9
Gas Explosions
9.1 Introduction
Two types of gas explosions are treated in this chapter. The first category includes gases formed from flashing inflammable liquids when their container collapses. Flashing liquids are liquids that, when stored under pressure, start to evaporate spontaneously when exposed to the atmosphere. The explosions of this category can be particularly violent because large quantities of material can be mixed with air in a short time. A well‐known type of explosion in this category is the BLEVE (boiling liquid expanding vapor explosion). Sections 9.2–9.7 deal with flashing inflammable liquids. The second category concerns the leaking of an inflammable gas into the atmosphere or into an enclosed space. That can occur when, e.g. a natural gas line is damaged. A substantial gas flow into the atmosphere can be dangerous. Even a relatively small gas flow leaking into an enclosed space can be dangerous because it is possible to obtain an explosive gaseous mixture. An example of the latter type of accident is discussed in Section 9.8.
Generally, accidents caused by flashing inflammable liquids are more serious than those by gas leakages.
9.2 Flashing Inflammable Liquids
The accidents described in Sections 9.3–9.7 occurred with flashing inflammable liquids. Those liquids are particularly hazardous materials, and this aspect is dealt with in this section. An accident in which such a liquid was involved is described in Section 1.4. The liquid is LPG in Sections 9.3, 9.4, and 9.6. LPG stands for liquid petroleum gas. The liquid is propylene in Section 9.5 and 1,3‐butadiene in Section 9.7. From a safety point of view, the transport, storage, and use of flashing inflammable liquids introduced a new dimension, compared to the transport, storage, and use of nonflashing inflammable liquids. Examples of the nonflashing inflammable liquids are petrol and diesel fuel. Propylene will be discussed as a typical example of a flashing inflammable liquid. The physical properties of propylene will be used for a calculation. The boiling point of propylene is −47.70 °C at atmospheric pressure. That means that propylene can only be kept in the liquid phase at ambient temperature under pressure. The pressure is dependent on the ambient temperature and will be in the range of 5–10 bara. When liquid propylene is ejected from a damaged vessel into the atmosphere, it is not in equilibrium with the atmosphere having atmospheric pressure. It can become in equilibrium therewith by cooling down to −47.70 °C. Its saturated vapor pressure is then equal to the atmospheric pressure. This cooling down is caused by rapid evaporation of a fraction of the propylene mass. That fraction will be estimated. It is assumed that the evaporation proceeds adiabatically, i.e. heat is not exchanged with the surroundings. The heat of evaporation of propylene at −47.70 °C is 437.49 kJ kg−1.
The specific heat (heat capacity) of liquid propylene at −50 °C is 2.08 kJ kg−1 K−1.
One kilogram of liquid propylene, on flashing, cools down from ambient temperature to −47.70 °C. That could mean a temperature drop of about 75 K. The sensible heat made available thereby is 1∙75∙2.08 = 156.0 kJ. That heat is used to evaporate propylene. The amount of evaporated propylene is ![]() .
.
Thus, the fraction of propylene evaporated is 0.357. In actual fact, the fraction will be higher because the evaporation does not proceed adiabatically. Liquid propylene on the ground will be warmed by the ground.
So, more than one third of liquid propylene evaporates almost instantaneously when liquid propylene is, at ambient temperature, exposed to the atmosphere. A further aspect is the entrainment of droplets enhancing a BLEVE's effect. They are ignited like inflammable dust particles at a dust explosion. The specific mass of gaseous propylene exceeds the air specific mass by a factor of 1.45. This statement is valid when air and gaseous propylene have the same temperature and pressure. Thus, in that case, gaseous propylene spreads over the ground while mixing with air.
The discussion will now be continued by considering flashing inflammable liquids in general. Bursting or ripping up of a vessel containing such a material may be caused by heat or mechanical impact.
First, the effect of heat is considered. When the vessel is exposed to a fire, the saturated vapor pressure of the liquid in the vessel rises and thus the vessel pressure. Simultaneously, the heating of the hull material causes a loss of its strength. A combination of both effects may cause bursting of the vessel, and this results in a sudden availability of a large amount of flashing inflammable material. A BLEVE then occurs when the vapor/air mixture is ignited. A BLEVE caused by a fire is called a warm BLEVE. The BLEVEs at Mexico City, see Section 9.3, and Nijmegen, see Section 9.4, were caused by fires and were thus warm BLEVEs. The accident at Mexico City concerned a storage and distribution center, whereas the accident at Nijmegen concerned a tank‐lorry.
