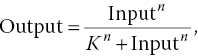3
Design Principles for Super Selectivity using Multivalent Interactions
Tine Curk1, Jure Dobnikar2, and Daan Frenkel1
1 Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK
2 Institute of Physics & School of Physical Sciences, Chinese Academy of Sciences, Beijing, 100190, China
3.1 Introduction
Multivalent particles have the ability to form multiple bonds to a substrate. Hence, a multivalent interaction can be strong, even if the individual bonds are weak. However, much more interestingly, multivalency greatly increases the sensitivity of the particle–substrate interaction to external conditions, resulting in an ultra‐sensitive and highly non‐linear dependence of the binding strength on parameters such as temperature, pH or receptor concentration.
In this chapter we focus on super selectivity: the high sensitivity of the strength of multivalent binding to the number of accessible binding sites on the target surface (see the schematic drawing in Figure 3.1). For example, the docking of a multivalent particle on a cell surface can be very sensitive (super selective) to the concentration of the receptors to which the multiple ligands can bind.

Figure 3.1 Selectivity denotes the ability of multivalent entities to distinguish between substrates depending on the surface density of binding sites.
We present a theoretical analysis of systems of multivalent particles and describe the mechanism by which multivalency leads to super selectivity. We introduce a simple analytical model that allows us to predict the overall strength of interactions based on physicochemical characteristics of multivalent binders. Finally, we formulate a set of simple design rules for multivalent interactions that yield optimal selectivity.
3.1.1 Background: Ultra‐sensitive Response
Many processes in biology depend ultra‐sensitively on variations in one or more of the parameters that control the process. Such ultra‐sensitivity manifests itself as an almost switch‐like, sigmoidal change in the ‘output’ when the control parameter crosses a threshold value. Understanding such switch‐like behaviour is obviously important to understand many regulatory processes in living systems, but such understanding will also help us design synthetic systems that combine weak supramolecular interactions with high selectivity.
The best known example of ultra‐sensitivity dates back to Hill who, in the beginning of the twentieth century, studied the binding of oxygen to haemoglobin. He found the that the relation between bound oxygen and partial pressure was sigmoidal [1]. Today this phenomenon is explained in terms of allosteric cooperativity whereby the four binding sites on haemoglobin do not act independently but are ‘cooperative’, that is binding of the first oxygen molecule increases the probability that the second oxygen molecule will bind. Hence, haemoglobin is likely to be either fully loaded with oxygen or empty, which makes haemoglobin an efficient transporter of oxygen between lungs and peripheral tissues. Other examples of ultra‐sensitivity include the switch‐like response of bacterial motors [2], or the switch‐like behaviour in gene regulation due to positive feedback loops in nucleosome modification [3]. For more information on this broad topic, the reader is referred to a review by Ferrell [4, 5, 6] and references therein.
Ultra‐sensitive response is usually characterized by a so‐called Hill curve:
where the Hill coefficient n quantifies the degree of cooperativity of the process: the higher the Hill coefficient, the more sensitive the response.1
Due to cooperativity, blocks that, individually, have limited selectivity can form units that interact selectively. For example, DNA base pairing is highly specific, even though underlying interactions (hydrogen bonding and base‐stacking) are not. Multivalent (or polyvalent) interactions can also lead to an ultra‐sensitive response, for example, the aggregation of multivalent DNA‐coated colloids depends sensitively on temperature [7]. Moreover, ligand–receptor or antibody–antigen interactions, are very sensitive to temperature, but also to ion concentration and pH. Internal protein interactions are also multivalent: protein folding and unfolding depend critically on temperature and other external conditions. The functioning of the biochemical machinery in cells relies (mostly) on multivalent supra‐molecular interactions. These interactions are very sensitive to external conditions which helps explain why the properties of living matter (cells, tissues) are very sensitive to temperature, while those of ‘formerly living’ matter (say, a piece of wood) are not.
In what follows, we focus on the ultra‐sensitivity of multivalent interactions to the density of ‘receptors’ on the substrate surface. In particular, we will derive expressions that show how the binding strength of a multivalent entity (say a ligand‐decorated nanoparticle or a multivalent polymer) to a substrate changes with the concentration of receptors2 on the substrate surface (Figure 3.2). It will turn out that multivalent interactions can be designed such that they result in an almost step‐like switch from unbound to bound as the receptor concentration exceeds a well‐defined threshold value. In the remainder of this chapter, we will use the term ‘super selectivity’ to denote this kind of sharp response.

Figure 3.2 Simulation snapshots comparing the targeting selectivity of monovalent and multivalent guest nanoparticles. We compare the adsorption onto two host surfaces with receptor concentrations (nR) that differ by a factor of three. (a) The monovalent guests provide little selectivity: increasing by three times the receptor coverage just increases the average number of bound guests by 1.8 (i.e. from 5.4 to 9.7 bound particles on average). (b) The multivalent nanoparticles behave super selectively: an increase of the receptor coverage by a factor three causes a 10‐fold increase in the average number of adsorbed particles. The multivalent guests have ten ligands per particle. The individual bonds of the multivalent case (b) are weaker than those in the monovalent case (a).
Source: Ref. [8]. Reproduced with permission of National Academy of Sciences of the United states of America.
The remainder of this chapter is structured as follows: First, we show how the description of simple chemical equilibria and Langmuir adsorption can be extended to multivalent interactions. We then discuss the conditions under which super selectivity appears and formulate simple design principles to achieve super selectivity. We include an appendix where we discuss how, in simple cases, our approach reduces to the widely used ‘effective molarity’ picture.
3.2 Super Selectivity: An Emergent Property of Multivalency
We first focus on a prototypical system of multivalent particles in solution that can adsorb to a receptor‐decorated surface (Figure 3.2). For simplicity, we assume that the surface is flat and much larger than the multivalent particles. Furthermore, we assume that these particles are larger than the surface receptors such that each particle can attach to many receptor sites simultaneously. Adsorption of particles is governed by the well‐known Langmuir isotherm which states that the fraction of the surface occupied by particles is
with ρ the molar concentration of particles in solution3, ![]() is the equilibrium avidity association constant of particles adsorbing to a surface. Note that
is the equilibrium avidity association constant of particles adsorbing to a surface. Note that ![]() is different from the affinity equilibrium constant KA which specifies chemical equilibria of individual ligand–receptor binding. Avidity (functional affinity) is the accumulated strength of multiple affinities [9].
is different from the affinity equilibrium constant KA which specifies chemical equilibria of individual ligand–receptor binding. Avidity (functional affinity) is the accumulated strength of multiple affinities [9].
We aim to understand how the overall avidity constant ![]() depends on the properties of the system, that is individual bond affinities KA4, the ligand valency k and number of receptors nR. The avidity constant includes all possible bound states, and is written as a sum over bonds
depends on the properties of the system, that is individual bond affinities KA4, the ligand valency k and number of receptors nR. The avidity constant includes all possible bound states, and is written as a sum over bonds
The first term on the right‐hand side takes into account all states with a single formed bond, the second term represents all doubly bound states, the third term triply bound states and so on Kintra is a constant specifying the internal equilibrium between singly and doubly bonded states. We have assumed that individual bonds form independently and Kintra is a constant, that is we ignore (allosteric) cooperative effects. We do this to clearly distinguish multivalent effects (the subject of this chapter) from cooperative effects [10].5
Ωi is the degeneracy pre‐factor, it measures the number of ways in which i bonds can be formed between two multivalent entities, see Figure 3.3 for representative cartoons. Degeneracy Ω is often labelled as a ‘statistical pre‐factor’ which denotes something that should be included for rigour but is otherwise not essential. However, as we will show, it is precisely this degeneracy that gives rise to super selectivity. The focus of the majority of theoretical papers [9, 11, 12, 13, 14] is on the calculation of the internal equilibrium constant Kintra. Here, instead, we focus on the degeneracy Ω. We will simply assume that Kintra is (or can be) known.

