for expensive treatments despite the clinical effectiveness of less costly alternatives” [28]. A salient point was the first explicit link between ad spend and drug price inflation.
The government followed suit, with the introduction of a bill aiming to limit DTC for three years after approval. Another bill was introduced with the objective of ending a tax deduction allowing biopharma companies to write off their DTC expenditures [29]. While these bills may not be finalized, the sheer scope of these protests across stakeholder groups may be a sufficient reason for biopharma companies to rethink their communication mix and resource allocation across all channels, with more focus on a product-neutral outreach that is globally acceptable and welcomed from a public health standpoint, such as awareness campaigns for vaccinations or underdiagnosed diseases.
In addition to this stakeholder resistance, multichannel strategies are meeting internal challenges. Because some companies still tend to be product-centric, a multichannel mindset faces barriers such as brand silos (each brand team is not incentivized to contribute to other brands) and a lack of information through a patient's journey, including the pre-diagnosis and post-treatment stages. What is needed is a balance between unbranded disease-oriented programs and brand-specific promotion [30].
Brand messages themselves should be tailored, not only to consumer segments, but also to the different perceived value of a brand by stakeholders. The rise of accountable care organizations (ACOs) has increased the importance of population-level benefits. While a community health center may value a good tolerability profile that supports patient adherence, a teaching institution may focus on an innovative mode of action because of its scientific value. To coordinate a multichannel outreach, marketing may be seen as a matrixed system of functions (innovation, strategic planning, positioning, and marketing mix) and talent factors (marketing as well as salesforce training) [31].
This also applies to new ways of leveraging mobile media, such as location-specific functionality, already used in consumer health. For its 2015 Neutrogena sunscreen campaign, Johnson & Johnson sent mobile banner ads when sun exposure and UV levels were high. The ads, targeting consumers close to beaches or pools, appeared amid beauty or fashion content on a range of websites. Nearly 60 percent of those surveyed said they would be more likely to try the sunscreen [32].
The application of these geofilters to biopharma products raises privacy issues, however, especially in the current context of a consumer backlash against digital intrusion. For this type of outreach, it would be prudent for brand teams to coordinate with medical and corporate affairs departments to ensure a stepwise approach with a possible pilot before a large rollout.
Content Marketing
For the best examples of content marketing to engage consumers with something relevant and directly useful, biopharmas would benefit from following the initiatives of healthcare systems such as the Mayo Clinic. In diabetes, Novo Nordisk has a disease focus and offers an online portfolio of personalized tools through its Cornerstones4Care support program. Although it includes diet, activity, and blood sugar tracking information, its “Diabetes 101” page remains fairly basic and lacks links to the literature. In addition, its “Medicines” section is limited to its own products, from NovoLog (insulin aspart) for types 1 and 2, to Victoza (liraglutide) for type 2 diabetes.
By contrast, the Mayo Clinic “Diseases and Conditions” website includes not only lifestyle and diet advice but also an extensive array of product-neutral information, from its Essential Diabetes Book to a DVD and a full list of type 1 and 2 treatments, from metformin and DPP-4 inhibitors like Merck's Januvia (sitagliptin) to GLP-1 receptor agonists like Novo Nordisk's Victoza (liraglutide), together with efficacy and side effects. The site also has a link to conference presentation summaries and other resources [33].
Alone among biopharmas, Merck has built a worldwide reputation among physicians as well as consumers for comprehensive medical information.
First published in 1899, the Merck Manual of Diagnosis and Therapy is the world's best-selling medical textbook, now published online in both professional and consumer versions. The manuals received five eHealthcare Leadership Awards, including a Gold Award for Best Healthcare Content for Professionals, and a Distinction Award for Best Overall Consumer Healthcare Site, at the 2015 Annual Healthcare Internet Conference. Merck has a full portfolio of publications, including a condensed reference guide, the Merck Manual of Patient Symptoms [34].
Given the current DTC controversy, it may be beneficial for biopharmas to shift more resources toward unbranded disease information that reflects patient needs through their journey and establishes them as a more trusted education source.
Salesforce Strategies
The traditional in-person sales model is no longer sufficient for many reasons.
While salesforces have shrunk and been outsourced, biopharmas remain dependent on representatives, often repurposed as key account managers (KAMs) for health system customers. However, a more comprehensive transformation is needed, given provider consolidation, the rising number of younger physicians joining group practices or networks that ban sales reps, and their demand for digital information. According to the 2015 ZS AccessMonitor survey, only 47 percent of US prescribers permit in-person sales details. In oncology, only 25 percent now see representatives [35].