Second, the effect of mechanical impact is considered. A distinction is made between a large damage of a vessel and a relatively small damage. In the case of a major damage, the vessel rips up and a BLEVE results. A BLEVE caused by mechanical impact is called a cold BLEVE. The BLEVE at Los Alfaques, see Section 9.5, was caused by the collision of a tank‐lorry and was thus a cold BLEVE. In the case of a relatively small damage or leakage, one or more vapor/air explosions may result when vapor/air mixtures are ignited by, e.g. a flame or a spark. That occurred at Viareggio, see Section 9.6. A hole of 15 cm in size was punched into the hull of a wagon containing LPG.
A flashing inflammable liquid was involved at the incident described in Section 9.7. A wagon containing 1,3‐butadiene was damaged; however, the liquid did not escape. We see a narrow escape.
A warm BLEVE is more serious than a cold BLEVE because, due to the heating of the flashing inflammable liquid, more energy is available.
A BLEVE produces a fireball. The duration of a fireball produced by a BLEVE depends on the amount of material involved and is typically in the range of 10–20 s [1]. A safe distance concerning a BLEVE of a tank‐lorry could be 400 m. These figures are empirical values, and theoretical calculations are very complex. A loaded tank‐lorry typically contains 30 m3 of liquid.
9.3 Mexico City in 1984
Event
The accident occurred in a large LPG storage and distribution center at San Juan Ixhuatepec, 20 km north of Mexico City in the early morning of November 19, 1984 [2–4]. The facilities were owned by the Pemex (Petróleos Mexicanos) State Oil Company and consisted of six spherical tanks (two with a volume of 2400 m3 and four of 1600 m3) and 48 horizontal cylindrical tanks of different sizes. The total capacity of the storage tanks was 16 000 m3. They contained 11 000–12 000 m3 of LPG, i.e. a mixture of propane and butane, prior to the accident. The accident started with a leakage of LPG. A cloud formed, an ignition took place and caused a vapor/air explosion and a fire. The explosion and the fire caused further damage at the site, and between 05.44 and 07.01 h nine explosions occurred. One of these explosions was identified as a warm BLEVE. The accident took the lives of five Pemex employees and injured two employees. In the neighborhood close to the site, 550 people died and approximately 7000 people were injured.
The LPG Storage and Distribution Center
See Figure 9.1 and Table 9.1. The storage and distribution center was built in 1962. The two large spheres were added in 1981. The storage location was divided into separate sections by means of concrete walls of about 1 m high. The supply of LPG took place by pipelines and the shipping was by pipelines, tank‐lorries, and wagons mainly. The site area was approximately 80 m long and 80 m wide.

Figure 9.1 Tankfarm of the Pemex LPG installation.
Source: Courtesy of Gelling Publishing, Nieuwerkerk aan den IJssel, The Netherlands.
Table 9.1 Cylindrical storage vessels of the Pemex LPG installation.
| Number of vessels | Volume per vessel (m3) | Section a |
| 4 | 270 | I |
| 14 | 180 | G, H |
| 6 | 54 | A, B |
| 3 | 45 | A, B |
| 21 | 36 | C, D, E |
a See Figure 9.1.
LPG: A Flashing Inflammable Liquid
LPG is a flashing inflammable liquid and a mixture of propane and butane. The percentages by weight of these two liquids in LPG vary. The boiling points at atmospheric pressure of propane, i‐butane, and n‐butane are −42, −11.7 and −0.5 °C, respectively. It can only be kept in the liquid phase at ambient temperature under pressure. The pressure is dependent on the ambient temperature and a typical pressure is 6 barg. All storage vessels at San Ixhuatepec were, at the top, equipped with relief valves. The pressure at which a relief valve on a spherical vessel at this location opened was 150 psig (10.2 barg). LPG leaving uncontrolled a damaged vessel in which it was kept is not in equilibrium with the atmosphere, having the atmospheric pressure. It can become in equilibrium with the atmosphere by cooling down to approximately −20 °C. This cooling down is accomplished by rapid evaporation of a substantial fraction of LPG. This fraction could be about 0.25 for LPG. See Section 9.2 where, for propylene, the fraction is estimated. Gaseous LPG is 1.5–2 times heavier than air and an LPG‐cloud spreads over the ground. In this stage, it does not yet mix properly with air. A fire is ignited when the spreading cloud meets an ignition source. An ignition source can be a flare or a running diesel motor. The fire then travels back to the point of release. The next phase is a vapor/air explosion because, by now, gaseous LPG and air are mixed at the point of release. The LEL (lower explosion limit) of propane is approximately 2% by volume in air. Next, the unvaporized portion of the LPG burns for, e.g. 20 min as a pool fire, and a huge conflagration sweeps the surroundings of the point of release. There is no crater, but at distances up to 100 m from the release point, pipelines are bent or torn from vessels due to the aforementioned explosion. The fire may lead to the collapse of storage vessels and BLEVEs may occur.