Figure 3.3 Entropic origin of super selectivity. The cartoons give a schematic representation of the simulation snapshots in Figure 3.2. The pictures show the binding of monovalent (a) and multivalent (b) entities (represented as a bar with attached flexible ligands). Receptors are shown as spheres tethered to the bottom surface. The left panels show a low receptor density ( ) and the panels on the right show a receptor density that is twice as high. In the monovalent case the number of distinct ways (Ω) to link ligands and receptors grows linearly with the number of receptors nR, while the multivalent case show a highly non‐linear response: changing nR from 3 to 6 increases Ω by a factor of 20. In general, the number of binding combinations (degeneracy) Ω is calculated using Eq. (3.6).
) and the panels on the right show a receptor density that is twice as high. In the monovalent case the number of distinct ways (Ω) to link ligands and receptors grows linearly with the number of receptors nR, while the multivalent case show a highly non‐linear response: changing nR from 3 to 6 increases Ω by a factor of 20. In general, the number of binding combinations (degeneracy) Ω is calculated using Eq. (3.6).
The degeneracy Ω depends on the spatial arrangement of both ligands and receptors. However, it is instructive to consider first the binding of flexible ligands, where all k ligands on a particle can bind to nR receptors (Figure 3.3b). In this case the degeneracy given by Eq. (3.6) becomes a very steep and non‐linear function of k and nR. This form was first considered by Kitov and Bundle [15] and has been applied, among others, to super‐selective targeting [8] and modelling the adhesion of influenza virus [16].
A low fraction of bound receptors in the system can arise either because the number of receptors is greater than the number of available ligands: ![]() or when individual bonds are weak:
or when individual bonds are weak: ![]() 6. In this case the avidity constant [Eq. (3.5)], using degeneracy [Eq. (3.6)], can be rewritten7 to yield a simple form:
6. In this case the avidity constant [Eq. (3.5)], using degeneracy [Eq. (3.6)], can be rewritten7 to yield a simple form:
where, as before, KA is the monomeric single‐bond affinity constant, Kintra the internal association constant, and nR and k are the number of receptors and ligands respectively. For our purpose it is important to note that for multivalent binding (![]() ),
), ![]() is a steep, non‐linear function of nR (Figure 3.4).
is a steep, non‐linear function of nR (Figure 3.4).

Figure 3.4 Adsorption profile of multivalent particles computed using Eqs (3.4) and (3.7). Monovalent adsorption (circles)  yields the familiar Langmuir isotherm. In contrast, multivalent particles display a steep, sigmoidal response. In the case shown, we have chosen the dimensionless activity in solution to be
yields the familiar Langmuir isotherm. In contrast, multivalent particles display a steep, sigmoidal response. In the case shown, we have chosen the dimensionless activity in solution to be  , the binding affinity of individual bonds decreases as the valency increases from mono‐valent to 10‐valent:
, the binding affinity of individual bonds decreases as the valency increases from mono‐valent to 10‐valent:  5, 1.5,
5, 1.5,  ,
,  , such that the overall avidity
, such that the overall avidity  at 50 % bound fraction (
at 50 % bound fraction ( ) is kept constant for all valencies.
) is kept constant for all valencies.
Equation (3.7) could have also been obtained directly by reasoning that for non‐saturated receptors (fraction of bound receptors is low), competition for the same receptor can be ignored. Each of the k ligands can then bind independently to any of the nR receptors with an equilibrium constant Kintra (weight nRKintra). Alternatively, the ligand is unbound (weight 1). Hence, for systems with a low fraction of bound receptors, the factor ![]() accounts (approximately) for all possible states. Furthermore, we subtract 1 because we use the convention that at least a single bond needs to be formed for the multivalent particle to be considered bound. The avidity constant has units of inverse molar concentration. To obtain the correct limiting behaviour in the limit
accounts (approximately) for all possible states. Furthermore, we subtract 1 because we use the convention that at least a single bond needs to be formed for the multivalent particle to be considered bound. The avidity constant has units of inverse molar concentration. To obtain the correct limiting behaviour in the limit ![]() , where
, where ![]() , we must multiply the expression in square brackets by
, we must multiply the expression in square brackets by  .
.
We note that the ratio  has the dimension of an effective volume veff. The form of Eq. (3.7) suggests that we can view the multivalent particle adsorption as a two‐step process. First, the particle adsorbs from the solution to the surface and comes into a position to start forming bonds, the equilibrium constant of this process is given by the ratio
has the dimension of an effective volume veff. The form of Eq. (3.7) suggests that we can view the multivalent particle adsorption as a two‐step process. First, the particle adsorbs from the solution to the surface and comes into a position to start forming bonds, the equilibrium constant of this process is given by the ratio  . Once the particle is in this position, all of the k ligands can independently form bonds with surface receptors.
. Once the particle is in this position, all of the k ligands can independently form bonds with surface receptors.
In the monovalent case (![]() ) the avidity constant reduces to
) the avidity constant reduces to ![]() and the standard Langmuir isotherm is obtained. Furthermore, expanding Eq. (3.7) in a binomial series and using a maximum term approximation we can insert the maximum term in Eq. (3.4) and obtain the phenomenological Hill equation [Eq. (3.1)]. In the case of very strong individual bonds (
and the standard Langmuir isotherm is obtained. Furthermore, expanding Eq. (3.7) in a binomial series and using a maximum term approximation we can insert the maximum term in Eq. (3.4) and obtain the phenomenological Hill equation [Eq. (3.1)]. In the case of very strong individual bonds (![]() ) virtually all k bonds are formed and the avidity becomes8:
) virtually all k bonds are formed and the avidity becomes8: ![]() .
.
We have shown how combinatorial entropy (also called ‘avidity entropy’ [9]) gives rise to sharp switching behaviour upon a change in receptor concentration nR (Figure 3.4). Next, we introduce a measure of the sensitivity of the binding of multivalent particles to the surface concentration of receptors:

α is the slope of the adsorption profile in a log–log plot (Figure 3.5). For mono‐valent binding the selectivity α is never larger than one, while in the multivalent case the selectivity can reach values greater than one, indicating a supra‐linear response. Note that for low surface coverage (![]() ) the selectivity α is equivalent to the effective Hill coefficient n from Eq. (3.1). However, because we consider all terms (all possible number of bonds) in calculating avidity [Eq. (3.7)], α is not a constant. At very low receptor concentrations the avidity shows a linear dependence on nR, and
) the selectivity α is equivalent to the effective Hill coefficient n from Eq. (3.1). However, because we consider all terms (all possible number of bonds) in calculating avidity [Eq. (3.7)], α is not a constant. At very low receptor concentrations the avidity shows a linear dependence on nR, and ![]() 9. Selectivity then grows with increasing receptor concentration nR until reaching a peak just before the saturation of the surface (
9. Selectivity then grows with increasing receptor concentration nR until reaching a peak just before the saturation of the surface (![]() ). We refer to the region with
). We refer to the region with ![]() as the ‘super‐selective’ region. In this region, a small change in the receptor density nR causes a faster‐than‐linear change in adsorption θ.
as the ‘super‐selective’ region. In this region, a small change in the receptor density nR causes a faster‐than‐linear change in adsorption θ.

