CHAPTER 13
SOLID PROPELLANTS
This is the second of four chapters dealing with solid propellant rocket motors. Here we describe several common solid rocket propellants, their principal categories, ingredients, hazards, manufacturing processes, and quality control. We also discuss liners and insulators, propellants for igniters, propellant tailoring, and propellants for gas generators.
Thermochemical analyses are needed to characterize the performance of any given propellant and specific methods are described in Chapter 5. Such analyses provide values for the effective average molecular mass, combustion temperature, average specific heat ratio, and characteristic velocity—these are all functions of propellant composition and chamber pressure. Specific impulses can also be computed for given nozzle configurations and exhaust conditions.
The term solid propellant has several connotations, including: (1) the rubbery or plastic‐like mixture of oxidizer, fuel, and other ingredients that have been processed (including curing) and constitute the finished grain; (2) the processed but uncured product; (3) a single ingredient, such as the fuel or the oxidizer. In this field, acronyms and chemical symbols are used indiscriminately as abbreviations for propellant and ingredient names; only some of these will be shown in this chapter.
13.1 CLASSIFICATION
Historically, the early rocket motor propellants used to be grouped into two classes: double‐base (DB)1 propellants were the first production propellants and subsequently the development of polymers as binders made the composite propellants feasible. Processed modern propellants are more finely classified as described below. Such classifications are helpful but they are neither rigorous nor complete. Sometimes the same propellant will fit into two or more classifications.
- Propellants are often tailored to and classified by specific applications, such as space launch booster propellants or tactical missile propellants, each having specific chemical ingredients, different burning rates, different physical properties, and different performance. Table 12–1 shows four rocket motor applications (each with somewhat different propellants), plus several gas generator applications and an artillery shell application. Propellants for rocket motors produce hot (over 2400 K) gases and are used for thrust, but gas generator propellants operate with lower‐temperature combustion gases (800 to 1200 K in order to use uncooled hardware) and are used to produce power, not thrust.
- Double‐base (DB)1 propellants form a homogeneous propellant grain, usually a nitrocellulose (NC)1—a solid ingredient that absorbs liquid nitroglycerine (NG), plus minor percentages of additives. The major ingredients are highly energetic materials and they contain both fuel and oxidizer. Both extruded double‐base (EDB) and cast double‐base (CDB) propellants have found extensive applications, mostly in small tactical missiles of older design. By adding crystalline nitramines (HMX or RDX)1 both performance and density can be improved; these are sometimes called cast‐modified double‐base propellants. Adding an elastomeric binder (rubber‐like, such as crosslinked polybutadiene) further improves the physical properties and allows more nitramine and thus increasing performance slightly. The resulting propellant is called elastomeric‐modified cast double‐base (EMCDB). These four classes of double‐base propellants have nearly smokeless exhausts. Adding some solid ammonium perchlorate (AP) and aluminum (Al) increases the density and the specific impulse slightly, but exhaust gases becomes smoky—such propellant is called composite‐modified double‐base propellant or CMDB.
Two operational systems produced by ATK that use double‐based propellants are the AGM‐114 Hellfire (which uses XLDB, a minimum smoke crosslinked propellant) and the Hydra 70 rocket (with a plateau burning propellant, see Fig. 12–6).
- Composite propellants form a heterogeneous propellant grain between oxidizer crystals and powdered fuel (usually aluminum) held together in a matrix of synthetic rubber (or plastic) binder, such as polybutadiene (HTPB).1 Composite propellants are cast from a mix of solid (AP crystals, Al powder) and liquid (HTPB, PPG)1 ingredients. The propellant is hardened by crosslinking or curing the liquid binder polymer with a small amount of curing agent, and curing it in an oven, where it becomes solid. In the past four decades composites have been the most commonly used class of propellant. Composites can be further subdivided:
- Conventional composite propellants, which usually contain between 60 and 72% AP as crystalline oxidizer, up to 22% Al powder as a metal fuel, and 8 to 16% of elastomeric binder (organic polymer) including its plasticizer.
- Modified composite propellant where an energetic nitramine (HMX or RDX) is added for obtaining some added performance and also a somewhat higher density.
- Modified composite propellant where an energetic plasticizer such as nitroglycerine (used in double‐base propellants) is added to give increased performance. Sometimes HMX is also added.
- High‐energy composite solid propellant (with added aluminum), where the organic elastomeric binder and the plasticizer are largely replaced by highly energetic materials and where some of the AP is replaced by HMX and RDX. Hexanitrohexaazaiso‐wurtzitane or CL‐20 is a recent propellant ingredient being used; it is produced outside of the United States (see Section 13–4 and Ref. 13–1). Some propellants are called elastomer‐modified cast double‐base propellants (EMCDB). Most are experimental propellants. Their theoretical specific impulse can be between 250 and 275 sec at standard conditions as explained below.
- Lower‐energy composite propellant, where ammonium nitrate (AN) is the crystalline oxidizer (not AP). These are used for gas generator propellants. When large amounts of HMX are added, they become minimum smoke propellants with fair performance.
- Propellants may also be classified by the smoke density in the exhaust plume as smoky, reduced smoke, or minimum smoke (essentially smokeless). Aluminum powder, a desirable fuel ingredient for performance, is oxidized to aluminum oxide during burning, which yields visible, small, solid smoky particles in the exhaust gas. Most composite propellants (e.g., AP) are also smoky. By replacing AP with HMX and RDX and by using energetic binders and plasticizers to compensate for eliminating aluminum, the amount of smoke may be considerably reduced in composite propellants. Carbon (soot) particles and metal oxides, such as zirconium oxide or iron oxide, are also visible in high enough concentrations. This is further discussed in Chapter 20.
- Safety ratings for detonation can distinguish propellants as a potentially detonable material (class 1.1) or as a nondetonable material (class 1.3), as described in Section 13.3. Examples of class 1.1 propellant are double‐base propellants and composite propellants containing a significant portion of a solid explosive (e.g., HMX or RDX), together with certain other ingredients.
- Propellants can be classified by some of the principal manufacturing processes used. A cast propellant is made by mechanically mixing solid and liquid ingredients, followed by casting and curing; it is the most common process for composite propellants. Curing of many cast propellants takes place through a chemical reaction between binder and curing agent at above ambient temperatures (45 to 150°C); however, there are some that can be cured at ambient temperatures (20 to 25°C) or hardened by nonchemical processes such as crystallization. Propellants can also be made by a solvation process (dissolving a plasticizer in a solid pelletized matrix, whose volume is expanded). Extruded propellants are made by mechanical mixing (rolling into sheets) followed by extrusion (pushing through a die at high pressure). Solvation and extrusion processes are applied primarily to double‐base propellants.
- Propellants have also been classified by their principal ingredient, such as the principal oxidizer (ammonium perchlorate propellants, ammonium nitrate propellants, or azide‐type propellants) or their principal binder or fuel ingredient, such as polybutadiene propellants or aluminized propellants. This classification of propellants by ingredients is further described later in Section 13.4 and Table 13–8.
- Propellants with toxic and nontoxic exhaust gases are discussed in more detail in Section 13.3.
- Experimental and/or production propellants. Such propellants are selected after extensive testing (preflight test, qualification tests) and demonstrated safety, life, and other essential properties. The culmination of any successful research and development (R&D) program is to have a propellant selected for production in a flight vehicle application.
Figures 13–1 and 13–2 show general regions for the specific impulse, burning rate, and density for the more common classes of propellants. The ordinate in these figures is an actual or estimated specific impulse at standard conditions (1000 psi chamber pressure and expansion to sea‐level pressure). These results do not reflect pressure drops in the chamber, nozzle erosion, or combustion losses and scaling assumptions. Composite propellants are shown to have a wide range of burning rates and densities; most of them have specific gravities between 1.75 and 1.81 and burning rates between 7 and 20 mm/sec. Composite propellants give higher densities, specific impulse, and a wider range of burning rates than others. Table 13–1 lists performance characteristics for several propellants. DB propellants and AN propellants have lower performance and density. Most composite propellants display similar performance and density but with a wider range of burning rates. The highest performance indicated is for a CMDB propellant whose ingredients are identified as DB/AP‐HMX/Al, though it is only 4% higher than the next.

Figure 13–1 Typical delivered specific impulse and burning rate for several solid propellant categories.
Adapted and reproduced from Ref. 13–2 with permission of the AIAA.
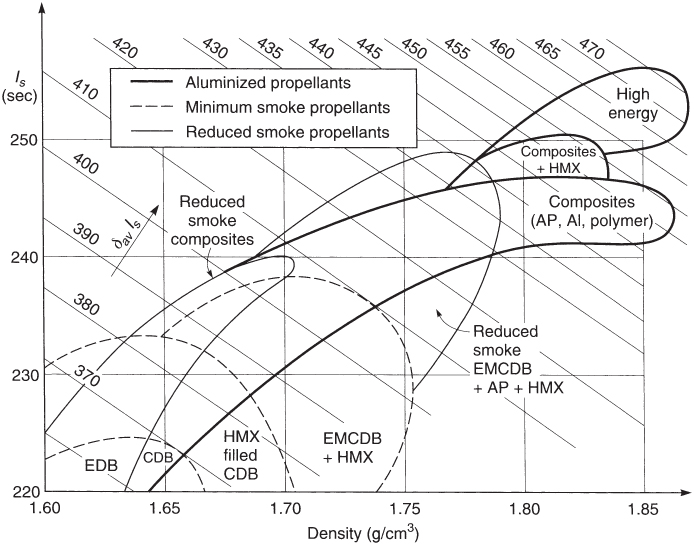
Figure 13–2 Typical delivered specific impulse and density‐specific impulse for several solid propellant categories with an expansion of 7 to 0.1 MPa.
Adapted and reproduced from Ref. 13–2 with permission of the AIAA.
Table 13–1 Characteristics of Some Operational Solid Propellants
| Flame Temperaturee | Density or Spec. Gravitye | Stress (psi)/Strain (%) | ||||||||||
| Propellant Typea | IS Range (sec)b | (°F) | (K) | (lbm/in3) | (SG) | Metal Content (mass %) | Burning Ratec,e (in./sec) | Pressure Exponente n | Hazard Classificationd | −60°F | +150°F | Typical Processing Method |
| DB | 220–230 | 4100 | 2550 | 0.058 | 1.61 | 0 | 0.05–1.2 | 0.30 | 1.1 | 4600/2 | 490/60 | Extruded |
| DB/AP/Al | 260–265 | 6500 | 3880 | 0.065 | 1.80 | 20–21 | 0.2–1.0 | 0.40 | 1.3 | 2750/5 | 120/50 | Extruded |
| DB/AP–HMX/Al | 265–270 | 6700 | 4000 | 0.065 | 1.80 | 20 | 0.2–1.2 | 0.49 | 1.1 | 2375/3 | 50/33 | Solvent cast |
| PVC/AP/Al | 260–265 | 5600 | 3380 | 0.064 | 1.78 | 21 | 0.3–0.9 | 0.35 | 1.3 | 369/150 | 38/220 | Cast or extruded |
| PU/AP/Al | 260–265 | 5700 | 3440 | 0.064 | 1.78 | 16–20 | 0.2–0.9 | 0.15 | 1.3 | 1170/6 | 75/33 | Cast |
| PBAN/AP/Al | 260–263 | 5800 | 3500 | 0.064 | 1.78 | 16 | 0.25–1.0 | 0.33 | 1.3 | 520/16 | 71/28 | Cast |
| (at −10°F) | ||||||||||||
| CTPB/AP/Al | 260–265 | 5700 | 3440 | 0.064 | 1.78 | 15–17 | 0.25–2.0 | 0.40 | 1.3 | 325/26 | 88/75 | Cast |
| HTPB/AP/Al | 260–265 | 5700 | 3440 | 0.067 | 1.86 | 4–17 | 0.25–3.0 | 0.40 | 1.3 | 910/50 | 90/33 | Cast |
| HTPE7/AP/Al | 248–269 | 5909 | 3538 | 0.07 | 1.70 | 0.4–0.7 | 0.50 | 1.3 | 174/44 | (77°F) | Cast | |
| PBAA/AP/Al | 260–265 | 5700 | 3440 | 0.064 | 1.78 | 14 | 0.25–1.3 | 0.35 | 1.3 | 500/13 | 41/31 | Cast |
| AN/Polymer | 180–190 | 2300 | 1550 | 0.053 | 1.47 | 0 | 0.06–0.5 | 0.60 | 1.3 | 200/5 | NA | Cast |
a Al, aluminum; AN, ammonium nitrate; AP, ammonium perchlorate; CTPB, carboxy‐terminated polybutadiene; DB, double‐base; HMX, cyclotetramethylene tetranitramine; HTPB, hydroxyl‐terminated polybutadiene; HTPE hydroxyl terminated polyether; PBAA, polybutadiene‐acrylic acid polymer; PBAN, polybutadiene–acrylic acid–acrylonitrile terpolymer; PU, polyurethane; PVC, polyvinyl chloride.
b At 1000 psia expanding to 14.7 psia, ideal or theoretical value at reference conditions.
c At 1000 psia.
d See hazard classification section.
e Is, flame temperature, density, burn rate, and pressure exponent will vary slightly with specific composition.