Detailed Description of the Event
November 19, 1984 was a Monday. The storage vessels had been filled during the weekend preceding November 19. The filling activities had been continued in the morning of that day. It is possible that a flange at one of the cylindrical vessels broke during the filling operation and that this failure caused the initial leakage. The ambient temperature was 7 °C at the time of the accident. There was a light north–east wind having a velocity of 0.4 m s−1. A ground‐level flare was burning all the time during the filling of the tanks in a device submerged in the ground for the burning off of excess gas. The flare was burning below the ground level, instead, as is usual, high above the ground. The strong winds prevailing locally could easily extinguish a flame above ground. The cloud formed by the leakage drifted toward a residential area and was possibly ignited by the ground‐level flare. The fire traveled back to the point of release and caused a vapor/air explosion. The first explosion was followed by a huge fire. The fire and the explosion damaged the tanks at the site. Some tanks could no longer keep the LPG contained. The second explosion was a violent one and is characterized as a warm BLEVE [5]. It is possible that, at the second explosion, two smaller spherical tanks burst due to a fire. The fire caused a pressure rise inside the spheres and also caused a loss of strength of the sphere material. More explosions followed. It is possible that the other two smaller spherical tanks fragmented prior to a BLEVE.
Relief valves on storage vessels probably have functioned at San Juan Ixhuatepec. Relieved material will have taken fire. However, the relief valves could not cope with the pressure increase due to the heat input by the fire.
Casualties and Damage
The reason for the large number of fatalities and injuries is that the built‐up area, with a high population density, was situated close to the site. When the plant was erected in 1962, the distance from the habitation was about 300 m. Under pressure from the large number of people moving in, the authorities had been unable to prevent a relatively primitive settlement from pushing ahead toward the depots. The shortest distance from the rows of houses to the storage tanks was reduced to 130 m. The majority of casualties occurred within the residential area close to the site, which reached out to roughly a distance of 300 m from the center of the storage location.
First, the damage at the site is discussed. See Figure 9.2. The two large spheres were not fragmented. Their supporting legs had collapsed due to fires. Both large spheres were damaged at the top and the contents had burnt away through the holes at the top. All four smaller spheres fragmented and some parts of these spheres had traveled about 400 m.

Figure 9.2 The Pemex site after the explosion.
Source: Courtesy of Gelling Publishing, Nieuwerkerk aan den IJssel, The Netherlands.
The cylindrical vessels had not been attached to their supporting structures. The vessels of Section I were relatively undamaged. Several vessels in Section G were displaced in a longitudinal direction. These vessels had kept their original order. Three vessels in Section H were, probably simultaneously, destroyed by fragmentation. The pressure wave of the explosion causing fragmentation displaced the mentioned vessels in Section G. The remaining four vessels in Section H were displaced sideways by the same pressure wave. One of these vessels was bent by the force of the explosion. The smaller vessels, i.e. the vessels in Sections A, B, C, D, and E were displaced. Twelve smaller cylindrical vessels were found outside the battery limits of the site. Most of these smaller cylindrical vessels exhibited a characteristic failure pattern. A circular front had been torn off. The vessel fragment lacking a front is called an “end tube.” The explanation of this phenomenon is as follows. Lines and valves are attached to one front of the vessel. That front is relatively weak and is removed from the vessel by the force exerted by the internal pressure. The vessel is then launched like a rocket. One vessel, having a volume of 45 m3, traveled 1200 m.
The specific area in square meter per kilogram product is, for vessels of the same form, inversely proportional to a linear dimension. Thus, the heat input by a fire is, per kilogram vessel content, greater for small vessels than for large vessels. It is possible that this phenomenon explains the greater damage suffered by small equipment than by large equipment at San Juan Ixhuatepec.
Cylindrical vessels will also have suffered from explosions.
It is likely that LPG has left the relatively undamaged cylindrical vessels through relief valves.
Neighboring companies also suffered considerable damage.
Two hundred and seventy houses were severely damaged by the explosions and the fire at San Ixhuatepec. The most extensive damage was done by fire. Vapor/air explosions occurred in some houses.
Remarks
The built‐up area and neighboring companies were too close to the site. This accident teaches that the distance between the battery limits and houses and other companies should have been at least 400 m. That distance would have saved the majority of the lives that were lost in this accident. It would also have prevented much of the damage suffered by neighboring companies. And 1.5 km would have been an even safer distance as one vessel traveled 1.2 km.
A flare at the Pemex site high above the ground with a protection against strong winds would have been better than the ground‐level flare present before the accident.