Figure 3.5 Selectivity. (a) shows the log–log plot of Figure 3.4, and (b) shows its slope, that is the selectivity  . We observe that selectivity is typically less than one for monovalent particles indicating at most linear response. Multivalent particles, on the other hand, exhibit a region with values of α significantly greater than one, thus demonstrating that the number of adsorbed ligands increases faster than linearly with the receptor concentration: in this regime, the system is super selective.
. We observe that selectivity is typically less than one for monovalent particles indicating at most linear response. Multivalent particles, on the other hand, exhibit a region with values of α significantly greater than one, thus demonstrating that the number of adsorbed ligands increases faster than linearly with the receptor concentration: in this regime, the system is super selective.
3.3 Multivalent Polymer Adsorption
To validate the model for super‐selective adsorption described above, we now compare its predictions with experimental data on polymer adsorption. Multivalent glycopolymers have been used as selective probes for protein–carbohydrate interactions in a biochemical setting [20, 21, 22]. More recently, super‐selective targeting was demonstrated in a synthetic system based on host–guest chemistry [17, 18]. We briefly describe multivalency effects in the case of polymers functionalized with many ligands.
We consider a flexible polymer with a contour length much larger than the persistence length. Ligands are randomly attached along the polymer chain (Figure 3.6a). Similar to the nanoparticles case above, a reasonable first assumption is that, due to polymer‐chain flexibility, all k ligands on a polymer can bind to any of the nR receptors within a domain on the surface with lateral dimensions comparable with those of the polymer. For simplicity, we describe the surface as a square lattice. The cells of the lattice have linear dimensions comparable with the radius of gyration Rg of the polymer. As in the case of soft multivalent particles, any ligand on the polymer can bind to any receptor in one (and only one) lattice cell, see Figure 3.6. The model is expected to offer a faithful description of the real system if the mean distance between ligands is larger than the Kuhn segment length such that even consecutive ligands along the polymer chain can be treated as uncorrelated.

Figure 3.6 Cartoon of the multivalent polymer model. (a) Flexible multivalent polymer close to the receptor decorated surface is modelled as (b) uncorrelated ligands within a lattice site with volume  and a the linear lattice size. The ligands can move and bind to receptors independently within the lattice site, but cannot escape the site individually.
and a the linear lattice size. The ligands can move and bind to receptors independently within the lattice site, but cannot escape the site individually.

Figure 3.7 Multivalent polymer adsorption. Experimental adsorption profiles (points with error bars) for hyaluronic acid polymers functionalized with β‐cyclodextrin hosts (HA‐β‐CD) binding to surface attached adamantene (affinity  ) or ferrocene (
) or ferrocene ( ) guests. As can be seen, the theoretical adsorption profiles (dashed, dotted or solid lines) match the experimental data well for all valencies (k), affinities (KD) and polymer concentration studies. In the key, ‘lc’ denotes lower concentration of polymers in solution. One parameter [Upoly in Eq. (3.11)] was fitted, the value
) guests. As can be seen, the theoretical adsorption profiles (dashed, dotted or solid lines) match the experimental data well for all valencies (k), affinities (KD) and polymer concentration studies. In the key, ‘lc’ denotes lower concentration of polymers in solution. One parameter [Upoly in Eq. (3.11)] was fitted, the value  provides a good fit to all data points.
provides a good fit to all data points.
Source: Adapted from Refs [17, 18].
The analytical expression given by Eq. (3.11) captures the essentials of multivalent polymer adsorption10. Importantly the model allows us to predict adsorption profiles and selectivities depending on the physicochemical properties of multivalent polymers, shown in Figure 3.7. Hence, use of the simple theoretical expression given by Eq. (3.11) allows us to design a multivalent polymer such that it will selectively target a desired receptor density. In other words, Eq. (3.11) offers a tool for the rational design of selective targeting.
3.4 Which Systems are Super Selective?
The discussion thus far has focused on selective adsorption of multivalent particles and polymers. We now generalize our treatment and discuss various practical systems. In particular, we will discuss the key role of disorder that is needed to observe super‐selective behaviour in multivalent interactions. Specifically, what is needed is that a multivalent entity can bind in many different ways to a receptor‐decorated substrate. This kind of disorder is usually not possible for multivalent interactions on the angstrom or nanometre scale, as the interacting units tend to be effectively rigid on that scale. In contrast, larger supramolecular systems (e.g. the binding of a multivalent polymer to a receptor decorated membrane) can sustain the ‘disordered’ interactions.
3.4.1 Rigid Geometry Interactions
A prototypical example of multivalent interactions is the fixed (rigid) geometry multivalency shown in Figure 3.8. Two rigid, multivalent entities bind via multiple bonds: as the geometry is rigid, individual bonds either fit together, or they do not. Examples of this kind of interaction include the base pairing between nucleotides in complementary sequences of single‐stranded DNA.

Figure 3.8 Rigid geometry multivalency. The cartoon presents a prototypical fixed geometry interaction where bonds are commensurate (e.g. DNA base pairing or enzyme–substrate interactions). Such systems generally do not exhibit super‐selective behaviour as we cannot increase the (binding site) density on one multivalent entity (substrate) without breaking the commensurability of the bonds. See also Eq. (3.12) and the discussion in the corresponding box.
Another well‐known example of a rigid multivalent interaction is the binding between an enzyme and a substrate. The interaction between a pair of proteins is multivalent, as it involves a number of local interactions of various types (hydrogen bonding, hydrophobic, Van der Waals, electrostatic etc.). To a first approximation the enzyme and substrate can be described as rigid objects. This is a simplification as proteins, even in their native state, are not entirely rigid. In any given relative orientation of the ligand to a substrate we find a two‐dimensional equivalent of the Figure 3.8. We name this class of multivalent interactions ‘rigid geometry multivalency’.
Due to the lack of flexibility of individual bonds, rigid multivalency will generally not show super‐selective behaviour. To understand this, consider a simple one‐dimensional example of a sequence of rigidly positioned ligands that bind to a commensurate sequence of receptors. One cannot increase the binding site density on the substrate without breaking the commensurability of the binding. Hence, increasing the receptor density will normally decrease the binding strength. In other words: commensurate lock‐and‐key interactions are not super selective. Interestingly, it seems that the ability of rigid multivalent particles to detect commensurate structures is exploited in nature, for instance in the activation of certain Toll‐like receptors [19].
3.4.2 Disordered Multivalency
Super‐selective behaviour can be exhibited by multivalent systems that can increase the number of possible bonds as the density of receptors increases. As we saw above, fully ordered multivalent systems only bind optimally to commensurate receptor arrangements. To achieve super selectivity, we typically need some kind of disorder or randomness in the geometry of binding. The ability to increase the number of bonds with increasing receptor density can be due to: (i) long, flexible binders; (ii) mobile receptors; or (iii) random binder positions. Figure 3.9 shows schematic examples of these three cases. Different types of bond disorder will result in different expressions for the bound partition functions (and therefore, for the avidity constants), see Eqs (3.13–3.15). However, they all show similar super‐selective behaviour (Figure 3.10).