Data from Ref. 13–3, CPIAC, and Orbital ATK.
Several of the above listed classifications tend to be confusing. The term composite‐modified double‐base propellant (CMDB) has been used for a DB propellant, where some AP, Al, and binder are added; alternatively, the same propellant could be classified as a composite propellant to which some double‐base ingredients have been added.
A large variety of chemical ingredients and propellant formulations have been synthesized, analyzed, and tested in experimental rocket motors. A typical solid propellant has between 4 and 12 different ingredients and this chapter discusses about a dozen basic propellant types, but many other types are still being investigated. Table 13–2 presents some advantages and disadvantages of selected propellant classes. Representative formulations for three types of propellant are given in Table 13–3. In actual practice, each propellant manufacturer uses its own formulation and processing procedures. The exact percentages of ingredients, even for a given propellant such as PBAN, can not only vary among manufacturers but often vary from one rocket motor application to another. The practice of adjusting mass percentages together with adding or deleting one or more of the minor ingredients (additives) is known as propellant tailoring. Tailoring is the practice of taking an established propellant and changing it slightly to fit a new application, different processing equipment, altered motor ballistics, storage life, temperature limits, and/or even a change in ingredient source.
Table 13–2 Characteristics of Selected Propellants
| Propellant Type | Advantages | Disadvantages |
| Double base (extruded) | Modest cost; nontoxic clean exhaust, smokeless; good burn rate control; wide range of burn rates; simple well‐known process; good mechanical properties; low‐temperature coefficient; very low pressure exponent; plateau burning is possible | Freestanding grain requires structural support; low performance, low density; high to intermediate hazard in manufacture; can have storage problems with NG bleeding out; diameter limited by available extrusion presses; class 1.1a |
| Double base (castable) | Wide range of burn rates; nontoxic smokeless exhaust; relatively safe to handle; simple, well‐known process; modest cost; good mechanical properties; good burn rate control; low‐temperature coefficient; plateau burning can be achieved | NG may bleed out or migrate; high to intermediate manufacture hazard; low performance; low density; higher cost than extruded DB; class 1.1a |
| Composite modified double base or CMDB with some AP and Al | Higher performance; good mechanical properties; high density; less likely to have combustion stability problems; intermediate cost; good background experience | Complex facilities; some smoke in exhaust; high flame temperature; moisture sensitive; moderately toxic exhaust; hazards in manufacture; modest ambient temperature range; the value of n is high (0.8–0.9); moderately high temperature coefficient |
| Composite AP, Al, and PBAN or PU or CTPB binder | Reliable; high density; long experience background; modest cost; good aging; long cure time; good performance; usually stable combustion; low to medium cost; wide temperature range; low to moderate temperature sensitivity; good burn rate control; usually good physical properties; class 1.3 | Modest ambient temperature range; high viscosity limits at maximum solid loading; high flame temperature; toxic, smoky exhaust; some are moisture sensitive; some burn‐rate modifiers (e.g., aziridines) are carcinogens |
| Composite AP, Al, and HTPB binder; most common composite propellant | Slightly better solids loading % and performance than PBAN or CTPB; wide ambient temperature limits; good burn‐rate control; usually stable combustion; medium cost; good storage stability; wide range of burn rates; good physical properties; good experience; class 1.3 | Complex facilities; moisture sensitive; fairly high flame temperature; toxic, smoky exhaust |
| Modified composite AP, Al, PB binder plus some HMX or RDX | Higher performance; good burn‐rate control; usually stable combustion; high density; moderate temperature sensitivity; can have good mechanical properties | Expensive, complex facilities; hazardous processing; harder‐to‐control burn rate; high flame temperature; toxic, smoky exhaust; can be impact sensitive; can be class 1.1,a high cost; pressure exponent 0.5–0.7 |
| Composite with energetic binder and plasticizer such as NG, and with AP, HMX | Highest performance; high density; narrow range of burn rates | Expensive; limited experience; impact sensitive; high‐pressure exponent; class 1.1a |
| Modified double‐base with HMX | Higher performance; high density; stable combustion; narrow range of burn rates | Same as CMDB above; limited experience; most are class 1.1a; high cost |
| Modified AN propellant with HMX or RDX added | Fair performance; relatively clean; smokeless; nontoxic exhaust | Relatively little experience; can be hazardous to manufacture; need to stabilize AN to limit grain growth; low burn rates; impact sensitive; medium density; class 1.1 or 1.3a |
| Ammonium nitrate plus polymer binder (gas generator) | Clean exhaust; little smoke; essentially nontoxic exhaust; low‐temperature gas; usually stable combustion; modest cost; low‐pressure exponent | Low performance; low density; need to stabilize AN to limitgrain growth and avoid phase transformations; moisture sensitive; low burn rates |
a Class 1.1 and 1.3—see Section 13.3 on Hazard Classification.
Table 13–3 Representative Propellant Formulations
Source: Courtesy of Air Force Phillips Laboratory, Edwards, California.
| Double Base (JPN Propellant) | Composite (PBAN Propellant) | Composite Double Base (CMDB Propellant) | |||
| Ingredient | Mass % | Ingredient | Mass % | Ingredient | Mass % |
| Nitrocellulose | 51.5 | Ammonium perchlorate | 70.0 | Ammonium perchlorate | 20.4 |
| Nitroglycerine | 43.0 | Aluminum powder | 16.0 | Aluminum powder | 21.1 |
| Diethyl phthalate | 3.2 | Polybutadiene–acrylic acid–acrylonitrile | 11.78 | Nitrocellulose | 21.9 |
| Ethyl centralite | 1.0 | Epoxy curative | 2.22 | Nitroglycerine | 29.0 |
| Potassium sulfate | 1.2 | Triacetin | 5.1 | ||
| Carbon black | <1% | Stabilizers | 2.5 | ||
| Candelilla wax | <1% | ||||
New propellant formulations are typically developed using laboratory‐size mixers, curing ovens, and related equipment with the propellant mixers (1 to 5 liters), and operated by remote control for safety reasons. Process studies usually accompany the development of new propellant formulations to evaluate the “processability” and to guide the design of any special production equipment needed in preparing ingredients, mixing, casting, or curing it.
Historically, black or gun powder (a pressed mixture of potassium nitrate, sulfur, and an organic fuel such as ground peach stones) was the very first propellant to be used. Many other types of propellants ingredients have been used in experimental motors, including fluorine compounds, propellants containing powdered beryllium, boron, hydrides of boron, lithium, or beryllium, or new synthetic organic plasticizer and binder materials with azide or nitrate groups. Most of these have not yet been considered satisfactory or practical for production in rocket motors.
13.2 PROPELLANT CHARACTERISTICS
Propellant selection is critical to rocket motor design. Desirable propellant characteristics are listed below and further discussed in other parts of this book. Many requirements for particular solid propellant rocket motors will influence priorities for choosing these characteristics:
- High performance or high specific impulse; this implies a high gas temperature and/or low exhaust gas molecular mass.
- Predictable, reproducible, and initially adjustable burning rate to fit grain‐design needs and thrust‐time requirements.
- For minimum variations in thrust or chamber pressure during burning, both the pressure or burning rate exponent and the temperature coefficient should be small.
- Adequate physical properties (including bond strengths) over the intended operating temperature range with allowance for some degradation due to cumulative damage.
- High density (resulting in a small‐volume rocket motor).
- Predictable, reproducible ignition qualities (such as acceptable ignition overpressures).
- Desirable aging characteristics and long life. Aging and life predictions depend on the propellant's chemical and physical properties, cumulative damage criteria with load cycling (see Section 12.4) and thermal cycling, and from actual tests on propellant samples and test data from failed motors.
- Low moisture absorption, because moisture often causes chemical deterioration.
- Simple, reproducible, safe, low‐cost, controllable, and low‐hazard manufacturing.
- Guaranteed availability of all raw materials and purchased components over the production and operating life of the propellant, and acceptable control over undesirable impurities.
- Low technical risk, such as a favorable history of prior applications.
- Relative insensitivity to certain external energy stimuli as described Section 13.3, the hazards section.
- Nontoxic and noncorrosive exhaust gases, also called green exhausts.
- Not prone to combustion instability (see Chapter 14).
- Equivalent composition, performance and properties with every new propellant batch.
- No slow or long‐term chemical reactions or migrations between propellant ingredients or between propellant and insulator/liner.
Some of these desirable characteristics will also apply to all materials and purchased components used in solid rocket motors, such as the igniter, insulator, case, or safe‐and‐arm device but several of these characteristics can sometimes be in conflict with each other. For example, increasing the physical strength (more binder and or more crosslinker) will reduce propellant performance and density. So a modification of the propellant for one of these characteristics may cause changes in a few others.
Several illustrations will now be given on how characteristics of a propellant change when the concentration of one of its major ingredients is changed. Figure 13–3 shows calculated variations in combustion or flame temperature, average product gas molecular mass, and specific impulse as a function of oxidizer concentration for composite propellants that use a polymer binder [hydroxyl‐terminated polybutadiene (HTPB)] and various crystalline oxidizers; these results are taken from Ref. 13–4, based on a thermochemical analysis as explained in Chapter 5. The maximum values of ![]() and
and ![]() occur at approximately the same concentration of oxidizer. For practical reasons, this optimum percentage for AP (about 90 to 93%) and AN (about 93%) cannot be implemented because concentrations greater than about 90% total solids (including the aluminum and solid catalysts) cannot be processed in a mixer—a castable slurry that will flow into a mold requires more than 10 to 15% liquid content.
occur at approximately the same concentration of oxidizer. For practical reasons, this optimum percentage for AP (about 90 to 93%) and AN (about 93%) cannot be implemented because concentrations greater than about 90% total solids (including the aluminum and solid catalysts) cannot be processed in a mixer—a castable slurry that will flow into a mold requires more than 10 to 15% liquid content.

Figure 13–3 Variation of combustion temperature, average molecular mass of combustion gases, and theoretical specific impulse (at frozen equilibrium) as a function of oxidizer concentration for HTPB‐based composite propellants. Data are for a chamber pressure of 68 atm and nozzle exit pressure of 1.0 atm.
Reproduced from Ref. 13–4 with permission of the AIAA.
A typical composition diagram for a composite propellant is depicted in Fig. 13–4. It shows how the specific impulse varies with changes in the composition of the three principal ingredients: the solid AP, solid Al, and viscoelastic polymer binder.

Figure 13–4 Composition diagram of calculated specific impulse for an ammonium perchlorate–aluminum–polyurethane propellant (PU is a polyester binder) at standard conditions (1000 psi and expansion to 14.7 psi). The maximum value of specific impulse occurs at about 11% PU, 72% AP, and 17% Al.
Reproduced from Ref. 13–5 with permission of the American Chemical Society.
For DB propellants variations of ![]() and
and ![]() are shown in Fig. 13–5 as a function of nitroglycerine (NG) concentration. The theoretical maximum specific impulse occurs at about 80% NG. In practice, NG, which is a liquid, is seldom found in concentrations over 60% because its physical properties are poor at the higher concentrations. Other major solid or soluble ingredients are also needed to make a usable DB propellant.
are shown in Fig. 13–5 as a function of nitroglycerine (NG) concentration. The theoretical maximum specific impulse occurs at about 80% NG. In practice, NG, which is a liquid, is seldom found in concentrations over 60% because its physical properties are poor at the higher concentrations. Other major solid or soluble ingredients are also needed to make a usable DB propellant.

Figure 13–5 Specific impulse and flame temperature versus nitroglycerine (NG) concentration of double‐base propellants.
Reproduced from Ref. 13–4 with permission of the AIAA.