New Roles for Medical Science Liaisons
In pre-launch stages, medical science liaisons (MSLs) can play a key role as a bridge between scientific and commercial teams, and in driving customer centricity. Far from being a secondary salesforce, MSLs can support existing KOLs and build new ones through investigator-initiated studies. They can communicate in-depth KOL profiles and develop engagement plans based on their information needs, which a representative has no time to assess. According to the Medical Science Liaison Society, the average time for a field representative with a physician is roughly two minutes, while it may be up to an hour for an MSL. This is especially relevant for rare diseases and breakthrough therapies with a new mode of action, where greater medical training is required and where the science must be branded well before launch.
Metrics for MSL effectiveness include quantitative variables (amount of face time) and qualitative ones (medical insights, competitive intelligence, and real-world evidence on patient outcomes). An effective MSL strategy must ensure that they are not siloed, and that they are aligned with medical affairs and marketing teams across all customer-facing channels [36].
As for salesforces, they must reach beyond the traditional “3R” model (right message, right frequency, right target) and engage customers, not only across channels, but also with new content. New channels range from KAM structures to patient/physician portals that may include disease information, social media and digital tools for education. New content may include:
- Budget/outcomes models helping ACOs assess the impact of a new therapy on relevant patient populations
- Value-added services such as education on companion diagnostics and facilitation of remote monitoring though biosensors
- Disease management services enabling coordination between patients, caregivers, nurses, and physicians, especially for chronic conditions
- E-detailing (virtual sales consultations), coordinated with other online brand and disease information
- Innovative pricing and contract agreements, in collaboration with payers and providers; ideally, joint analytic studies with them and possibly with patient communities for real-world evidence on outcomes and comparative value [37]
In sum, successful prelaunch and launch strategies entail a close coordination across all functions, as early as possible in product development. At the preclinical stage (at least seven years before launch), a key factor is the understanding of global unmet needs and the identification of patient and physician KOLs to ensure co-creation of a new product. An assessment of payer views, including a definition of comparators of standard of care, is also needed to undertake possible head-to-head studies.
As a product enters the clinic in Phase I, real-world evidence is possible through patient communities. Global KOL input helps provide an early definition of target product profiles (TPPs). By Phase II, trials should be leveraged as the most effective way to brand the science and, if applicable, the new mode of action. For critical areas, approval may be granted in Phase IIb, and Phase III may serve as part of post-launch studies.
At that stage, efficacy and safety should be well validated, and ease of use, delivery mode, and formulation should have been determined with patient input. Forecasts and pharmacoeconomic dossiers should have been finalized, together with a donation/discount program, in consultation with patient advocacy groups.
Finally, multipronged monitoring should be conducted post-launch, watching patient outcomes, physician and consumer reactions, and competitor and payer responses on a worldwide basis. Multiple follow-on trials and related publications should include new indications and product combinations, both on a randomized and observational basis. This process is shown in Figure 5-8.

Figure 5-8 Pre-launch and launch strategies
In addition to these launch success factors, product sustainability over time depends on the early planning of strategies ranging from portfolio diversification to franchise management, through successor molecules and combination therapies.
Sustainability Strategies: Beyond the Life Cycle
The traditional life cycle approach limiting a product to its introduction, growth, maturity, and decline can be optimized from early R & D to the post-patent stage.
In the same way as launches are actually won in early clinical stages, life cycles can be extended through portfolio and franchise management approaches. This is especially relevant given the patent expiries of many biologics. The European Medicines Agency has approved 20 biosimilars, and the first US approval occurred in March 2015 with the launch of Sandoz's Zarxio as the biosimilar version of Amgen's Neupogen (filgrastim) [38].
Portfolio Diversification and Franchise Building
Many companies are struggling with dependence on one flagship product, such as AbbVie's Humira (adalimumab). To counter patent loss, the company is counting on its portfolio of more than 70 patents to protect it until 2022. However, generic companies such as Teva have increased in recent years their launches “at risk,” that is, before the originator's patent expiry.
The first line of defense is clearly portfolio diversification, as Roche has done with its monoclonal antibodies and its oncology franchise. At the portfolio level, a strategy increasingly used is an asset swap, such as that between Novartis and GlaxoSmithKline (GSK), with Novartis selling its vaccine business to GSK in exchange for GSK's oncology business.