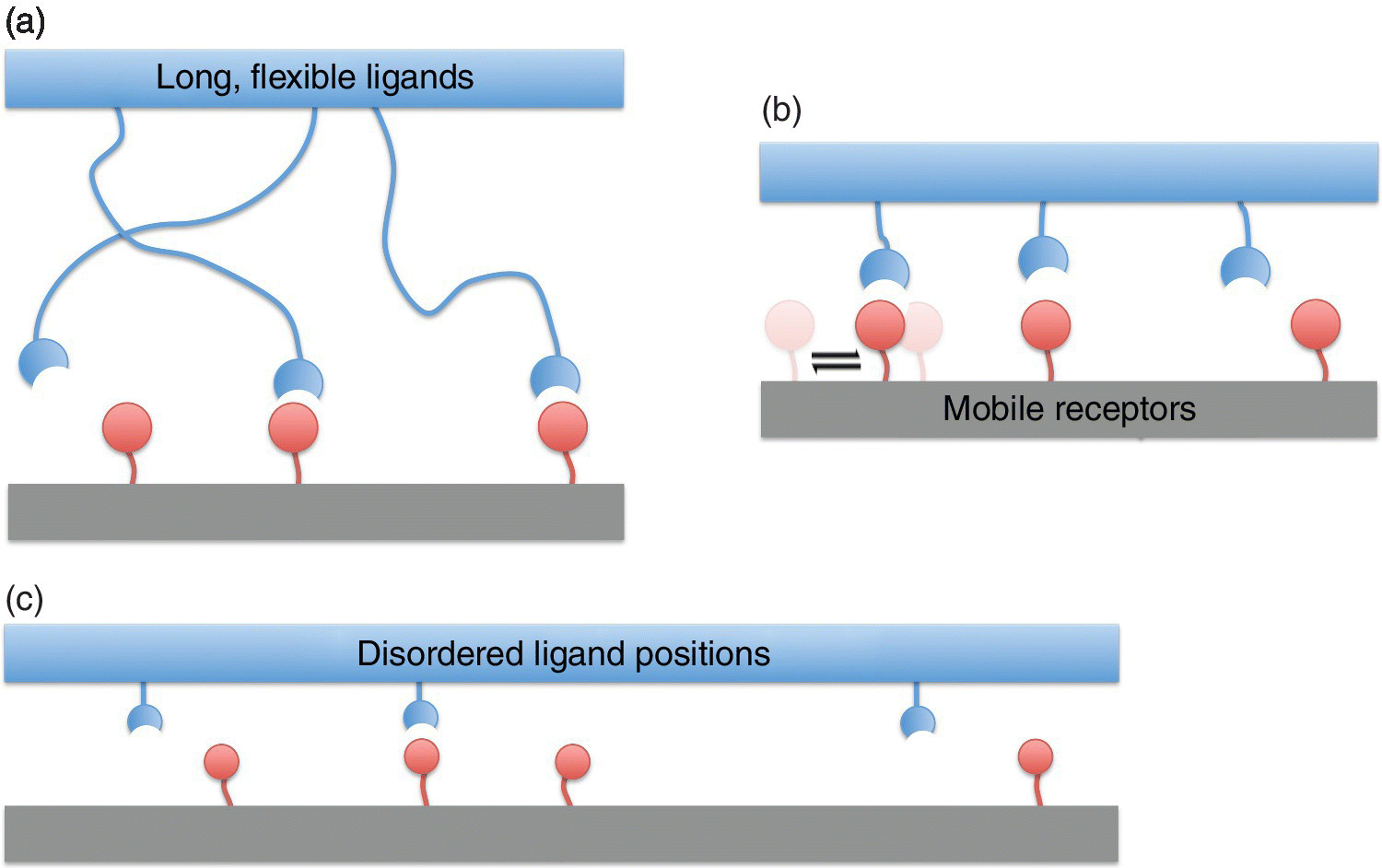
Figure 3.9 Disordered multivalent systems. Three characteristic types of multivalent interactions are shown: (a) long, flexible binders, (b) mobile receptors, (c) disordered, random positions of individual binders. Different types can behave slightly differently, see the adsorption profiles in Figure 3.10. However, they all exhibit super selectivity, and consequently, any practical system that is similar to at least one of them, will be super selective.

Figure 3.10 Adsorption profile for different disordered systems, depicted in Figure 3.9. Different systems show qualitatively similar super‐selective behaviour. For large number of bonds the adsorption profiles converge. The plots shown were generated using the Langmuir expression for the adsorption isotherm Eq. (3.8) with activity  . We used expressions (3.13, 3.14, 3.15) with
. We used expressions (3.13, 3.14, 3.15) with  and
and  to compute the adsorption isotherm in the case of a few strong bonds (solid lines). To represent the case of many weak bonds (dashed lines), we used the same equations but assumed
to compute the adsorption isotherm in the case of a few strong bonds (solid lines). To represent the case of many weak bonds (dashed lines), we used the same equations but assumed  and
and  .
.
At first sight, it would seem that the case of mobile receptors shown in Figure 3.9b should be rather different from the immobile case. However, since the receptors are mobile, each ligand can, in principle, bind to any receptor. In this light the two problems become very similar. Another way of looking at the system with mobile receptors is to consider the receptors as a (two‐dimensional) ‘ideal gas’ of particles that can bind to the ligands with an interaction strength f. Up to a concentration‐independent term ![]() , the chemical potential of these receptors is given by
, the chemical potential of these receptors is given by ![]() . A small change in the receptor concentration nR leads to a small change in the chemical potential μR, which alters the probability of each and every individual ligand binding. For multivalent particles a small change per ligand adds up to a large change per particle.11 Clearly, the binding probability depends on nR, see Refs [19, 23] for practical examples of super selectivity with mobile receptors. We note that for dilute receptors the chemical potential is dominated by the translational entropy. Hence, the origin of super selectivity is entropic, also for mobile receptors.
. A small change in the receptor concentration nR leads to a small change in the chemical potential μR, which alters the probability of each and every individual ligand binding. For multivalent particles a small change per ligand adds up to a large change per particle.11 Clearly, the binding probability depends on nR, see Refs [19, 23] for practical examples of super selectivity with mobile receptors. We note that for dilute receptors the chemical potential is dominated by the translational entropy. Hence, the origin of super selectivity is entropic, also for mobile receptors.
Finally, for immobile randomly distributed binders shown in Figure 3.9c the intuitive reasoning for super selectivity follows from our initial discussion in the introduction. Let us consider two ligand/receptor‐decorated multivalent nanoparticles, A and B that can attach through ligand–receptor binding. The binding moieties are randomly distributed on both nanoparticles. From a point of view of a particular ligand on particle A, the probability of it binding, p1A, is to a first approximation linear in the density nR of complementary receptors on particle B. The number of possible bonds in the contact area is proportional to the number of ligands k in that area. The net result is that the binding probability depends exponentially on the product of k and nR, as would follow from Eq. (3.15).
We note that in the cases of fixed short ligands we have only illustrated and discussed the two limiting cases: (i) perfectly complementary rigid interaction (Figure 3.8); and (ii) disordered interaction case (Figure 3.9c). Practical systems will fall between these two extremes. As a rule of thumb, small molecules and macromolecules, such as DNA or proteins, or virus capsids have a rather well defined geometry and we expect their interactions to be closer to the rigid geometry case. On the other hand, the spatial distribution of binders (ligands) on entities larger than a few nanometres is, in general, more disordered; be they man‐made such as DNA coated colloids [24, 25, 26], or natural such as cells.
We have presented simple analytical models that can be used to rationalize and understand super selectivity in various multivalent systems. In the case of polymers, the simple model works very well (3.7). However, certain systems have been studied in a greater detail. For these cases, more sophisticated (and more complex) models have been developed. For example, cell endocytosis of a virus is mediated by a multivalent interaction between membrane proteins (receptors) and virus capsid proteins (ligands). But to model the process, one should account for membrane elasticity and, in some cases, also for active processes [27]. More detailed models of multivalent polymer adsorption have recently been developed [28, 29]. A theory of valence‐limited interactions explicitly taking into account specific positions and different types of tethered binders requires the self‐consistent solution of a system of equations [30, 31], the framework was also extended to mobile ligands [32]. A complementary approach is based on a saddle‐point approximation for the binding free energy [33]. We note that the results presented in these papers support the conclusions about super‐selective behaviour that we have obtained here using much simpler models.