For CMDB propellants the addition of either AP or a reactive nitramine such as RDX allows for higher ![]() than with ordinary DB (where AP or RDX percent is zero), as shown in Fig. 13–6. Both AP and RDX greatly increase the flame temperature and thus make the effects of heat transfer more critical. The maximum values of
than with ordinary DB (where AP or RDX percent is zero), as shown in Fig. 13–6. Both AP and RDX greatly increase the flame temperature and thus make the effects of heat transfer more critical. The maximum values of ![]() occur at about 50% AP and at 100% RDX (an impractical propellant concentration because it cannot be manufactured and will not have reasonable physical properties). At high concentrations of AP or RDX, the exhaust gases contain considerable H2O and O2 (as shown in Fig. 13–7); these enhance erosion rates in carbon‐containing insulators or nozzle materials. In Fig. 13–7, the toxic HCl gas is present in concentrations between 10 and 20%, but in practical propellants it can seldom exceed 14%.
occur at about 50% AP and at 100% RDX (an impractical propellant concentration because it cannot be manufactured and will not have reasonable physical properties). At high concentrations of AP or RDX, the exhaust gases contain considerable H2O and O2 (as shown in Fig. 13–7); these enhance erosion rates in carbon‐containing insulators or nozzle materials. In Fig. 13–7, the toxic HCl gas is present in concentrations between 10 and 20%, but in practical propellants it can seldom exceed 14%.

Figure 13–6 Specific impulse and flame temperature versus AP or RDX concentration of AP–CMDB propellants.
Reproduced from Ref. 13–4 with permission of the AIAA.

Figure 13–7 Calculated combustion products of composite propellant with varying amounts of AP or RDX.
Adapted from Chapter 1 of Ref. 13–4 with permission of the AIAA.
Nitramines such as RDX or HMX contain relatively few oxidizing radicals and the binder surrounding the nitramine crystals cannot be fully oxidized. As the binder decomposes at the combustion temperature, it releases gases rich in hydrogen and carbon monoxide (which reduce the molecular mass), cooling the gas mixture to lower combustion temperatures. The exhaust gases of AP‐based and RDX‐based CMDB propellant are shown in Fig. 13–7 where it can be seen that solid carbon particles disappear when the RDX content is above 85%.
13.3 HAZARDS
With proper precautions, training and equipment, all common propellants can be manufactured, handled, and fired safely. It is necessary and imperative to fully understand all hazards and methods for preventing dangerous situations from arising. Each material has its own set of hazards; some of the more common ones are described briefly below and also in Refs. 13–6 and 13–7. Not all apply to every propellant.
Inadvertent Ignition
If a rocket motor is unexpectedly ignited and starts combustion, the very hot exhaust gases may cause burns and local fires, or ignition of adjacent rocket motors. Unless the motor is constrained or fastened down, its thrust will accelerate it to unanticipated high velocities or erratic flight paths that can cause much severe damage. Its exhaust cloud can be toxic and corrosive. Inadvertent ignitions may be caused by the following effects:
- Stray or induced currents that activate the igniter.
- Electrostatic charging causing unintended sparks or arc discharges.
- Fires excessively heating rocket motor exteriors, raising the solid propellant temperature above its ignition point.
- Impacts (bullet penetration or dropping the rocket motor onto a hard surface).
- Energy absorption from prolonged mechanical vibrations that overheat the propellant (e.g., transport over rough roads).
- Radiation from nuclear explosions.
An electromechanical system called safe and arm system is usually included to prevent stray currents from activating the igniter. It averts ignition induced by currents in other wires of the vehicle, radar‐ or radio‐frequency‐induced currents, electromagnetic surges, or pulses from a nuclear bomb explosion. It prevents any electric currents from reaching the igniter circuit during its “unarmed” condition. When in the “arm” position, it accepts and transmits a start signal to the igniter.
Electrostatic discharges (ESD) may be caused by lightning, friction in insulating materials, or by the moving separation of two insulators. The buildup of high electrostatic potentials (thousands of volts) may, upon discharge, allow rapid increases in electric current, which in turn may lead to arcing or exothermic reactions along the current's path. For this reason all propellants, liners, or insulators need to have sufficient electric conductivity to prevent any buildup of such an electrostatic charge. The well‐known inadvertent ignition of a Pershing ground‐to‐ground missile is believed to have been caused by an electrostatic discharge while in the transporter‐erector vehicle. ESD capabilities depend on materials, their surface and volume resistivities, dielectric constants, and the breakdown voltages.
Viscoelastic propellants are excellent absorbers of vibration energy and can become locally hot when oscillating for extensive periods at particular frequencies. This can happen in designs where a segment of the grain is not well supported and is free to vibrate at its natural frequencies. Because propellants can be accidentally ignited by extraneous means (such as mechanical friction or bullet impacts, or accidentally dropping a rocket motor) standard tests have been developed to measure the propellant's resistance to such energy inputs. Considerable effort is spent in developing new propellants resistant to these energy inputs.
Aging and Useful Life
This topic is briefly discussed in the section on Structural Design in the previous chapter. The aging of a propellant can be measured with test motors and propellant sample tests when the loading history during the life of the motor can be correctly anticipated. It has then been possible to estimate and predict the useful shelf or storage life of a rocket motor (see Refs. 13–7 and 13–8). When changes in physical properties, caused by estimated thermal or mechanical load cycles (cumulative damage), reduce the safety margin on stresses and/or strains to a danger point, the rocket motor is no longer considered to be safe to be ignited or operated. Once this age limit or its predicted, weakened condition is reached, the rocket motor has a high probability of failure and should be removed from any ready inventory and the aged propellant removed and replaced.
The life of a particular motor depends on its propellant composition, the frequency and magnitude of imposed loads or strains, and the design among other factors. Typical life values range from 5 to 25 years. Shelf life can usually be increased by increasing the physical strength of the propellants (e.g., by increasing the amount of binder), selecting chemically compatible, stable ingredients with minimal long‐term degradation, and/or by minimizing the vibration loads, temperature limits, or number of cycles (i.e., controlling the storage and transport environments).
Case Overpressure and Failure
A rocket motor case will break and/or explode during operation when the chamber pressure exceeds the case's burst pressure. The release of high‐pressure gases can cause explosions where motor pieces are thrown out into adjacent areas. Any sudden depressurization from chamber pressure to ambient pressure (which is usually below the deflagration limit) would normally stop the burning in a class 1.3 propellant (see Hazard Classification). Large pieces of unburned propellant are often found after violent case bursts. Case overpressure rocket motor failure may be caused by one of the following:
- The grain is overaged, porous, or severely cracked and/or has major unbonded areas due to severe accumulated damage.
- There have been a significant chemical changes in the propellant due to migration or slow, low‐order chemical reactions; these can reduce the allowable physical properties, weakening the grain, so that it will crack or cause unfavorable increases in the burning rate. In some cases chemical reactions create gaseous products, which produce many small voids and raise the pressure in sealed stored rocket motors.
- The rocket motor has not properly been manufactured. Obviously, careful fabrication and inspection are a must.
- The motor has been damaged. For example, a nick or dent in the case caused by improper handling will reduce the case strength. This can be prevented by careful handling and repeated inspections.
- An obstruction plugs the nozzle (e.g., a loose large piece of insulation) causing a rapid increase in chamber pressure.
- In propellants that contain hygroscopic ingredients, moisture absorption can degrade strength and strain capabilities by factors of 3 to 10. Rocket motors are routinely sealed to prevent humid air access.
Detonation versus Deflagration
When the burning rocket motor propellant is overpressurized, it may either continue to deflagrate (burn) or detonate (explode violently), as described in Table 13–4. In a detonation, the chemical reaction energy of the entire grain is released in a very short time (microseconds), and in effect it becomes an exploding bomb. Detonations happen with some propellants that contain certain ingredients (e.g., nitroglycerine or HMX as described later in this chapter). Proper designs, correct manufacture, and safe handling and operating procedures are essential in order to minimize or totally avoid detonations.
Table 13–4 Comparison of Burning (Deflagration) and Detonation
| Characteristic | Burning with Air | Deflagration within Rocket Motors | Detonation of Rocket Motor |
| Typical material | Coal dust and air | Propellant, no air | Rocket propellant or explosives |
| Common means of initiating reaction | Heat | Heat | Shock wave; sudden pressure rise plus heat |
| Linear reaction rate (m/sec) | 10−6 (subsonic) | 0.2 to 5 × 10−2 (subsonic) | 2 to 9 × 103 (supersonic) |
| Shock waves | No | No | Yes |
| Time for completing reaction (sec) | 10−1 | 10−2–10−3 | 10–6 |
| Maximum pressure [MPa (psi)] | 0.07–0.14 (10–20) | 0.7–100 (100–14,500) | 7000–70,000 (106−107) |
| Process limitation | By heat transfer at burning surface | Strength of case | By physical and chemical properties of material, (e.g., density, composition) |
| Increase in burning rate can result in: | Potential furnace failure | Overpressure and explosive failure of motor case | Motor case failure and violent rapid explosion of all the propellant |
| Remainder | Unburnt coal dust pockets may continue to burn | After case failure, unused propellant pieces will usually stop burning | No propellant remainder to burn |
| Classification | None | Class 1.3 | Class 1.1 |
Any given propellant material may either burn or detonate depending on its chemical formulation, physical properties (such as density or porosity) the type and intensity of the initiation, the degree of confinement, and the geometric characteristics of the rocket motor. It is also possible for certain burning propellants to change suddenly from an orderly deflagration to a detonation. A simplified explanation of this transition is as follows: normal burning starts at the rated chamber pressure; then hot gases penetrate some existing but unknown pores or small cracks in the unburned propellant, where gas confinement causes the pressure to become locally very high; the combustion front then attains shock wave speeds with a low‐pressure differential and then it accelerates further to the strong, fast, high‐pressure shock wave, characteristic of detonations. The degree and rigidity of the motor geometric confinement and some scale factor (e.g., larger‐diameter grain) influence the severity and occurrence of detonations.
Hazard Classification
Propellants that may transition from deflagration to detonation are considered more hazardous and are usually designated as class 1.1‐type propellants. Class 1.3 propellants will not detonate even if while burning the case bursts as the chamber pressure becomes too high. The required tests and rules for determining this hazard category are treated in Ref. 13–9. Propellant samples are subjected to various tests, including impact tests (dropped weight) and card gap tests (which determine the force needed to initiate a propellant detonation when a sample is subjected to a blast from a known booster explosive). Even when the case bursts violently with a class 1.3 propellant, much of the remaining unburnt propellant is ejected and then usually stops burning. With a class 1.1 propellant, a powerful detonation can sometimes ensue, which rapidly gasifies all the remaining propellant, and is much more powerful and destructive than the bursting of the case under high pressure. Unfortunately, the term explosion has been used to describe both case bursting with its remaining unburned propellant fragmentation, and also the higher rate of energy release in a detonation which leads to more rapid and much more energetic fragmentation of the rocket motor.
The Department of Defense (DOD) classification of 1.1 or 1.3 determines the method of labeling and the cost of shipping rocket propellants, loaded military missiles, explosives, or ammunitions; it also defines required limits on propellant amounts that may be stored or manufactured in any one site and the minimum separation distance of that site to the next building or site. The DOD system (Ref. 13–9) is the same as that which has been used by the United Nations.
Insensitive Munitions
Any accidental ignition or otherwise unplanned rocket or military missile operation or explosion may cause severe damage to equipment and injure or kill personnel. This may be avoided or minimized by making rocket motor designs and their propellants insensitive to a variety of energetic stimuli. The worst scenario is propellant detonation, releasing all the propellant's energy explosively, and this scenario must be avoided at all cost. Missiles and their rocket motors must undergo a series of prescribed tests to determine their resistance to inadvertent ignition using the most likely energy inputs during any possible battle situation. Table 13–5 describes a series of tests called out in military specifications, which are detailed in Refs. 13–3 and 13–10. Other tests besides those listed in Table 13–5 are sometimes needed, such as friction tests and drop tests. A threat hazard assessment must be made prior to any such tests, to evaluate the logistic and operational threats during the missile's life cycle. This may result in modifications to the test setups, changes to acceptance criteria, and/or the skipping of some such tests.