At the franchise level, launching new molecules does not preclude the continued promotion of late-stage brands, as MSLs and salesforces can communicate new prescribing developments. Lower-cost digital programs can target brand-loyal physicians and patient communities and can leverage a brand's name recognition among patients who may be averse to switching to other therapies 39. A franchise approach may also be useful as a company launches new dosing regimens and enters new markets. Examples include Pfizer's launch of Viagra (sildenafil) as Revatio, with different dosages for erectile dysfunction and pulmonary arterial hypertension, and Merck's extension of Proscar (finasteride), for enlarged prostate, into Propecia for hair loss.
The need for distinct value propositions also applies in emerging markets with high cost constraints and price sensitivity. While Sanofi launched its antimalaria drug as ASAQ in collaboration with the nonprofit Drugs for Neglected Diseases Initiative, which qualified it for the WHO requirements, it sold it as Coarsucam in private markets at a higher price. Similarly, as Plavix (clopidogrel) faced patent loss in 2012, Sanofi launched in Indonesia a lower-priced branded generic version.
In emerging markets, in addition to affordable pricing, biopharmas must also ensure broad utilization, often through distribution partnerships. AstraZeneca entered an agreement in India aiming to market its platelet aggregation inhibitor Brilinta (ticagrelor) through Sun Pharma with the new brand name Axcer 40.
An example of effective franchise management is Roche's approach for its HER2 breast cancer line. Its succession plan includes new molecules like Kadcyla (ado-trastuzumab emtansine), the antibody drug conjugate version of Herceptin, and Perjeta (pertuzumab), which has helped Roche maintain share in the neoadjuvant breast cancer market in the United States. The new drug positioning has been both evidence based, with a trial of a Herceptin/Perjeta combination for second-line therapy, and experience-based, as some patients have switched to the subcutaneous version [41].
Building the Market: New Indications and Formulations
Key success factors are early development of multiple indications and launch of a new molecule well before the initial brand's patent loss to allow for patient base conversion. This is a high-cost/high-reward approach, with the significant expenditure of new trials, but the benefits include long-term added exclusivity, penetration of new segments, and the potential strengthening of physician loyalty through a “one-stop shop” approach.
New formulations should also be planned early, with the potential benefit of dual patents (such as compound and device patents for a smart insulin pen or an asthma inhaler) and the building of an “umbrella equity.” Combined products are widely used in areas such as HIV and oncology, but they yield a lower return if one product is externally sourced, as profits are shared.
Mature Strategy: Optimized Customer Penetration
As products reach maturity, an effective approach is to assess value leakages along the patient journey and address them with added services to increase adherence. In diabetes, these leakages include lack of coordinated care, suboptimal treatment of comorbidities, reduced quality of life, and the burden of glucose monitoring. Intervention opportunities include personalized counseling on disease management, co-creation of new formulations and delivery modes, diet and exercise coaching and related apps, and virtual consultations with health professionals [44].
Renewal Strategies: From Patent Protection to Branded Generics
Finally, late-life cycle strategies include maximizing patents and changing product status to branded generics or, if applicable, switches to over-the-counter (OTC) status (Figure 5-9). The most defensible patents are product patents applying to new entities. Formulation and process patents are less protective, and method of use patents covering different indications are least effective, because competing products may be prescribed off-label for the same use.

Figure 5-9 Sustainability strategies
As noted earlier, orphan drug status yields a seven-year extension in the United States, but it applies only to rare diseases. A trend across countries is for patent litigation to favor generic companies, as most infringement suits fail, and generic challenges increasingly occur before the originator's patent expiry.
Branded generics have been used for decades by companies such as the Novartis Sandoz division and now extend to biologics. Sandoz was first to gain approval for growth hormone Omnitrope, and many other biosimilars have been approved in Europe.
A post-patent strategy with inherent limitations is a switch to OTC status. This only applies to limited cases that meet multiple criteria:
- Can the condition be self-diagnosed and treated?
- Can the patient apply the label properly and monitor the condition?
- Is the product safe and effective, and is the dosage easily delivered?
While this increases patient access and decreases office visit costs, it has risks such as inaccurate diagnosis and drug interactions. A dual status, with different strengths or formulations for prescription and OTC forms, may mitigate risks and reinforce equity [45].
A special case of late-life cycle management concerns the emergence of biosimilars in major markets.