3.5 Design Principles for Super‐Selective Targeting
Clearly, super‐selective targeting has important practical applications (as even viruses seem to ‘know’). It is therefore important to formulate design principles for achieving optimal super selectivity. To formulate design rules, we start once again from the simple model described above: multivalent particle docking to a receptor‐decorated surface (e.g. a cell). The density of receptors on the surface is again measured by nR, the mean number of receptors in the contact area (i.e. the area accessible to a docked particle). In many cases of practical interest, we aim to target only those surfaces (e.g. a cell surface) that have a receptor concentration above a certain threshold. How should we design the particle to target this surface optimally? Our control parameters are the valency k, the ligand–receptor binding strength f, and the activity of particles in solution z.
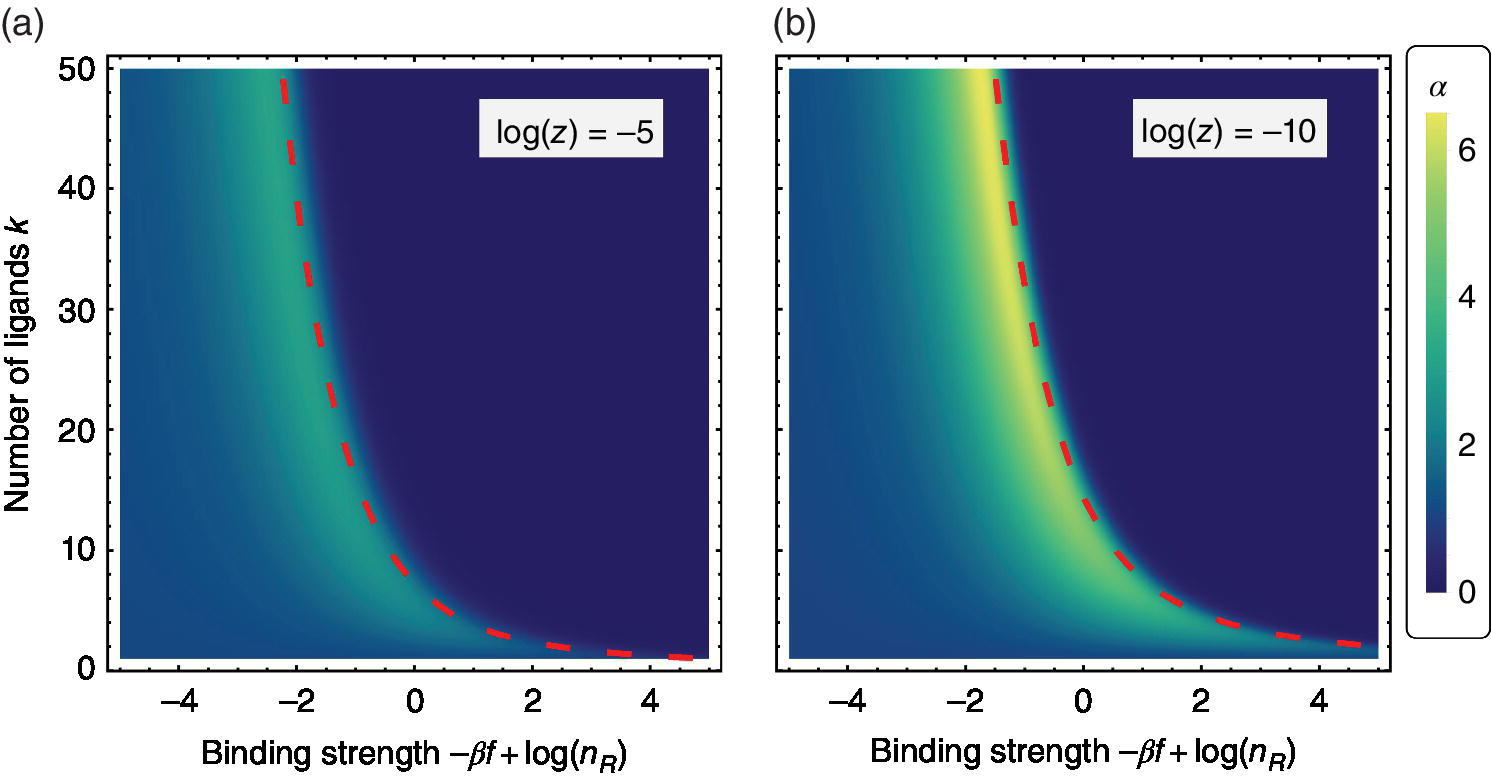
Figure 3.11 The selectivity landscape as function of the valency k and the rescaled binding strength  . The landscape was obtained by calculating the selectivity α using Eq. (3.16). The activity of multivalent particles was chosen as: (a)
. The landscape was obtained by calculating the selectivity α using Eq. (3.16). The activity of multivalent particles was chosen as: (a)  ; and (b)
; and (b)  . Both plots use the same colour scale. The dashed curves represent the approximate optimal selectivity relation given by Eq. (3.17), which rather accurately fits the maximum selectivity region.
. Both plots use the same colour scale. The dashed curves represent the approximate optimal selectivity relation given by Eq. (3.17), which rather accurately fits the maximum selectivity region.
The landscape plots of selectivity as a function of the valency k and bond strength f are shown in Figure 3.11. We immediately notice three features: (i) High selectivity appears only in a small region of the parameter space, along the curve predicted by Eq. (3.17). (ii) The selectivity reaches a plateau value at large valencies k and weak individual bonds. (iii) Maximum selectivity is limited by the activity z; lowering the activity (or density) of multivalent particles yields a higher selectivity.
The dimensionless activity  depends on the density ρ, but also on the ratio of the equilibrium constants for the formation of the first bond, and for the formation of subsequent ligand–receptor bonds in a particle–substrate complex [see Eq. (3.5)]. Therefore, even at large densities, selectivity can be substantial if the ratio
depends on the density ρ, but also on the ratio of the equilibrium constants for the formation of the first bond, and for the formation of subsequent ligand–receptor bonds in a particle–substrate complex [see Eq. (3.5)]. Therefore, even at large densities, selectivity can be substantial if the ratio  is small. This can be achieved by adding a non‐specific repulsion between the multivalent entities (for instance, by coating the particle with inert polymer that provides steric repulsion [34]). Such a repulsion would present a barrier to particle association but would not prevent additional bonds from forming once the barrier is overcome: the result would be a reduction in KA due to repulsion, but as Kintra would be less affected, this steric repulsion would decrease the ratio
is small. This can be achieved by adding a non‐specific repulsion between the multivalent entities (for instance, by coating the particle with inert polymer that provides steric repulsion [34]). Such a repulsion would present a barrier to particle association but would not prevent additional bonds from forming once the barrier is overcome: the result would be a reduction in KA due to repulsion, but as Kintra would be less affected, this steric repulsion would decrease the ratio  .
.
Our calculations show that selectivity is suboptimal when using high affinity bonds. However, strong affinity multivalent constructs can still behave super selectively (![]() ) if their activity (concentration) in the solution is low enough, see Eq. (3.19). This suggests that, although in principle it is possible to design a super‐selective system based on very strong affinity interactions, such as the biotin–streptavidin pair, such a system would only be super selective at extremely low concentrations where the kinetics would be too slow for practical applications.