Table 13–5 Typical Testing for Insensitivity of Rockets and Missiles
| Test | Description | Criteria for Passing |
| Fast cook‐off | Build a fire (of jet fuel or wood) underneath the missile or its rocket motor | No reaction more severe than burning propellant |
| Slow cook‐off | Gradual heating (6°F/hr) to failure | Same as above |
| Bullet impact | One to three 50‐caliber bullets fired at short intervals | Same as above |
| Fragment impact | Small high‐speed steel fragment | Same as above |
| Sympathetic detonation | Detonation from an adjacent similar motor or a nearby specific munition | No detonation of test motor |
| Shaped explosive charge impact | Blast from specified shaped charge in specified location | No detonation |
| Spall impact | Several high‐speed spalled fragments from a steel plate which is subjected to a shaped charge | Fire, but no explosion or detonation |
In all prescribed tests, the missiles together with their rocket motors are destroyed. If the rocket motor should detonate (an unacceptable result), the motor must be redesigned and/or undergo a change in propellant. There are some newer propellants that are more resistant to external stimuli and are therefore preferred for tactical missile applications, even though there may be a penalty in propulsion performance. If explosions (not detonations) occur, it may be possible to redesign the rocket motor and mitigate the explosion effects (make it less violent). For example, motor cases can have a provision for venting prior to explosion. Changes to shipping containers can also mitigate some of these effects. If the result is a fire (a sometimes acceptable result), it should be confined to the particular grain or rocket motor. Under some circumstances a burst failure of the case may also be acceptable. In the past decade a new class of insensitive propellants has been developed with new types of binders. Their purpose remains to minimize any drastic consequences (e.g., detonations) when the rocket motor is exposed to various unexpected energetic stimuli, such as external fires, and impact or pressure waves (see Table 13–5). Recently, insensitive propellants have been selected for some military missions, reflecting an important milestone in their development. The leading example is a recent composite propellant with HTPE (hydroxyl‐terminated polyether) as the binder, see Ref. 13–11. HTPE has been qualified and applied to the MK 134 rocket motor for the ship‐launched Evolved Sea Sparrow Missile (ESSM). This motor is co‐manufactured by ATK in the United States and NAMMO Raufoss in Norway for member nations of the NATO Sea Sparrow Consortium. A relatively insensitive propellant has also been developed in France, namely, a nonmigrating ferrocene‐grafted HTPB binder called Butacene. It has been qualified for a few systems in the United States and other countries; see Ref. 13–11.
Upper Pressure Limit
When the pressure‐rise rate and the absolute pressure become sufficiently high (as in some impact tests or in the high acceleration of a gun barrel), some propellants will detonate. For many propellants these pressures are above approximately 1500 MPa or 225,000 psi, but for others they can be lower (as low as 300 MPa or 45,000 psi). The values quoted represent defined upper pressure limits beyond which such a propellant should not operate.
Toxicity
Many solid propellants do not have any significant toxicity problem. A number of propellant ingredients (e.g., some crosslinking agents and burning rate catalysts) and a few of the plastics used in fiber‐reinforced cases can be dermatological or respiratory toxins; a few are carcinogens or suspected carcinogens. They, and the mixed uncured propellant containing these materials, must be handled carefully to prevent operator exposure. This means using gloves, face shields, good ventilation, and, with some high‐vapor‐pressure ingredients, gas masks. Usually, the finished or cured grain or motor is not toxic.
Exhaust plume gases can be very toxic if they contain beryllium or beryllium oxide particles, chlorine gas, hydrochloric acid gas, hydrofluoric acid gas, or some other fluorine compounds. When an ammonium perchlorate oxidizer is used, the exhaust gas may contain up to about 14% hydrochloric acid, which is a toxic gas. For large rocket motors this can mean many tons of highly toxic gas. Test and launch facilities for rockets with toxic plumes require very special precautions and occasionally decontamination processes, as explained in Chapter 21.
Safety Rules
Among the most effective ways to control hazards and prevent accidents are: (1) to acquaint personnel of the hazards of each propellant being handled by teaching them how to properly avoid hazardous conditions and to prevent accidents and how to recover from them; (2) to design the rocket motors, their fabrication as well as test facilities and equipment to be as safe as possible; and (3) to institute and enforce rigid safety rules during design, manufacture, and operation. There are many such rules. Examples include avoiding smoking (and matches) in areas where there are propellants or loaded motors, wearing spark‐proof shoes and using spark‐proof tools, shielding all electrical equipment, providing a water‐deluge fire extinguishing system in test facilities to cool motors or extinguish burning, and/or proper grounding of all electrical equipment and items that could build up static electrical charges.
13.4 PROPELLANT INGREDIENTS
A number of relatively common propellant ingredients are listed in Table 13–6 for double‐base propellants and for composite‐type solid propellants in Table 13–7. They are categorized by major function, such as oxidizer, fuel, binder, plasticizer, curing agent, and so on, and each category is briefly described later in this section. However, several ingredients can have more than one function. These lists are not complete because over 200 other ingredients have been tried in experimental rocket motors. Most ingredients in Table 13–6 have a shelf life of over 30 years.
Table 13–8 shows a classification for modern propellants (including some new types that are still in the experimental phase) according to their binders, plasticizers, and solid ingredients; these solids may be an oxidizer, a solid fuel, or a combination or a compound of both.
Ingredient properties and impurities both may have a profound effect on propellant characteristics. A seemingly minor change in one ingredient can cause measurable changes in ballistic properties, physical properties, migration, aging, and/or ease of manufacture. When the propellant's performance or ballistic characteristics have tight tolerances, ingredient purity and properties must conform to equally tight tolerances and careful handling (e.g., no exposure to moisture). In the remainder of this section, a number of the important ingredients, grouped by function, are briefly discussed.
Inorganic Oxidizers
Some thermochemical properties of several oxidizers and radical‐containing oxygen compounds are listed in Table 13–9. Listed values depend on the chemical nature of each ingredient.
Table 13–6 Typical Ingredients of Double‐Base (DB) Propellants and Composite‐Modified Double‐Base (CMDB) Propellants

|
Table 13–7 Typical Ingredients of Composite Solid Propellants

|

|
Table 13–8 Classification of Solid Rocket Propellants Used in Flying Vehicles According to Their Binders, Plasticizers, and Solid Ingredients
| Designation | Binder | Plasticizer | Solid Oxidizer and/or Fuel | Propellant Application |
| Double base, DB | Plasticized NC | NG, TA, etc. | None | Minimum signature and smoke |
| CMDBa | Plasticized NC | NG, TMETN, TA, BTTN, etc. | Al, AP, KP | Booster, sustainer, and spacecraft |
| Same | Same | HMX, RDX, AP | Reduced smoke | |
| Same | Same | HMX, RDX, azides | Minimum signature, gas generator | |
| EMCDBa | Plasticized NC + elastomeric polymer | Same | Like CMDB above, but generally superior mechanical properties with elastomer added as binder | |
| Polybutadiene | HTPB | DOA, IDP, DOP, DOA, etc. | Al, AP, KP | Booster, sustainer, or spacecraft; used extensively in many applications |
| HTPB | DOA, IDP, DOP, DOA, etc. | AN, HMX, RDX, some AP | Reduced smoke, gas generator | |
| CTPB, PBAN, PBAA | All like HTPB above, but somewhat lower performance due to higher processing viscosity and consequent lower solids content. Still used in applications with older designs. | |||
| Polybutadyene with HMX or RDX | HTPB | DOA, IDP, DOP, DOA, etc. | AP, Al, HMX, or RDX | High energy vehicles |
| Polyether and polyesters | PEG, PPG, PCP, PGA, HTPE,b and mixtures | DOA, IDP, TMETN, DEGDN, etc. | Al, AP, KP, HMX, BiO3 | Booster, sustainer, or spacecraft |
| Energetic binder (other than NC) | GAP, PGN, BAMO/ NMMO, BAMO/AMMO | TMETN, BTTN, etc. GAP‐azide, GAP‐nitrate, NG | Like polyether/polyester propellants above, but with slightly higher performance. Experimental propellant. | |
a CMDB, composite‐modified double‐base; EMCDB, elastomer‐modified cast double‐base. For definition of acronyms and abbreviations of propellant ingredients see Tables 13–2 and 13–3.
b HTPE, hydroxyl‐terminated polyether, binder for propellant developed for Insensitive Munitions by Orbital ATK.
Table 13–9 Comparison of Crystalline Oxidizers
| Oxidizer | Chemical Symbol | Molecular Mass (kg/kg‐mol) | Density (kg/m3) | Total Oxygen Content (mass%) | Available Oxygen Content (mass%) | Remarks |
| Ammonium perchlorate | NH4ClO4 | 117.49 | 1949 | 54.5 | 34.0 | Low n, low cost, readily available, high performance |
| Potassium perchlorate | KClO4 | 138.55 | 2519 | 46.2 | 40.4 | Low burning rate, medium performance |
| Sodium perchlorate | NaClO4 | 122.44 | 2018 | 52.3 | Hygroscopic, high performance, bright flame | |
| Ammonium nitrate | NH4NO3 | 80.0 | 1730 | 60.0 | 20.0 | Smokeless, medium performance, low cost |
Ammonium perchlorate (NH4ClO4) is the most widely used crystalline oxidizer in solid propellants. It dominates the solid oxidizer field because of its desirable characteristics that include compatibility with other propellant materials, good performance, satisfactory quality and uniformity, low impact and friction sensitivities, and good availability. Other solid oxidizers, particularly ammonium nitrate and potassium perchlorate, have occasionally been used in production rockets but are now replaced by more modern propellants containing ammonium perchlorate. None of the many other oxidizer compounds that were investigated during the 1970s have reached production status.
The oxidizing potential of the perchlorates is generally high, which makes this material suited for high‐specific‐impulse propellants. Both ammonium and potassium perchlorate are only slightly soluble in water, a favorable propellant trait. All the perchlorate oxidizers generate gaseous hydrogen chloride (HCl) and other toxic and corrosive chlorine compounds in their reaction with fuels. In the firing of rockets, care is required, particularly for very large rockets, to safeguard operating personnel and/or communities in the path of exhaust gas clouds. Ammonium perchlorate (AP) is supplied in the form of small white crystals. Particle size and shape influences their manufacturing process and propellant burning rate. Therefore, close control of crystal sizes and size distributions present in a given quantity or batch is required. AP crystals are rounded (to nearly ball shape) to make for easier mixing than with sharp, fractured crystals. From the factory, they come in sizes ranging from about 600 µm (1 µm = 10−6 m) diameter to about 80 µm. Diameters below about 40 µm are considered more hazardous (e.g., can easily be ignited and sometimes detonate) and are not shipped; instead, propellant manufacturers take the larger crystals and grind them (at a motor factory) to smaller sizes (down to 2 µm) just before they are incorporated into a propellant.
Inorganic nitrates are relatively low‐performance oxidizers compared with perchlorates. However, ammonium nitrate (AN) is used in some applications because of its very low cost as well as its smokeless and relatively nontoxic exhaust. Its principal use is in low‐burning‐rate, low‐performance rocket and gas generator applications. Ammonium nitrate changes its crystal structure at several phase transformation temperatures. These changes cause slight changes in volume. One phase transformation at 32°C causes about a 3.4% change in volume. Repeated temperature cycling through this transition temperature creates tiny voids in the propellant, resulting in growth in the grain and a change in physical and/or ballistic properties. The addition of a small amount of stabilizer such as nickel oxide (![]() ) or potassium nitrate (
) or potassium nitrate (![]() ) appears to change this transition temperature to above 60°C, a high enough value so that normal ambient temperature cycling will no longer cause recrystallization (Refs. 13–12 and 13–13). With such an additive, AN is known as phase‐stabilized ammonium nitrate (PSAN). Moreover, AN is hygroscopic and any moisture absorption will degrade and destabilize propellants containing AN.
) appears to change this transition temperature to above 60°C, a high enough value so that normal ambient temperature cycling will no longer cause recrystallization (Refs. 13–12 and 13–13). With such an additive, AN is known as phase‐stabilized ammonium nitrate (PSAN). Moreover, AN is hygroscopic and any moisture absorption will degrade and destabilize propellants containing AN.
Fuels
This section discusses solid fuels of which powdered spherical aluminum is the most common. It consists of small spherical particles (5 to 60 µm diameter) and is used with a wide variety of composite and composite‐modified double‐base propellant formulations, usually constituting 14 to 20% of the propellant by weight. Small aluminum particles can burn in air and aluminum powder is mildly toxic if inhaled. During rocket combustion this fuel is oxidized to aluminum oxide. Such oxide particles tend to agglomerate and form larger particles. Aluminum increases the heat of combustion, the propellant density, the combustion temperature, and thus the specific impulse. The oxide starts in liquid droplet form during combustion but solidifies in the nozzle as the gas temperature drops. When in the liquid state, the oxide can form a molten slag, which can accumulate in pockets (e.g., around an improperly designed submerged nozzle), thus adversely affecting the vehicle's mass ratio. It also can deposit on walls inside the combustion chamber, as described in Refs. 13–14 and 13–15. Reference 13–16 addresses important issues related to adding aluminum as fuel to solid propellants,
Boron is a high‐energy fuel that is lighter than aluminum and has a high melting point (2304°C). It is difficult to burn with high efficiency in combustion chambers of practical lengths. However, it can be efficiently oxidized if the boron particle size is sufficiently small. Boron has been used advantageously as a propellant in a rocket combined with an air‐burning engine, where there is adequate combustion volume and atmospheric oxygen.