Addressing the Challenge of Biosimilars
The US approval of Sandoz's Zarxio in March 2015 as the biosimilar version of Amgen's Neupogen (filgrastim) was the first step in a potential wave of such products under the new 351(k) pathway set up as part of the 2010 Affordable Care Act. Since 2006, a few products, such as Sandoz's Omnitrope, a biosimilar of Pfizer's growth hormone Genotropin, had been approved in the United States under different pathways—505(b)(2) in the case of Omnitrope. However, Europe leads in terms of regulation and market uptake, as a formal pathway was set up in 2006, and many biosimilars have since been approved by the European Medicines Agency, from filgrastim and epoetin to monoclonal antibodies such as infliximab.
Biosimilars have more limitations than small-molecule generics, requiring at least one head-to-head trial to confirm biosimilarity, and a lack of therapeutic interchangeability allowing automatic substitution by pharmacists. Market uptake varies across Europe. While a generic may reach a 90 percent market share within a year of entry in the United States, biosimilar erythropoietins only gained a 37 percent share across Europe within two years of launch. Germany has seen the highest penetration, partly due to minimum-level quotas set by its 200 sickness funds. By contrast, the United Kingdom has generally seen lower adoption levels, partly due to lower originator prices. Across Europe, discounts range from 15 to 30 percent, and they are expected to be from 25 to 35 percent in the United States.
There are a number of drivers, but also barriers, to biosimilar adoption. While payer pressure will drive them, physicians and patients will face trade-offs. Physicians may be reluctant to switch good responders and, thus, prescribe biosimilars only to new patients. Consumers may welcome lower copays but have safety concerns. An added issue is the extrapolation of original indications. While the FDA approved all five Neupogen indications in the case of Zarxio, for other products this may depend on the existence of real-world evidence. Biosimilars may thus be required to undergo wide post-marketing surveillance to monitor safety, efficacy, and quality of manufacturing, which has been shown to vary for biologics [46].
Further limitations apply to orphan biosimilars. An entire wave of these will lose exclusivity in the next decade. Enzyme replacement therapies such as Sanofi's Cerezyme (imiglucerase) and Fabrazyme (agalsidase alfa) have largely lost patents, as well as protein-based therapies such as Pfizer's Genotropin. Some critical questions apply to orphan biologics, from patient identification and trial recruitment to real-world evidence, reimbursement incentives, and market size threshold. Physician and patient loyalty may also protect originators, as their manufacturers often supply extensive clinical and reimbursement support services. For payers, trade-offs include cost savings versus low total budget impact and safety and efficacy concerns in critical therapeutic areas.
In Europe, regulation varies by country. In Germany, orphan drugs face fairly low scrutiny and there is little intent to cap prices. In the United Kingdom, the centralized National Health Service, rather than regional Clinical Commissioning Groups, regulates orphan drug costs. In France, however, the Paris public hospital system extracted a 45 percent discount on Celltrion's Inflectra, a biosimilar of Janssen's Remicade (infliximab), in exchange for an exclusive contract 47.
While payers may be the most influential drivers of biosimilars, their actions will be mitigated by physician and patient appreciation of the support services of originator companies and by a risk-averse attitude to switching in the critical therapeutic areas that apply to most orphan diseases.
Summary Points
- Consumer, regulator, and payer trends are driving the need for precision marketing as a counterpart to precision medicine.
- Payers, physicians, and consumers increasingly demand an evidence-based strategy, whereby product positions are supported by clinical and economic data.
- Biopharmas need to balance evidence and experience across therapeutic areas, with evidence more dominant in oncology and experience more salient in noncritical areas such as allergy, but with the support of evidence as a differentiator.
- Consumer-centered strategies depend on a broad understanding of the patient journey, from pre-diagnosis to post-treatment; new segmentation bases may include genotypes as well as technographics.
- Dual branding models now coexist, from targeted therapies with small salesforces and little or no use of mass media to primary care products with large populations.
- Launch success drivers range from co-creation with patients to the capture of physician KOLs, payer partnerships, cross-functional teams, and global planning.
- Multichannel communications must take into account consumers' resistance to digital intrusion and favor a more product-neutral outreach that meets their needs.
- Salesforce strategies may shift from traditional detailing to new structures such as key account management and value-added services such as patient education and remote monitoring.
- Sustainability strategies go well beyond the traditional life cycle and include portfolio diversification and franchise management, with the early planning of successor molecules, new indications, formulations, and combination therapies, as well as comprehensive patent portfolios.