) if their activity (concentration) in the solution is low enough, see Eq. (3.19). This suggests that, although in principle it is possible to design a super‐selective system based on very strong affinity interactions, such as the biotin–streptavidin pair, such a system would only be super selective at extremely low concentrations where the kinetics would be too slow for practical applications.
Multivalency leads to super selectivity, but it also leads to high sensitivity of binding to the variation in other relevant quantities. Therefore, in practical applications, it is important to control (or, at least know) parameters such as temperature, pH, ionic binding strength when using multivalent particles for selective targeting. The parameter range that yields high selectivity is rather small, see Figure 3.11b). A brute‐force ‘random’ search in design‐parameter space is, therefore, unlikely to find the optimal selectivity region. We hope that the theoretical guidelines and design principles set forth in this chapter will enable a more rational design of particles for super‐selective targeting.
We condense the results shown in Figure 3.11 and our theoretical considerations, Eq. (3.18), in a set of simple design rules for multivalent binding that yield maximum selectivity. We use our dimensionless statistical mechanics notation, which can be straightforwardly converted to chemical equilibrium units using  and
and ![]() , as discussed in the Notation box.
, as discussed in the Notation box.
- The maximal possible selectivity α is limited by the activity of multivalent particles in solution:
 so the activity z of multivalent binders should be small.
so the activity z of multivalent binders should be small. - Many weak bonds are better than a few strong ones. The selectivity is also limited by the valency k, until a point of saturation given by
 . The first two design rules together state that the maximal selectivity is limited by either the valency k or the
. The first two design rules together state that the maximal selectivity is limited by either the valency k or the  , whichever is smaller.
, whichever is smaller. - The relationship between the ligand number k and binding strength f should be obeyed:
 . Together with the above rule, this one states that to achieve maximal selectivity individual bonds should be very weak
. Together with the above rule, this one states that to achieve maximal selectivity individual bonds should be very weak 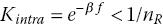 . In other words, the fraction of bound receptors/ligands should always remain small.
. In other words, the fraction of bound receptors/ligands should always remain small.
The main assumptions used to arrive at these design rules are: (i) ligands are identical and bind independently; (ii) all ligands of a (surface bound) multivalent construct can reach all surface attached receptors within a lattice site, but cannot bind to any receptor outside of the site (Figure 3.6); and (iii) receptors, ligands or particles have no interactions except for the steric repulsion and ligand–receptor affinity.
3.6 Summary: It is interesting, but is it useful?
We have shown that weak, multivalent interactions can result in a super‐selective behaviour where the overall interaction strength becomes very sensitive to the concentration of individual binders (receptors). We presented a simple yet powerful analytical model with good predictive power for designing multivalent interactions. We expect that, even in cases where the simple model fails quantitatively, the above design rules will still provide a good starting point for designing super selectivity in practical multivalent systems. Figure 3.12 summarizes advantages of weak multivalent interactions in selective targeting.

Figure 3.12 Advantages of using weak bonds. Contrary to strong monovalent antibody–antigen interactions and covalent bonds, multiple weak complexes can be disassembled (one by one) using different environmental stimuli (temperature, interaction strength, pH, light), which provides flexibility and reversibility. Examples of systems that exploit multivalency are dendrimers [35], stimulus‐responsive coatings [36], renewable sensors for biomolecules [37], reversible gels [38] and gel‐particle glue [39]. Importantly, external stimuli can be used to tune the super‐selectivity region to the desired surface density of receptors. For example, one could exploit the acidic extracellular environment of tumour tissues to improve the efficiency of drug targeting using multivalent particles.
We can imagine effective purification devices where nano objects of different valencies are passed through super‐selective sieves. In the field of material self‐assembly, multivalent supramolecular entities could be designed to hierarchically assemble depending on the valency, thus enhancing the precision of self‐assembled constructs [25].
The ability to target diseased cells pathogens based on the surface concentration of certain (over)expressed receptors would be of huge practical importance. At present, the delivery of pharmaceutical compounds to specific cells is usually based on the existence of a specific marker (e.g. a sugar or a peptide fragment) that is unique to the targeted cell type. The current wisdom seems to be to functionalize drugs or drug carriers such that they bind strongly to the specific marker. This strategy is fine if the target cells (e.g. bacteria) are very different from the cells of the host, and carry very different markers.
However, the strong‐binding strategy becomes problematic if one wishes to target, say, cancerous cells, which are usually very similar to our healthy cells. Cancerous cells typically over‐express markers that are also present, be it in smaller quantities, on healthy cell surfaces. Examples are the CD44 (‘don’t eat me’ receptor) or the folic receptor. In such cases, a compound that binds strongly to the over‐expressed marker will also bind to (and kill) healthy cells. The insensitivity of strong binders to the surface concentration of markers is one of the main reasons why antibiotics can be efficient with few side effects (in most patients), while chemotherapy is directly harmful to our body.
As outlined in this chapter, carefully designed multivalent drugs could be targeted super selectively only to cells with cognate receptor concentration above a certain threshold value [8, 40, 41]. Furthermore, in a living cell, receptor interactions and signalling play a major role which can further enhance the non‐linear response of the system [42, 43, 44, 45, 46].
Multivalency extends the sensitivity of interactions into the receptor density domain. Moreover, it enables the design of specific, highly selective interactions based on the concentration of ligands or binders, as well as on their chemical nature, thus opening up the possibility for selective targeting with minimal side effects.
Appendix 3.A: What Is Effective Molarity?
Effective molarity (EM) is an empirical concept that is commonly used to relate the kinetics and equilibria of intramolecular and intermolecular reactions [9, 10, 11]. It is defined as
where KAintra and KAinter are the equilibrium association constants. EM has units of molar concentration and is a useful measure of multivalent interactions efficacy, see Figure 3.A.1. For example, when the concentration ρ of multivalent ligands in solution is high ![]() multivalent effects are suppressed and ligands will bind monovalently. On the other hand when
multivalent effects are suppressed and ligands will bind monovalently. On the other hand when ![]() multivalent interactions dominate over monovalent binding. Additionally, EM allows us to de‐convolute the intra equilibrium constant into a simple part (KA) due to bond formation, and a complicated part (EM) related to the change of conformational entropy and free energy upon binding, see Refs [9, 10, 12, 13] for more discussion.
multivalent interactions dominate over monovalent binding. Additionally, EM allows us to de‐convolute the intra equilibrium constant into a simple part (KA) due to bond formation, and a complicated part (EM) related to the change of conformational entropy and free energy upon binding, see Refs [9, 10, 12, 13] for more discussion.

Figure 3.A.1 The concept of effective molarity. The above cycle shows the three different states that divalent ligands (BB) can bind to two receptors (AA) (unbound state is omitted). We have three distinct states and, therefore, need two equilibrium constants to characterize the equilibrium properties of the system, KA and EM. A product of the two is often called an intra association constant  . A useful reference point is that for a divalent ligand/receptor system and saturated receptors, EM determines the concentration of divalent ligands [BB] in solution at which we expect equal number of singly and doubly bonded ligands.
. A useful reference point is that for a divalent ligand/receptor system and saturated receptors, EM determines the concentration of divalent ligands [BB] in solution at which we expect equal number of singly and doubly bonded ligands.
However, it is important not to over‐interpret the meaning of ‘effective’ concentrations. The name suggests that we can calculate the internal chemical equilibria of multivalent interactions simply by using some effective concentrations of ligands. That, however, is not quite the case, as the expressions for association equilibrium between two compounds do not carry over to the situation when the numbers involved are small.