Beryllium burns much more easily than boron, improving the specific impulse of a solid propellant motor by about 15 sec, but both beryllium and its highly toxic oxide powders are absorbed by animals and humans when inhaled. The technology with composite propellants using powdered beryllium fuel has been experimentally proven, but its severe toxicity makes any earth‐bound application unlikely.
Binders
In composite propellants, binders provide the structural matrix or glue with which the solid granular ingredients are held together. In raw form, these materials are liquid prepolymers or monomers. Polyethers, polyesters, and poly‐butadienes have been used (see Tables 13–6 and 13–7). After they are mixed with their solid ingredients, cast and cured, they form a hard rubber‐like material that constitutes the grain. Polyvinylchloride (PVC) and polyurethane (PU) (Table 13–1) were used 50 years ago and are still used in a few rocket motors, mostly of old design. Binder materials also act as fuels for solid propellant rockets and are oxidized in the combustion process. The binding ingredient, typically a polymer of one type or another, has a primary effect on motor reliability and its mechanical properties, propellant processing complexity, storability, aging, and costs. Some polymers undergo complex chemical reactions, crosslinking, and branch chaining during curing of the propellant. HTPB has been a favorite binder in recent years, because it allows somewhat higher solids fraction (88 to 90% of AP and Al), a small performance improvement, and relatively good physical properties at the temperature limits. Several common binders are listed in Tables 13–6 , and 13–7. Elastomeric binders can be added to plasticized double‐base‐type nitrocellulose to improve its physical properties. Polymerization occurs when the binder monomer and its crosslinking agent react (beginning in the mixing process) to form long chains and complex three‐dimensional polymers. Other types of binders, such as PVC, cure or plasticize without molecular reactions (see Refs. 13–4, 13–5, and 13–15). Often called plastisol‐type binders, these form very viscous dispersions of powdered polymerized resins in nonvolatile liquids; they polymerize slowly.
Burning‐Rate Modifiers
A burning‐rate catalyst or burning‐rate modifier helps to accelerate or decelerate combustion at the burning surface and thus increases or decreases the propellant burning rate. It permits tailoring of the burning rate to fit a specific grain design and thrust–time curve. Several are listed in Tables 13–6 and 13–7. Some, like iron oxide or lead stearate, increase the burning rate; however, others, like lithium fluoride, will reduce the burning rate of some composite propellants. Inorganic catalysts do not contribute to the combustion energy, but consume energy by being heated to combustion temperatures. These modifiers are effective because they can change combustion mechanisms, these are mentioned in Chapter 14 (examples of modifiers that change the burning rate of composite propellants are given in Chapter 2 of Ref. 13–4).
Burning rate is defined in Section 12.1; it is a strong function of the propellant composition. For composite propellants it may be increased by changing the propellant characteristics as follows:
- Introduce a burning rate catalyst, or burning rate modifier (0.1 to 3.0% of propellant) or increase percentage of existing catalyst.
- Decrease the oxidizer particle size.
- Increase oxidizer percentage.
- Increase the heat of combustion of the binder and/or the plasticizer.
- Imbed high‐conductivity wires or metal staples in the propellant.
Plasticizers
A plasticizer is usually a relatively low‐viscosity, liquid organic ingredient, which also acts as fuel. It is added to improve the elongation of the propellant at low temperatures and to improve its processing properties, such as lower viscosity for casting or longer pot life of the mixed but uncured propellants. The plasticizers listed in Tables 13–6, 13–7 and 13–8 represent several examples.
Curing Agents or Crosslinkers
A curing agent or crosslinker causes prepolymers to form longer chains of larger molecular mass and interlocks between chains. Even though these curing agents are present in small amounts (0.2 to 3%), a minor change in their percentage can have a major effect on the propellant physical properties, manufacturability, and aging. They are used primarily with composite propellants and cause the binder to solidify and become hard. Several curing agents are listed in Table 13–7.
Energetic Binders and Plasticizers
Energetic binders and/or plasticizers are used in lieu of the conventional organic materials. They contain oxidizing species (such as azides or organic nitrates) as well as other organic species. They add some energy to the propellant causing a modest increase in performance. They serve also as binders to hold other ingredients, or as energetic plasticizers liquid. They can self‐react exothermally and burn without a separate oxidizer. Glycidyl azide polymer (GAP) is an example of an energetic, thermally stable, hydroxyl‐terminated prepolymer that can be polymerized. It has been used in experimental propellants. Other energetic binder or plasticizer materials are listed in Tables 13–6, 13–7, and 13–8.
Organic Oxidizers or Explosives
Organic oxidizers are highly energetic compounds with –NO2 radicals or other oxidizing fractions incorporated into their molecular structure. References 13–4 and 13–15 describe their properties, manufacture, and applications. They are used with high‐energy propellants and/or with smokeless propellants. They can be crystalline solids, such as the nitramines HMX or RDX, fibrous solids such as NC, or energetic plasticizer liquids such as DEGDN or NG. These materials can react or burn by themselves when initiated with enough activating energy and all may detonate under certain conditions. Both HMX and RDX are stoichiometrically balanced materials and their addition into either fuel or oxidizer reduces the values of T1 and Is. Therefore, when binder fuels are added to hold the HMX or RDX crystals in a viscoelastic matrix, it is also necessary to add an oxidizer such as AP or AN.
RDX and HMX are quite similar in structure and properties. Both are white crystalline solids that can be made in different sizes. For safety, they are shipped in a desensitizing liquid, which is removed prior to propellant processing. HMX has a higher density, a higher detonation rate, yields more energy per unit volume, and has a higher melting point than RDX. Also extensively used in military and commercial explosives are NG, NC, HMX, and RDX. To achieve higher performance or other desirable characteristics, HMX or RDX can be included in DB, CMDB, or composite propellants. The amount added can range up to 60% of the propellant. Processing propellant with these or similar ingredients can be hazardous and the necessary extra safety precautions make the processing more expensive.
Liquid nitroglycerine (NG) by itself is very sensitive to shock, impact, or friction. It is an excellent plasticizer for propellants when desensitized by the addition of other liquids (like triacetin or dibutyl phthalate) or by compounding it with nitrocellulose. It readily dissolves in many organic solvents, and in turn it acts as a solvent for NC and other solid ingredients (Ref. 13–15).
Nitrocellulose (NC) is a key ingredient in DB and CMDB propellants. It is made by the acid nitration of natural cellulose fibers from wood or cotton and is a mixture of several organic nitrates. Although crystalline, it retains the fiber structure of the original cellulose (see Ref. 13–15). The nitrogen content is important in defining the significant properties of nitrocellulose, which can range from 8 to 14%, but the grades used for propellant are usually between 12.2 and 13.1%. Since it is impossible to make NC from natural products with an exact nitrogen content, the required properties are achieved by careful blending. Since the solid‐fiber‐like NC material is difficult to make into a grain, it is usually mixed with NG, DEGDN, or other plasticizers to gelatinize or solvate it when used with DB and CMDB propellants.
A newly identified organic oxidizer, hexanitrohexaazaiso‐wurtzitane (also known as HNIW or CL‐20), is being extensively investigated for its potential as a practical propellant, see Refs. 13–1 and 13–17. It may yield the highest specific impulse to date, slightly higher than currently modified solid propellants with HMX, and can act as a most powerful explosive (20% more power than HMX). To date (in 2015), it has only been produced in small laboratory quantities. Two major factors have restrained the full implementation of Cl‐20, namely, its high cost (up to $ 570/pound in 2013) and its high sensitivity—impact and friction tests indicate that CL‐20 is less stable than HMX. CL‐20's density is somewhat higher than any other explosive ingredients (2.04 g/cm3). Investigations on its potential uses are continuing.
Additives
Additives perform many functions, including accelerating or lengthening curing times, improving rheological properties (easier casting of viscous raw mixed propellant), improving some physical properties, adding opaqueness to a transparent propellant to prevent radiation heating at places other than the burning surface, limiting migration of chemical species from the propellant to the binder or vice versa, minimizing any slow oxidations or chemical deterioration during storage, and improving aging characteristics or moisture resistance. Bonding agents are additives that enhance adhesion between the solid ingredients (AP or Al) and the binder. Stabilizers are intended to minimize slow chemical or physical reactions that may occur in propellants. Catalysts are sometimes added to crosslinker or curing agents to slow down curing rates. Lubricants are an aid the extrusion process. Desensitizing agents help to make propellants more resistant to inadvertent energy stimuli. Such additives are usually included in very small quantities.
Particle‐Size Parameters
The size, shape, and size distribution of solid particles like AP, Al, or HMX within the propellant can have a major influence on composite propellant characteristics. These particles are made spherical in shape to allow for easier mixing and to attain higher solid percentages in the propellant than shapes of sharp‐edged natural crystals. Normally, ground AP oxidizer crystals are rated according to particle size ranges as follows:
| Coarse | 400 to 600 µm (1 µm = 10−6 m) |
| Medium | 50 to 200 µm |
| Fine | 5 to 15 µm |
| Ultrafine | sub micrometer to 5 µm |
Coarse and medium‐grade AP crystals are handled as class 1.3 materials, whereas the fine and ultrafine grades are considered as class 1.1 high explosives and are usually manufactured on‐site from medium or coarse grades, (see Section 13.3 for a definition of these explosive hazard classifications). Most propellants use a multimodal blend of oxidizer particle sizes to maximize the amount of oxidizer per unit volume of propellant, with the small particles filling part of the voids between the larger particles. A monomodal propellant has one size of solid oxidizer particles, a bimodal has two sizes (say, 20 and 200 µm), and a trimodal propellant has three sizes; multiple modes allow a larger mass of solids to be placed into a given volume. Problem 13–1 has a sketch depicting how voids between the largest particles are filled with smaller particles.
Figure 13–8 shows the influence of varying the ratio of coarse to fine oxidizer particle sizes on propellant burning rate together with the influence of a burning rate additive. Figure 13–9 shows that the effect of particle size of aluminum fuels on propellant burning rate is much less pronounced than that of oxidizer particle size (Fig. 13–8 also shows an effect of particle size). Particle size, range, and shape for both the oxidizer (usually ammonium perchlorate AP]) and solid fuel (usually aluminum) have significant effects on solid packing fractions and on rheological properties (associated with the flowing or pouring of viscous liquids) of uncured composite propellants. By definition, packing fraction is the volume fraction of all solids when packed to minimum volume (a theoretical condition). High packing fractions make mixing, casting, and handling during propellant fabrication more difficult. Figure 13–10 shows a resulting distribution of AP particle size using a blend of sizes; the shape of this curve can be altered drastically by controlling size ranges and ratios. Also, solid particle size, range and shape affect the solids loading ratio, which is the mass ratio of solid to total ingredients in the uncured propellants. Computer‐optimizing methods exist for adjusting particle‐size distributions to improve the loading of solids, which can be as high as 90% in some composite propellants. Even though high solids loadings are desirable for high performance, they often introduce complexity and higher costs into the processing of propellant. Trade‐offs among ballistic (performance) requirements, processability, mechanical strength, rejection rates, and facility costs are an ever‐present concern with many high‐specific‐impulse composite propellants. References 13–4 and 13–15 report on the influence of particle size on motor performance.

Figure 13–8 Typical effect of oxidizer (ammonium perchlorate) particle size mixture and burning rate additive on the burning rate of a composite propellant.
From NASA report: 260‐SL‐3 Motor program, Volume 2, 260‐SL‐3 motor propellant development, NASA‐CR‐72262, AGC‐7096, Jul 1967, 110 pp. Accession Number: N68‐16051. http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19680006582_1968006582.pdf.

Figure 13–9 Typical effect of aluminum particle size on propellant burning rate for a composite propellant.
From NASA report: Solid propellant processing factor in rocket motor design, NASA‐SP‐8075, 82 pp. (Oct 1972); N72‐31767. http://hdl.handle.net/2060/19720024117; NTRS Document ID: 19720024117.

Figure 13–10 The oxidizer (AP) particle size distribution is a blend of two or more different particle sizes; this particular composite propellant consists of a narrow cut at about 10 µm and a broad region from 50 to 200 µm.