Let us consider a prototypical system: Only two particles (ligands) in a box with volume V. The particles can associate with an equilibrium constant KA that was predetermined for us, see Figure 3.A.2. We wish to calculate the association probability of these two particles. To obtain the correct result we can calculate the partition functions of the bound/unbound state.

Figure 3.A.2 Dimerization reaction in a small box. We have two particles (a single A‐type and a single B‐type) in a box with volume V. We assume that, although the particles can bind, they do otherwise behave as an ideal gas. We wish to calculate the relation between the probability of dimerization and equilibrium association constant KA. Simply calculating effective concentrations of [A], [B] and [AB], and using standard chemical equilibrium equation 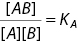 gives a wrong answer, see boxes on dimer reactions.
gives a wrong answer, see boxes on dimer reactions.
On the other hand, if we naively make use of the expression for chemical equilibrium in a bulk mixture binary chemical equilibrium, we do not reproduce the correct result.
Treating the system as a bulk binary reaction is not valid for only two dimerizing particles. The approach is valid in the thermodynamic limit where the chemical potential of a molecular species can be related to the logarithm of its concentration. What it boils down to is that Stirling’s approximation is valid only for large number of particles ![]() , it is clearly wrong when N equals 1 or 2. The same problem occurs when trying to calculate equilibrium constant from molecular dynamics simulations using small system sizes [47].
, it is clearly wrong when N equals 1 or 2. The same problem occurs when trying to calculate equilibrium constant from molecular dynamics simulations using small system sizes [47].
The above example might seem rather abstract. However, it exposes a potential pitfall of misusing ‘effective’ concentrations. The same pitfall is encountered when calculating binding probabilities of multivalent ligands, because the reactions shown in Figures 3.A.1 and 3.A.2 are very similar. For example, one could naively argue that both the unbound ligand (A) and receptor (B) in Figure 3.A.1 are flexible and can explore some effective volume V and have some effective concentration within this volume. One then applies a ‘Local chemical equilibrium’ (LCE) assumption [24, 48] which, in our simple system is given by Eqs (3.A.5, 3.A.6). But this procedure does not generally give a correct result. It becomes a good approximation only in the limit of weak binding12 or a very large valency where the Stirling’s approximation becomes applicable.
It should be clear that effective molarity is not really a concentration.13 Rather, it is a quantity with the dimensions of concentration, defined by Eq. (3.A.1). We can view the effective molarity as a measure for the probability that an unbound ligand and receptor would overlap in space (and hence come into position to bind). In an idealized system, neglecting the effects of the linker and orientational correlations in the unbound state, this probability is related to an effective concentration of, say, a ligand (B) as experienced by its complementary receptor (A) [9, 12, 14]. This is exactly the ‘cloud of ideal ligands’ approximation we have used as a starting point for our theory of multivalent polymer adsorption, Eq. (3.11). In the case of our simplified system of two dimerizing particles (Figure 3.A.2) the effective concentration ceff of type‐A, as experienced by type‐B (or vice versa) is
where we recall that V is the box volume. We can think of particle A adsorbing to particle B and the ratio of probabilities of being bound to unbound becomes
which is consistent with the correct result, Eq. (3.A.4). We could view ceff pu as the concentration of unbound A.
Applying this concept to dimer adsorption (Figure 3.A.1) we would find that the empirically calculated effective molarity [Eq. (3.A.1)] is similar to the theoretical effective concentration ![]() (in our idealized system they are equal). Therefore, effective concentration, when applied properly, is a useful concept when attempting to theoretically predict equilibria of multivalent binding.
(in our idealized system they are equal). Therefore, effective concentration, when applied properly, is a useful concept when attempting to theoretically predict equilibria of multivalent binding.
Acknowledgements
We thank Galina V. Dubacheva and Stefano Angioletti‐Uberti for useful suggestions for the manuscript and help with designing figures. This work on multivalency was supported by the ERC Advanced Grant 227758 (COLSTRUCTION), ITN grant 234810 (COMPPLOIDS) and by EPSRC Programme Grant EP/I001352/1. T.C. acknowledges support form the Herchel Smith fund.
References
- 1 Hill, A.V. (1910) Proceedings of the Physiological Society: January 22, 1910. The Journal of Physiology, 40 (suppl.), i–vii.
- 2 Tu, Y. (2008) The nonequilibrium mechanism for ultrasensitivity in a biological switch: Sensing by Maxwell’s demons. Proceedings of the National Academy of Sciences, 105 (33), 11 737–11 741.
- 3 Sneppen, K., Micheelsen, M.A. and Dodd, I.B. (2008) Ultrasensitive gene regulation by positive feedback loops in nucleosome modification. Molecular Systems Biology, 4 (1), 182.
- 4 Ferrell, J.E. and Ha, S.H. (2014) Ultrasensitivity part I: Michaelian responses and zero‐order ultrasensitivity. Trends in Biochemical Sciences, 39 (10), 496–503.
- 5 Ferrell, J.E. and Ha, S.H. (2014) Ultrasensitivity part II: multisite phosphorylation, stoichiometric inhibitors, and positive feedback. Trends in Biochemical Sciences, 39 (11), 556–569.
- 6 Ferrell, J.E. and Ha, S.H. (2014) Ultrasensitivity part III: cascades, bistable switches, and oscillators. Trends in Biochemical Sciences, 39 (12), 612–618.
- 7 Dreyfus, R., Leunissen, M.E., Sha, R., Tkachenko, A.V., Seeman, N.C., Pine, D.J. and Chaikin, P.M. (2009) Simple quantitative model for the reversible association of DNA coated colloids. Physical Review Letters, 102, 048 301.
- 8 Martinez‐Veracoechea, F.J. and Frenkel, D. (2011) Designing super selectivity in multivalent nano‐particle binding. Proceedings of the National Academy of Sciences of the United States of America, 108 (27), 10 963–10 968.
- 9 Krishnamurthy, V.M., Estroff, L.A. and Whitesides, G.M. (2006) Multivalency in Ligand Design, Wiley‐VCH Verlag GmbH and Co. KGaA, pp. 11–53.
- 10 Ercolani, G. and Schiaffino, L. (2011) Allosteric, chelate, and interannular cooperativity: A mise au point. Angewandte Chemie International Edition, 50 (8), 1762–1768.
- 11 Hunter, C. and Anderson, H. (2009) What is cooperativity? Angewandte Chemie International Edition, 48 (41), 7488–7499.
- 12 Huskens, J., Mulder, A., Auletta, T., Nijhuis, C.A., Ludden, M.J.W., and Reinhoudt, D.N. (2004) A model for describing the thermodynamics of multivalent host‐guest interactions at interfaces. Journal of the American Chemical Society, 126 (21), 6784–6797.
- 13 Mulder, A., Huskens, J. and Reinhoudt, D.N. (2004) Multivalency in supramolecular chemistry and nanofabrication. Organic & Biomolecular Chemistry, 2, 3409–3424.
- 14 Krishnamurthy, V.M., Semetey, V., Bracher, P.J., Shen, N. and Whitesides, G.M. (2007) Dependence of effective molarity on linker length for an intramolecular protein‐ligand system. Journal of the American Chemical Society, 129 (5), 1312–1320.
- 15 Kitov, P.I. and Bundle, D.R. (2003) On the nature of the multivalency effect: A thermodynamic model. Journal of the American Chemical Society, 125 (52), 16 271–16 284.
- 16 Xu, H. and Shaw, D.E. (2016) A simple model of multivalent adhesion and its application to influenza infection. Biophysical Journal, 110 (1), 218–233.
- 17 Dubacheva, G.V., Curk, T., Mognetti, B.M., Auzély‐Velty, R., Frenkel, D. and Richter, R.P. (2014) Superselective targeting using multivalent polymers. Journal of the American Chemical Society, 136 (5), 1722–1725.
- 18 Dubacheva, G.V., Curk, T., Auzély‐Velty, R., Frenkel, D. and Richter, R.P. (2015) Designing multivalent probes for tunable superselective targeting. Proceedings of the National Academy of Sciences, 112 (18), 5579–5584.
- 19 Schmidt, N.W., Jin, F., Lande, R., Curk, T., Xian, W., Lee, C., Frasca, L., Frenkel, D., Dobnikar, J., Gilliet, M. and Wong, G.C.L. (2015) Liquid‐crystalline ordering of antimicrobial peptide‐DNA complexes controls TLR9 activation. Nature Materials, 14 (7), 696–700.
- 20 Mortell, K.H., Weatherman, R.V., and Kiessling, L.L. (1996) Recognition specificity of neoglycopolymers prepared by ring‐opening metathesis polymerization. Journal of the American Chemical Society, 118 (9), 2297–2298.
- 21 Carlson, C.B., Mowery, P., Owen, R.M., Dykhuizen, E.C. and Kiessling, L.L. (2007) Selective tumor cell targeting using low‐affinity, multivalent interactions. ACS Chemical Biology, 2 (2), 119–127.
- 22 Kiessling, L.L. and Grim, J.C. (2013) Glycopolymer probes of signal transduction. Chemical Society Reviews, 42, 4476–4491.
- 23 Albertazzi, L., Martinez‐Veracoechea, F.J., Leenders, C.M.A., Voets, I.K., Frenkel, D., and Meijer, E.W. (2013) Spatiotemporal control and superselectivity in supramolecular polymers using multivalency. Proceedings of the National Academy of Sciences, 110 (30), 12 203–12 208.
- 24 Rogers, W.B. and Crocker, J.C. (2011) Direct measurements of DNA‐mediated colloidal interactions and their quantitative modeling. Proceedings of the National Academy of Sciences, 108 (38), 15 687–15 692.
- 25 van der Meulen, S.A.J. and Leunissen, M.E. (2013) Solid colloids with surface‐mobile DNA linkers. Journal of the American Chemical Society, 135 (40), 15 129–15 134.
- 26 Wang, Y., Wang, Y., Zheng, X., Ducrot, É., Yodh, J.S., Weck, M. and Pine, D.J. (2015) Crystallization of DNA‐coated colloids. Nature Communications, 6, 7253 EP –.
- 27 Gao, H., Shi, W. and Freund, L.B. (2005) Mechanics of receptor‐mediated endocytosis. Proceedings of the National Academy of Sciences of the United States of America, 102 (27), 9469–9474.
- 28 Gernier, R.D., Curk, T., Dubacheva, G.V., Richter, R.P. and Mognetti, B.M. (2015) A new configurational bias scheme for sampling supramolecular structures. Journal of Chemical Physics, 141, 244909.
- 29 Tito, N.B. and Frenkel, D. (2014) Optimizing the selectivity of surface‐adsorbing multivalent polymers. Macromolecules, 47 (21), 7496–7509.
- 30 Varilly, P., Angioletti‐Uberti, S., Mognetti, B.M. and Frenkel, D. (2012) A general theory of DNA‐mediated and other valence‐limited colloidal interactions. The Journal of Chemical Physics, 137 (9), 094108.
- 31 Angioletti‐Uberti, S., Varilly, P., Mognetti, B.M., Tkachenko, A.V. and Frenkel, D. (2013) Communication: A simple analytical formula for the free energy of ligand–receptor‐mediated interactions. The Journal of Chemical Physics, 138 (2), 021102.
- 32 Angioletti‐Uberti, S., Varilly, P., Mognetti, B.M. and Frenkel, D. (2014) Mobile linkers on DNA‐coated colloids: Valency without patches. Physical Review Letters, 113, 128 303.
- 33 Tito, N.B., Angioletti‐Uberti, S., and Frenkel, D. (2016) Communication: Simple approach for calculating the binding free energy of a multivalent particle. The Journal of Chemical Physics, 144 (16), 161101.
- 34 Wang, S. and Dormidontova, E.E. (2012) Selectivity of ligand‐receptor interactions between nanoparticle and cell surfaces. Physical Review Letters, 109, 238 102.
- 35 Ling, X.Y., Reinhoudt, D.N., and Huskens, J. (2008) Reversible attachment of nanostructures at molecular printboards through supramolecular glue. Chemistry of Materials, 20 (11), 3574–3578.
- 36 Dubacheva, G.V., Heyden, A.V.D., Dumy, P., Kaftan, O., Auzély‐Velty, R., Coche‐Guerente, L. and Labbé, P. (2010) Electrochemically controlled adsorption of Fc‐functionalized polymers on beta‐CD‐modified self‐assembled monolayers. Langmuir, 26 (17), 13 976–13 986.
- 37 Dubacheva, G.V., Galibert, M., Coche‐Guerente, L., Dumy, P., Boturyn, D. and Labbe, P. (2011) Redox strategy for reversible attachment of biomolecules using bifunctional linkers. Chemical Communications, 47, 3565–3567.
- 38 Yamaguchi, H., Kobayashi, Y., Kobayashi, R., Takashima, Y., Hashidzume, A. and Harada, A. (2012) Photoswitchable gel assembly based on molecular recognition. Nature Communications, 3, 603.
- 39 Rose, S., Prevoteau, A., Elziere, P., Hourdet, D., Marcellan, A. and Leibler, L. (2014) Nanoparticle solutions as adhesives for gels and biological tissues. Nature, 505 (7483), 382–385.
- 40 Duncan, G.A. and Bevan, M.A. (2015) Computational design of nanoparticle drug delivery systems for selective targeting. Nanoscale, 7, 15 332–15 340.
- 41 Satav, T., Huskens, J. and Jonkheijm, P. (2015) Effects of variations in ligand density on cell signaling. Small, 11 (39), 5184–5199.
- 42 Grove, J. and Marsh, M. (2011) The cell biology of receptor‐mediated virus entry. The Journal of Cell Biology, 195 (7), 1071–1082.
- 43 Duke, T.A.J. and Bray, D. (1999) Heightened sensitivity of a lattice of membrane receptors. Proceedings of the National Academy of Sciences, 96 (18), 10 104–10 108.
- 44 Monine, M.I., Posner, R.G., Savage, P.B., Faeder, J.R. and Hlavacek, W.S. (2010) Modeling multivalent ligand‐receptor interactions with steric constraints on configurations of cell‐surface receptor aggregates. Biophysical Journal, 98 (1), 48–56.
- 45 Wu, H. (2013) Higher‐order assemblies in a new paradigm of signal transduction. Cell, 153 (2), 287–292.
- 46 Collins, B.E. and Paulson, J.C. (2004) Cell surface biology mediated by low affinity multivalent protein‐glycan interactions. Current Opinion in Chemical Biology, 8 (6), 617–625.
- 47 De Jong, D.H., Schäfer, L.V., De Vries, A.H., Marrink, S.J., Berendsen, H.J.C. and Grubmüller, H. (2011) Determining equilibrium constants for dimerization reactions from molecular dynamics simulations. Journal of Computational Chemistry, 32 (9), 1919–1928.
- 48 Angioletti‐Uberti, S., Mognetti, B.M. and Frenkel, D. (2016) Theory and simulation of DNA‐coated colloids: a guide for rational design. Physical Chemistry Chemical Physics, 18, 6373–6393.