13.5 OTHER PROPELLANT CATEGORIES
Gas Generator Propellants
Gas generator propellants are used to produce hot gases, not thrust. They generally have a low combustion temperature (800 to 1600 K), and most do not require internal insulators when used with metal cases. Typical applications of gas generators are listed in Table 12–1. Of the large variety of propellants utilized to for gas generators only a few will be mentioned.
Stabilized AN‐based propellants have been used for many years with various binder ingredients. They give a clean, essentially smokeless exhaust and operate at a low combustion temperature. Because of their low burning rate they are useful for long‐duration gas generator applications, say 30 to 300 sec. Typical compositions are given in Ref. 13–13 and Table 13–10 describes a propellant representative of early gas generators. It is often of interest to add some fuel to AN that acts as a coolant.
Table 13–10 Typical Gas Generator Propellant Using Ammonium Nitrate Oxidizer
| Ballistic Properties | |
| Calculated flame temperature (K) | 1370 |
| Burning rate at 6.89 MPa and 20°C (mm/sec) | 2.1 |
| Pressure exponent n (dimensionless) | 0.37 |
| Temperature sensitivity σp (%/K) | 0.22 |
| Theoretical characteristic velocity, |
1205 |
| Ratio of specific heats | 1.28 |
| Molecular mass of exhaust gas | 19 |
| Composition (Mass Fraction) | |
| Ammonium nitrate (%) | 78 |
| Polymer binder plus curing agent (%) | 17 |
| Additives (processing aid, stabilizer, antioxidant) (%) | 5 |
| Oxidizer particle size, (µm) | 150 |
| Exhaust Gas Composition (Molar %) | |
| Water (steam) | 26 |
| Carbon monoxide | 19 |
| Carbon dioxide | 7 |
| Nitrogen | 21 |
| Hydrogen | 27 |
| Methane | Trace |
| Physical Properties at 25°C or 298 K | |
| Tensile strength (MPa) | 1.24 |
| Elongation (%) | 5.4 |
| Modulus of elasticity in tension (N/m2) | 34.5 |
| Specific gravity | 1.48 |
One method of reducing flame temperatures is to burn conventional AP propellants hot and then to add water to bring combustion gas temperatures down to where uncooled metals can contain them. This is used on the MX missile launcher tube gas generator (Ref. 13–18). Another formulation uses HMX or RDX with an excess of polyether‐ or polyester‐type polyurethane.
For the inflation of automobile collision safety bags the combusted gas must be nontoxic, smoke free, have a low temperature (so as not to burn people), be quickly initiated; the propellant must be storable for rather long times without degrading and reliably available. One solution is to use alkali metal azides (e.g., ![]() or
or ![]() ) with an oxide and an oxidizer. The resulting nitrates or oxides are solid materials that are removed by filtering, and the gas is clean largely composed of moderately hot nitrogen. In one model, air can be aspirated into the air bag by the hot, high‐pressure gas (see Ref. 13–19). One particular composition uses 65 to 75%
) with an oxide and an oxidizer. The resulting nitrates or oxides are solid materials that are removed by filtering, and the gas is clean largely composed of moderately hot nitrogen. In one model, air can be aspirated into the air bag by the hot, high‐pressure gas (see Ref. 13–19). One particular composition uses 65 to 75% ![]() , 10 to 28%
, 10 to 28% ![]() , and 5 to 16%
, and 5 to 16% ![]() as an oxidizer, a burn rate modifier, and a small amount of SiO2 for moisture absorption. The resultant solid nitride slag is caught in a filter.
as an oxidizer, a burn rate modifier, and a small amount of SiO2 for moisture absorption. The resultant solid nitride slag is caught in a filter.
The ideal power ![]() delivered by a gas generator can be expressed as (see Chapters 3 and 5)
delivered by a gas generator can be expressed as (see Chapters 3 and 5)
where ![]() is the mass flow rate,
is the mass flow rate, ![]() and
and ![]() are the enthalpies per unit mass respectively (at the gas generator chamber and exhaust pressure conditions),
are the enthalpies per unit mass respectively (at the gas generator chamber and exhaust pressure conditions), ![]() is the flame temperature in the gas generator chamber,
is the flame temperature in the gas generator chamber, ![]() is the gas constant,
is the gas constant, ![]() is the reciprocal of the pressure ratio through which these gases are expanded, and k the specific heat ratio. Because flame temperatures are relatively low, there is no appreciable dissociation and frozen equilibrium calculations are usually adequate.
is the reciprocal of the pressure ratio through which these gases are expanded, and k the specific heat ratio. Because flame temperatures are relatively low, there is no appreciable dissociation and frozen equilibrium calculations are usually adequate.
Smokeless or Low‐Smoke Propellant
Several types of DB propellant, DB modified with HMX, nitramine (HMX or RDX) based composites, AN composites, and/or combinations of these have very few or no solid particles in their exhaust gases. They do not contain aluminum or AP (generally resulting in lower specific impulses than comparable propellants with AP) and have very little primary smoke, but they may produce secondary smoke in unfavorable weather. Several of these propellants have been used in tactical missiles. For certain military applications smokeless propellants are needed as discussed in Chapter 20.
It is difficult to make a solid propellant that produces truly smokeless exhaust gases. A distinction must be made, therefore, between low‐smoke also called minimum‐smoke (almost smokeless) and reduced‐smoke propellants, which have a faintly visible plume. Visible smoke trails typically originate from solid metal oxide particles in the plume, such as aluminum oxide. With enough of these, the exhaust plume will scatter and/or absorb light and become as visible as primary smoke sources. Exhaust particles can also act as nuclei for moisture condensation, which occurs in saturated air or under high‐humidity and low‐temperature conditions. Moreover, vaporized plume molecules such as water or hydrochloric acid may condense in cold air to form droplets and thus a cloud trail. These processes create a vapor trail or secondary smoke.
As stated, minimum‐smoke propellants are not a special class with a peculiar formulation but a variation of one of the classes mentioned previously. Propellants containing Al, Zr, ![]() (a burn rate modifier), and/or other metallic species will form visible and often undesirable clouds in the exhaust. Reduced‐smoke propellants are usually composite propellants with low concentrations of aluminum (1 to 6%) that results in having low percentages of aluminum oxide in their exhaust plume; they are faintly visible as primary smoke but may precipitate heavy secondary smoke in unfavorable weather. Their specific impulse is much better than that of minimum‐smoke propellants, as seen in Fig. 13–1.
(a burn rate modifier), and/or other metallic species will form visible and often undesirable clouds in the exhaust. Reduced‐smoke propellants are usually composite propellants with low concentrations of aluminum (1 to 6%) that results in having low percentages of aluminum oxide in their exhaust plume; they are faintly visible as primary smoke but may precipitate heavy secondary smoke in unfavorable weather. Their specific impulse is much better than that of minimum‐smoke propellants, as seen in Fig. 13–1.
Igniter Propellants
The process of propellant ignition is discussed in Section 14.2, and several types of igniter hardware are discussed in Section 15.3. Propellants for igniters, a specialized field of propellant technology, are briefly described here. Requirements for igniter propellants include the following:
- Fast high heat release and high gas evolution per unit propellant mass to allow rapid filling of grain cavity with hot gas and to partially pressurize the chamber.
- Stable initiation and operation over a wide range of pressures (from subatmospheric to the high chamber pressures) and smooth burning at low pressures with no ignition overpressure surges.
- Rapid initiation of igniter propellant burning and low ignition time delays.
- Low sensitivity of burn rate to ambient temperature changes and low burning rate pressure exponent.
- Proper start, operation and storage over the required ambient temperature ranges.
- Safe and easy to manufacture, and safe to ship and handle.
- Satisfactory aging characteristics and long life.
- Minimal moisture absorption or degradation with time.
- Low cost of ingredients and fabrication.
- Low or no toxicity and low corrosive effects.
Some igniters not only generate hot combustion gases but also can produce hot solid particles or hot liquid droplets, which radiate heat and impinge on the propellant surface in the chamber where they embed themselves and assist in propellant burning on the exposed grain surface.
There is a wide variety of igniter propellants and their development has been largely empirical. Black powder, which was used in early rocket motors, is no longer favored because its properties are difficult to duplicate. Extruded double‐base propellants are now frequently utilized, usually in the form of a large number of small cylindrical pellets. In some instances, rocket propellants used in the main grain are also used for the igniter grain, sometimes slightly modified. They are made in the form of a small rocket motor within the larger motor that is to be ignited. A common igniter formulation uses 20 to 35% boron and 65 to 80% potassium nitrate with 1 to 5% binder. Binders typically include epoxy resins, graphite, nitrocellulose, vegetable oils, polyisobutylene, and other binders listed in Table 13–7. Another formulation contains magnesium with a fluorocarbon (Teflon); this gives hot particles and hot gases (Refs. 13–20 and 13–21). Other igniter propellants are listed in Ref. 13–11.
13.6 LINERS, INSULATORS, AND INHIBITORS
Liners, insulators, and inhibitors represent three layer types that reside at grain interfaces and are defined in Section 12.3. Their materials do not contain any oxidizing ingredients but they may ablate, cook, char, vaporize, or disintegrate in the presence of hot gases. Many will actually burn if the hot combustion gases contain even small amounts of oxidizing species, but they will not usually burn by themselves. Liners, internal insulators, or inhibitors must be chemically compatible with the propellant and with each other to avoid migration (described below) and/or changes in material composition; they must have good adhesive strength so that they stay bonded to the propellant, or to each other. The temperatures at which they experience damage or large surface regressions should be adequately high. They should all have low densities in order to reduce inert mass. Typical materials are neoprene (specific gravity 1.23), butyl rubber (0.93), a synthetic rubber called ethylene propylene diene or EPDM (0.86), or a propellant binder such as polybutadiene (0.9 to 1.0); these values are low relative to propellant specific gravities of 1.6 to 1.8. For low‐smoke propellant these three rubber‐like materials should only give off some gases but few, if any, solid particles (see Ref. 13–22).
The liners principal purpose is to provide a proper bond between the grain and the case, or between the case and any internal thermal insulation. In addition to the desired characteristics listed in the previous paragraph, the liner should be a soft stretchable rubber‐type thin material (typically 0.02 to 0.04 in. thick with 200 to 450% elongation) to allow relative movement along the bond line between the grain and the case. This differential expansion is needed because the thermal coefficient of expansion of the grain is typically an order of magnitude higher than that of the case. A liner will also seal fiber‐wound cases (particularly thin cases), which are often porous, so that high‐pressure hot gases cannot escape. Typical liners for tactical guided missiles have been made from polypropylene glycol (about 57%) with a titanium oxide filler (about 20%), a di‐isocyanate crosslinker (about 20%), and minor ingredients such as an antioxidant. Rocket motor cases have to be preheated to about 82°C prior to liner application. Often used as polymer for liners is ethylene propylene diene monomer (EPDM) linked into ethylene propylene diene terpolymer to form a synthetic rubber; it adheres and elongates nicely.
In some present‐day motors, internal insulators not only provide for thermal protection of the case (from the hot combustion gases) but also often serve as a liner providing good bonding between propellant and insulator or insulator and case. Most motors today still have a separate liner and an insulating layer. Internal thermal insulation should also fulfill these additional requirements:
- It must be erosion resistant, particularly at the motor aft‐end or blast tube. This may be achieved in part by using tough elastomeric materials, such as neoprenes or butyl rubbers that are chemically resistant to the hot gases and to the impact of particulates. Such surface integrity is also achieved when a porous black‐carbon layer formed on a heated surface (called a porous char layer) remains after some interstitial materials have been decomposed and vaporized.
- It must provide good thermal resistance and have low thermal conductivity to limit heat transfer to the case and thus keep the case below its maximum allowable temperature, which is usually between 160 and 350°C for the plastic in composite material cases and about 550 and 950°C for most steel cases. These are accomplished by filling the insulator either with silicon oxide, graphite, Kevlar, or ceramic particles. Asbestos, an excellent filler material, is no longer used because of health hazards.
- It should allow large‐deformations or strains to accommodate grain deflections upon pressurization or temperature cycling, and should transfer loads between the grain and the case.
- Surface regression should be minimal so as to retain much of its original geometric surface contour and allow for thin insulation.
A simple relationship for the internal insulation thickness ![]() at any location in the rocket motor depends on the exposure time
at any location in the rocket motor depends on the exposure time ![]() , the erosion rate
, the erosion rate ![]() (obtained from erosion tests at the likely gas velocity and temperature), and the safety factor f which can range from 1.2 to 2.0:
(obtained from erosion tests at the likely gas velocity and temperature), and the safety factor f which can range from 1.2 to 2.0:
For small test rocket motors, some designers use the rule that the insulation depth should be twice the charred depth of the insulation.
Usually, the thickness of insulators cannot be uniform, varying by factors up to 20. It has to be thicker at locations near the nozzle where it is exposed to longer intervals and higher scrubbing velocities than at insulator locations protected by bonded propellant. Before making any material selection, it is necessary to evaluate the burned‐propellant flow field and thermal environment (combustion temperature, gas composition, pressure, exposure duration, and internal ballistics) in order to carry out proper thermal analyses (erosion predictions and estimated thickness of insulator). Evaluation of loads and deflections under loads at different motor locations are also needed to estimate shear and compression stresses. When high stresses or relief flaps are involved, structural analyses are also needed. Various software codes, such as those mentioned in Refs. 13–23 and 13–24, have been used.
Inhibitors are usually made of the same materials as internal insulators. They are applied (bonded, molded, glued, or sprayed) to grain surfaces that should not burn. In a segmented rocket motor, for example (see Fig. 15–2), where burning is allowed only on the internal port area, the faces of the cylindrical grain sections may be inhibited.
Migration describes the transfer of mobile (liquid) chemical species from the solid propellant to the liner, insulator, or inhibitor, and vice versa. Liquid plasticizers such as NG or DEGDN or unreacted monomers or liquid catalysts are known to migrate. This migratory transfer occurs very slowly but may cause dramatic changes in physical properties (e.g., the propellant next to the liner becomes brittle or weak), and there are several instances where nitroglycerine that migrated into an insulator made it flammable. Migration can be prevented or inhibited by using (1) propellants without plasticizers, (2) insulators or binders with plasticizers identical to those used in propellants, (3) a thin layer of an impervious material or a migration barrier (such as PU or a thin metal film), and (4) an insulator material that will not allow migration (e.g., PU) (see Ref. 13–25).
The graphite–epoxy case of the launch‐assist rocket motors used to boost the Delta launch vehicle utilizes a three‐layer liner: EPDM (ethylene propylene diene terpolymer) as a thin primer to enhance bond strength, a polyurethane barrier to prevent migration of the plasticizer into the EPDM liner, and a plasticized HTPB‐rich liner to prevent burning next to the case–bond interface. Composite AP–Al propellants also use the same HTPB binder.
Liners, insulators, and/or inhibitors may be applied to the grain in several ways: by painting, coating, dipping, spraying, or by gluing sheets or strips to the case or the grain. Often automated, robotic machines are used to achieve uniform thicknesses and high quality. Reference 13–22 describes the manufacture of particular insulators.
External insulation is often added to the outside of the motor case when it is part of the vehicle's structure, particularly in tactical missiles or high‐acceleration launch boosters. Such insulation reduces the heat flow from any hot boundary layers outside the vehicle surface (aerodynamic heating) to the case, and then to the propellant. It may thus prevent fiber‐reinforced plastic cases from becoming weak or propellants from becoming soft or, in extreme situations, from being ignited. Such insulator must withstand oxidation from hot air flows, have good adhesion, have structural integrity to loads imposed by the flight or launch, and must have reasonable cure temperatures. Materials ordinarily used as internal insulators are unsatisfactory because they burn in the atmosphere generating additional heat. The best is a nonpyrolyzing, low‐thermal‐conductivity refractory material (Ref. 13–26) such as certain high‐temperature paints. Any internal and external insulation also helps to reduce grain temperature fluctuations and thus thermal stresses imposed by thermal cycling, such as day–night variations or high‐ and low‐altitude temperature variations for airborne missiles.
13.7 PROPELLANT PROCESSING AND MANUFACTURE
The manufacture of solid propellant involves many complex physical and chemical processes. In the past, propellants have been produced by several different processes such as compaction or pressing of powder charges, extrusion of propellant through dies under pressure using heavy presses, and mixing with a solvent which is later evaporated. Even for the same type of propellant (e.g., double‐base, composite, or composite double‐base), fabrication processes are usually not identical for different manufacturers, motor types, sizes, or propellant formulation, and no single simple generalized process flowsheet or fabrication technique prevails. Most rocket motors in production today use composite‐type propellants and therefore more emphasis on this process is given here.
Figure 13–11 is a representative flowsheet for the manufacture of a complete solid rocket motor with a composite propellant made by batch processes. Processes marked with an asterisk are potentially hazardous, are usually operated or controlled remotely, and are usually performed in buildings designed to withstand potential fires or explosions. Mixing and casting processes are the most complex, being more critical than other processes in determining quality, performance, burn rate, and physical properties of the resulting propellant.
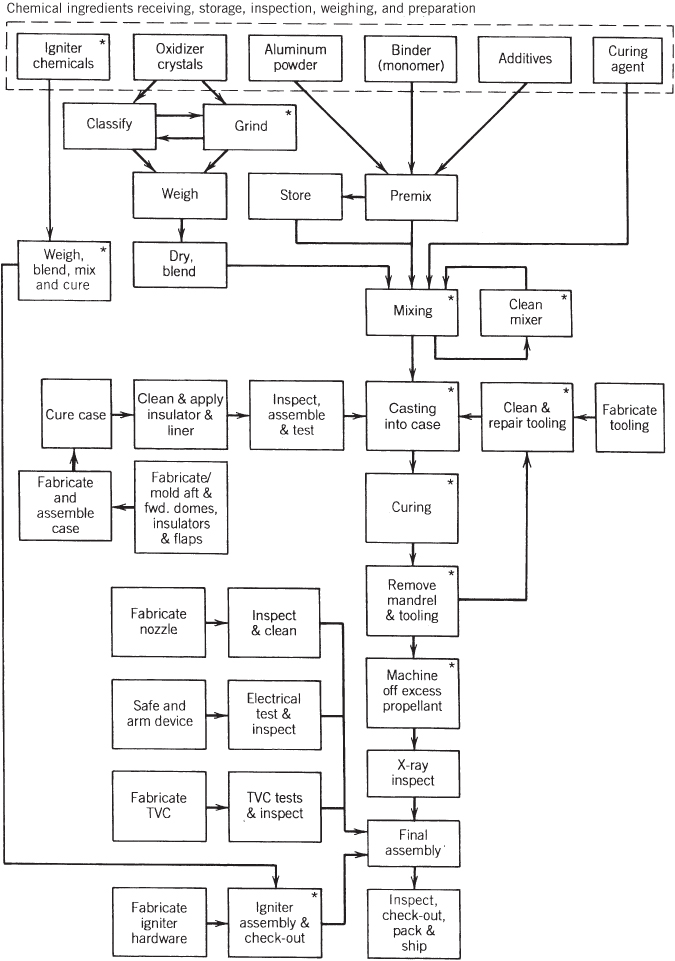
Figure 13–11 Simplified manufacturing process flow diagram for a rocket motor and its composite solid propellant. An asterisk means it is a more hazardous operation.
The rheological properties of uncured propellants (e.g., their flow properties in terms of shear rate, stress, and time) are all‐important to their processability and these properties usually change substantially throughout the path of the processing line. Batch‐type processing of propellants, including the casting (pouring) of propellants into motors that serve as their own molds, is the most common method. For very large motors several days are needed for casting up to perhaps 40 batches into a single case to form a single grain. Vacuum is almost always imposed on the propellant during the mixing and casting operations to remove air and other dispersed gases and to avoid air bubbles in the grain. Viscosity measurements of the mixed propellant (in the range of 10,000 to 20,000 poise) are made for quality control. Vacuum, temperature, vibration, energy input to the mixer, and time are some factors affecting the viscosity of the uncured propellant. Time is important in terms of pot life, that is, that period of time the uncured propellant remains reasonably fluid after mixing before it cures and hardens. Short pot lives (a few hours) require fast operations in emptying mixers, measuring for quality control, transporting, and casting into motors. Some binder systems, such as those using PVC, present a very long pot life and avoid the urgency or haste in the processing line. References 13–15, 13–11, and 13–27 give details on propellant processing techniques and equipment.
Double‐base propellants and modified double‐base propellants are manufactured by a different set of processes. The key goal here is the diffusion of liquid nitroglycerine into the fibrous solid matrix of nitrocellulose, thus forming, by means of solvation, a fairly homogeneous, well‐dispersed, relatively strong solid material. Several processes for making double‐base rocket propellant are in use today, including extrusion and slurry casting. In the slurry casting process the case (or the mold) is filled with solid casting powder (a series of small solid pellets of nitrocellulose with a small amount of nitroglycerine) and the case is then flooded with liquid nitroglycerine, which then solvates the pellets. Figure 13–12 shows a simplified diagram of a typical setup for a slurry cast process. Double‐base propellant manufacturing details are shown in Refs. 13–5 and 13–15.

Figure 13–12 Basic diagram of one system for slurry casting and initial curing of a double‐base solid propellant.
Mandrels are used during casting and curing to assure the proper internal cavity or perforation pattern. They are made of metal in the shape of the internal bore (e.g., star or dogbone) and are often slightly tapered and coated with a nonbonding material, such as Teflon, to facilitate the withdrawal of the mandrel after curing without tearing the grain. For complicated internal passages, such as a conocyl, complex built‐up mandrels, which can be withdrawn through the nozzle flange opening in smaller pieces or which can be collapsed are necessary. Some manufacturers have had success in making permanent mandrels (that are not withdrawn but stay with the motor) out of a lightweight foamed propellant that burn very quickly once ignited.
An important objective in processing is to produce a propellant grain free of cracks, low‐density areas, voids, or other flaws. In general, voids and other flaws degrade the ballistic and mechanical properties of the propellant grain. Many small dispersed gas bubbles in a propellant grain may result in an abnormally high burning rate, one so high as to cause excessive pressure and catastrophic case failure.
The finished grain (or rocket motor) is usually inspected for defects (cracks, voids, and debonds) using X‐rays, ultrasonics, heat conductivity probes, and/or other nondestructive inspection techniques. Propellant samples are taken from each batch, tested for rheological properties, and cast into physical property specimens and/or small rocket motors, which are then cured and subsequently tested. Any determination of the sensitivity of rocket motor performance, including possible failure, to propellant voids and other flaws often requires the test firing of motors with known defects. Data from such tests are important in establishing inspection criteria for accepting and rejecting production rocket motors.
Special processing equipment is required in the manufacture of propellants. For composite propellants this includes mechanical mixers (usually with two or three blades rotating on vertical shafts agitating propellant ingredients in a mixer bowl under vacuum), casting equipment, curing ovens, and/or machines for automatically applying the liner or insulation to the case, see Ref. 13–11. Double‐base processing requires equipment for mechanically working the propellant (rollers, presses) or special tooling for allowing a slurry cast process. Computer‐aided filament winding machines are used for laying the fibers of fiber‐reinforced plastic cases and nozzles.
Casting of propellant into large boosters and strap‐ons very near the launch site and the vertical keeping of rocket motors has the potential to decrease problems associated with handling and transportation, as well as with propellant slump. This decreases the time from manufacturing to launch but is not recommended for long storage times in any vertical position.
PROBLEMS
- Ideally, solid oxidizer particles in a propellant may be treated as spheres of uniform size. Three sizes of particles are available: Coarse at 500 µm, medium at 50 µm, and fine at 5 µm, each at a specific gravity of 1.95, and a viscoelastic fuel binder is available at a specific gravity of 1.01. Assume that these materials can be mixed and vibrated so that the solid particles will touch each other so that there are no voids in the binder, and that the particles occupy a minimum of space similar to the sketch of the cross section shown here. It is desired to put 94 wt‐% of oxidizer into the propellant mix, as this will give maximum performance. (a) Determine the maximum weight percentage of oxidizer if only coarse crystals are used or if only medium‐sized crystals are used. (b) Determine the maximum weight of oxidizer if both coarse and fine crystals are used, with the fine crystals filling the voids between the coarse particles. What is the optimum relative proportion of coarse and fine particles to give a maximum of oxidizer? (c) Same as part (b), but use coarse and medium crystals only. Is this better and, if so, why? (d) Using all three sizes, what is the ideal weight mixture ratio and what is the maximum oxidizer content possible and the theoretical maximum specific gravity of the propellant? (Hint: The centers of four adjacent coarse crystals form a tetrahedron whose side length is equal to the diameter.)

- Suggest one or two specific applications (e.g., anti‐aircraft, intercontinental missile, space launch vehicle upper stage, etc.) for each of the propellant categories listed in Table 13–2 and explain why it was selected in comparison to other propellants.
- Prepare a detailed outline of a procedure to be followed by a crew operating a propellant mixer. This 1‐m3 vertical solid propellant mixer has two rotating blades, a mixing bowl, a vacuum pump system to allow mix operations under vacuum, feed chutes or pipes with valves to supply the ingredients, and variable‐speed electric motor drive, a provision for removing some propellant for laboratory samples, and a double‐wall jacket around the mixing bowl to allow heating or cooling. It is known that composite propellant properties are affected by mix time, small deviations from the exact composition, temperature of the mix, the mechanical energy added by the blades, the blade speed, and the sequence in which the ingredients are added. It is also known that bad propellant would result if there are leaks that destroy the vacuum, if the bowl, mixing blades, feed chutes, and so on, are not clean but contain deposits of old propellant residue from a prior mix on their walls, if they are not mixed at 80°C, or if the viscosity of the mix becomes excessive. Usually, the sequence of loading ingredients needs to be: (1) prepolymer binder, (2) plasticizer, (3) minor liquid additives, (4) solid consisting of first powdered aluminum followed by mixed bimodal or trimodal AP crystals, and (5) finally the polymerizing agent or crosslinker. Refer to Fig. 13–11. Samples of the final liquid mix are taken to check for viscosity and density. Please list all the sequential steps that the crew should undertake before, during, and after the mixing operation. Each major step in this outline of the procedure for mixing a composite propellant has a purpose or objective that should be stated. Any specific data for each step (e.g., mass, duration, temperature, blade speed, etc.) is not required to be listed, but if known can be stated. Mention all instruments (e.g., thermometers, wattmeter, etc.) that the crew should have and identify those that they must monitor closely. Assume that all ingredients were found to be of the desired composition, purity, and quality.
- Determine the longitudinal growth of a 24‐in.‐long freestanding grain with a linear thermal coefficient of expansion of 7.5 × 10−5/°F for temperature limits of −40 to 140°F.Answer: 0.32 in.
- The following data are given for an internally burning solid propellant grain with inhibited end faces and a small initial port area:
Determine the initial forces on the propellant supports produced by the pressure differential and by the vehicle acceleration.Answer: 19,600 lbf, 5090 lbf.Length 40 in. Port area 27 in.2 Propellant weight 240 lbf Initial pressure at front end of chamber 1608 psi Initial pressure at nozzle end of chamber 1412 psi Propellant density 0.060 lbm/in.3 Vehicle acceleration 21.2g0 - A solid propellant rocket motor with an end‐burning grain has a thrust of 4700 N and a14 sec duration. Four different burning rate propellants are available, all with approximately the same performance and the same specific gravity, but different AP mix and sizes and different burning rate enhancing ingredients. They are 5.0, 7.0, 10, and 13 mm/sec. The preferred
 is 2.60, but values of 2.2 to 3.5 are acceptable. The impulse‐to‐initial‐weight ratio is 96 at an
is 2.60, but values of 2.2 to 3.5 are acceptable. The impulse‐to‐initial‐weight ratio is 96 at an  of 2.5. Assume optimum nozzle expansion at sea level. Chamber pressure is 6.894 MPa or 1000 psia and the operating temperature is 20°C or 68°F. Determine grain geometry, propellant mass, approximate hardware mass, and initial mass.
of 2.5. Assume optimum nozzle expansion at sea level. Chamber pressure is 6.894 MPa or 1000 psia and the operating temperature is 20°C or 68°F. Determine grain geometry, propellant mass, approximate hardware mass, and initial mass. - For the rocket in Problem 6 determine the approximate chamber pressure, thrust, and duration at 245 and 328 K. Assume the temperature sensitivity (at a constant value of
 ) of 0.01%/K does not change with temperature.
) of 0.01%/K does not change with temperature. - A fuel‐rich solid propellant for a gas generator drives a turbine of a liquid propellant turbopump. Determine its mass flow rate. The following data are given:
Windage and bearing friction is 10 kW. Neglect start transients.Answer:Chamber pressure 
Combustion temperature 
Specific heat ratio 
Required pump input power 970 KW Turbine outlet pressure 0 psia Turbine efficiency 65% Molecular mass of gas 22 kg/kg‐mol Pressure drop between gas generator and turbine nozzle inlet 0.10 MPa  .
. - A gas generator propellant the following characteristics:
Determine the size of an end‐burning cylindrical grain.Answer: Single end‐burning grain 27.2 cm in diameter and 31.9 cm long, or two end‐burning opposed grains (each 19.6 cm diameter × 37.3 cm long) in a single chamber with ignition of both grains in the middle of the case.Burn rate at standard conditions 4.0 mm/sec Burn time 110 sec Chamber pressure 5.1 MPa Pressure exponent n 0.55 Propellant specific gravity 1.47
REFERENCES
- 13–1. O. Bolton et al., “High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL‐20:HMX,” Crystal Growth, Design, Vol. 12, No. 9, August 2012, pp. 4311–4314. doi:10.1021/cg3010882.
- 13–2. A. Davenas, “Solid Rocket Motor Design,” Chapter 4 of G. E. Jensen and D. W. Netzer (Eds.), Tactical Missile Propulsion, Vol. 170, Progress in Astronautics and Aeronautics, AIAA, Reston, VA, 1996.
- 13–3. “Hazards Assessment Tests for Non‐Nuclear Ordnance,” Military Standard MIL‐STD‐2105B (government‐issued specification), 1994; M. L. Chan et al., “Advances in Solid Propellant Formulations,” Chapter 1.7 in V. Yang, T. B. Brill, and W.‐Z. Ren (Eds.), Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, Progress in Astronautics and Aeronautics, Vol. 185, AIAA, Reston VA, 2000, pp. 185–206.
- 13–4. N. Kubota, Chapter 1, “Survey of Rocket Propellants and Their Combustion Characteristics,” in K. K. Kuo and M. Summerfield (Eds.), Fundamentals of Solid‐Propellant Combustion, Progress in Astronautics and Aeronautics, Vol. 90, American Institute of Aeronautics and Astronautics, New York, 1984; see also Chapters 1.1 to 1.8 in V. Yang, T. B. Brill, and W.‐Z. Ren (Eds.), Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, Progress in Astronautics and Aeronautics, Vol. 185, AIAA, Reston VA, 2000.
- 13–5. C. Boyars and K. Klager, Propellants: Manufacture, Hazards and Testing, Advances in Chemistry Series 88, American Chemical Society, Washington, DC, 1969.
- 13–6. Chemical Propulsion Information Agency, Hazards of Chemical Rockets and Propellants, Vol. II, Solid Rocket Propellant Processing, Handling, Storage and Transportation, NTIS AD‐870258, May 1972.
- 13–7. H. S. Zibdeh and R. A. Heller, “Rocket Motor Service Life Calculations Based on First Passage Method,” Journal of Spacecraft and Rockets, Vol. 26, No. 4, July–Aug. 1989, pp. 279–284; doi: 10.2514/3.26067.
- 13–8. D. I. Thrasher, Chapter 9, “State of the Art of Solid Propellant Rocket Motor Grain Design in the United States,” in Design Methods in Solid Rocket Motors, Lecture Series LS 150, AGARD/NATO, Apr. 1988.
- 13–9. “Explosive Hazard Classification Procedures,” DOD, U.S. Army Technical Bulletin TB 700‐2, updated 1989; T. L. Boggs et al., “Hazards Associated with Solid Propellants,” Chapter 1.9 in V. Yang, T. B. Brill, and W.‐Z. Ren (Eds.), Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, Progress in Astronautics and Aeronautics, Vol. 185, AIAA, Reston VA, 2000. http://oai.dtic.mil/oai/oai?&verb=getRecord&metadataPrefix=html&identifier=ADA407332
- 13–10. “Ammunition and Explosive Hazard Classification Procedures,” U.S. Department of Defense, U.S. Army TB 700‐2, U.S. Navy NAVSEAINST 8020.8, U.S. Air Force TO 11A‐1‐47, Defense Logistics Agency DLAR 8220.1, Jan. 1998 rev.
- 13–11. A. Davenas, Solid Rocket Propulsion Technology, Elsevier Science, New York, 1992 (originally published in French); see also A. Davenas, “Development of Modern Solid Propellants,” Journal of Propulsion and Power, Vol. 19, No. 6, Nov.–Dec. 2003, pp. 1108–1128; doi: 10.2514/2.6947.
- 13–12. G. M. Clark and C. A. Zimmerman, “Phase Stabilized Ammonium Nitrate Selection and Development,” JANNAF Propellant Char. Subc. Mtg., CPIA Publication 435, Oct. 1985, pp. 65–75; AD‐B099756L.
- 13–13. J. Li and Y. Xu, “Some Recent Investigations in Solid Propellant Technology for Gas Generators,” AIAA Paper 90‐2335, Jul. 1990; doi: 10.2514/6.1990-2335.
- 13–14. S. Boraas, “Modeling Slag Deposition in the Space Shuttle Solid Motor,” Journal of Spacecraft and Rockets, Vol. 21, No. 1, Jan.–Feb. 1984, pp. 47–54.
- 13–15. V. Lindner, “Explosives and Propellants,” Kirk‐Othmer, Encyclopedia of Chemical Technology, Vol. 9, pp. 561–671, John Wiley & Sons, New York, 1980.
- 13–16. R. Geisler, “A Global View of the Use of Aluminum Fuel in Solid Rocket Motors,” AIAA Paper 2002‐3748, Jul. 2002.
- 13–17. U, R, Nair et al., “Hexanitrohexaazaisowurtzitane (CL‐20) and CL‐20 Based Formulations,” Combustion Explosives and Shock Waves, Vol. 41, No. 2, 2005, pp. 121–132.
- 13–18. J. A. McKinnis and A. R. O'Connell, “MX Launcher Gas Generator Development,” Journal of Spacecraft and Rockets, Vol. 20, No. 3, May–Jun. 1983; doi: 10.2514/3.25590.
- 13–19. F. McCullough, W. F. Thorn, L. B. Katter, and E. W. Schmidt, “Gas Generator and Aspirator for Automatic Occupant Restraint Systems,” SAE Technical Paper 720413, 1972, doi: 10.4271/720413.
- 13–20. A. Peretz, “Investigation of Pyrotechnic MTV Compositions for Rocket Motor Igniters,” Journal of Spacecraft and Rockets, Vol. 21, No. 2, Mar.–Apr. 1984, pp. 222–224; doi: 10.2514/3.8639.
- 13–21. P. Gillard and F. Opdebeck, “Laser Diode Ignition of B/KNO3 Pyrotechnic Mixture,” Combustion Science and Technology, Vol. 179, No. 8, Aug. 2007, pp. 1667–1699; doi: 10.1080/00102200701259833; G. Frut, “Mistral Missile Propulsion System,” AIAA Paper 89–2428, Jul. 1989; doi: 10.2514/6.1989‐2428.
- 13–22. J. L. Laird and R. J. Becker, “A Novel Smokeless Non‐flaking Solid Propellant Inhibitor,” Journal of Propulsion and Power, Vol. 2, No. 4, Jul.–Aug. 1986, pp. 378–379; doi: 10.2514/3.22898.
- 13–23. M. Q. Brewster, “Radiation–Stagnation Flow Model of Aluminized Solid Rocket Motor Insulation Heat Transfer,” Journal of Thermophysics and Heat Transfers, Vol. 3, No. 2, Apr. 1989, pp. 132–139; doi: 10.2514/3.139.
- 13–24. A. Truchot, Chapter 10, “Design of Solid Rocket Motor Internal Insulation,” in Design Methods in Solid Rocket Motors, Lecture Series LS 150, AGARD/NATO, Brussels, Apr. 1988.
- 13–25. M. Probster and R. H. Schmucker, “Ballistic Anomalies in Solid Propellant Motors Due to Migration Effects,” Acta Astronautica, Vol. 13, No. 10, 1986, pp. 599–605; doi: 10.1016/0094-5765(86)90050-0.
- 13–26. L. Chow and P. S. Shadlesky, “External Insulation for Tactical Missile Motor Propulsion Systems,” AIAA Paper 89–2425, Jul. 1989; doi: 10.2514/6.1989-2425.
- 13–27. W. W. Sobol, “Low Cost Manufacture of Tactical Rocket Motors,” Proceedings of 1984 JANNAF Propulsion Meeting, Vol. II, Chemical Propulsion Information Agency CPIA Publ. 390, Vol. 2, Johns Hopkins University, Columbia, MD, 1984, pp. 219–226; AD‐A143025.