A final remark is that the storage vessels were too close to each other. A bursting vessel could now, probably more than once, involve one or more neighboring vessels in an explosion.
9.4 Nijmegen in The Netherlands in 1978
Event
A tank‐lorry filled with LPG arrived at a filling station at Nijmegen in The Netherlands at approximately 08.00 h on December 18, 1978 [6]. The total tank volume was 31.7 m3. It was the first discharge of the day for the tank‐lorry, so its degree of filling was probably 85%. The tank‐lorry thus probably contained 27 m3 of LPG. The driver of the tank‐lorry started the transfer of LPG to an open‐air storage vessel at 08.23 h. The tank‐lorry motor was kept running because the motor drove the transfer pump. Most probably because of an incorrect connection, LPG started to leak and the hot motor ignited a gaseous LPG/air mixture. The fire heated the LPG in the tank‐lorry, causing a pressure rise in the tank‐lorry hull. At the same time the fire heated the hull material, causing a weakening of that material. Both effects caused bursting of the hull and a warm BLEVE. The driver of the tank‐lorry and the operator of the filling station had fled from the filling station by car and remained unharmed. Other personal damage did not occur. The tank‐lorry was destroyed by the explosion and the filling station was seriously damaged. Further material damage did not occur. The distance between the filling station and the built‐up area was 500 m. The fire‐brigade had blocked the area.
The Location and the Tank‐lorry
See Figure 9.3. The filling station serves one direction of a four‐lane highway. There is a verge between the two lanes of that direction and the two lanes of the other direction. A railway track is at the other side of the highway. The distance between the tank‐lorry and the open‐air storage vessel was approximately 10 m. The filling station is surrounded by pastures.
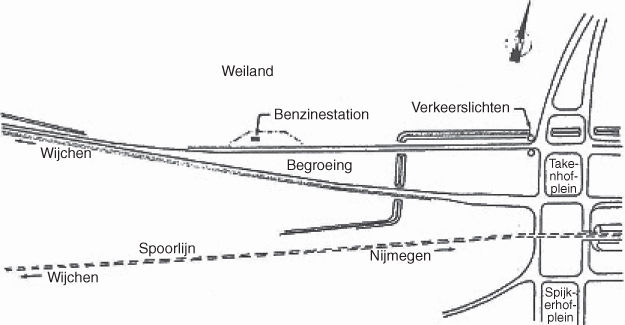
Figure 9.3 Location of the filling station at Nijmegen. A translation of the words in the figure follows: Text of the figure from top to bottom and from the left to the right: Weiland, pasture; Benzinestation, gas station; Verkeerslichten, traffic lights; Begroeing, overgrowth; Spoorlijn, railroad; Wijchen and Nijmegen are, respectively, a village and a town; Takenhofplein and Spijkerhofplein are street names.
Source: Courtesy of Gelling Publishing, Nieuwerkerk aan den IJssel, The Netherlands.
The diameter of the tank of the tank‐lorry was 2 m and its length was 10.73 m. The hull's wall thickness was 12.5 mm.
Detailed Description of the Event
The ambient temperature was −4 °C. The saturated vapor pressure of LPG is approximately 4 bara at this temperature. The driver entered the shop of the filling station shortly after 08.20 h. Shortly thereafter, he and the filling station operator noticed a fire under the tank‐lorry. They then left the shop to try to extinguish the fire by means of hand‐operated fire extinguishers. They concluded on the spot that extinguishing would not be successful because of the size of the fire and the limited amount of extinguishing material. They then fled by car after having asked for the assistance of the fire‐brigade by telephone at 08.24 h. The fire‐brigade decided to not approach the filling station as there were no humans at the filling station. They blocked the highway and stopped train traffic. The fire‐brigade noticed that the relief valve on the storage vessel was relieving and that the relieved material burned. The tank‐lorry did not have a relief valve. The tank‐lorry burst with a longitudinal crack at 08.45 h and a BLEVE occurred. Remains of the tank‐lorry were displaced over 4 m. A front of the tank traveled approximately 50 m and internal partitions of the tank traveled approximately 125 m. A shock wave was not noticed. The fire‐brigade went to the site after the explosion and extinguished the fire. The outdoor storage vessel was cooled with water and subsequently emptied by means of hoses into a pasture.
Discussion of the Event
The tank‐lorry probably contained 85% of 31.7 m3, i.e 27 m3. The amount of LPG in the tank‐lorry was about 14 t. The saturated vapor pressure of liquid propane at 55 °C is 22 bara. The hull of the tank may have burst because of a comparable high pressure and because the fire had weakened the steel of the hull. The specific mass of gaseous LPG exceeds the specific mass of air at the same temperature and pressure by a factor of 1.5–2. So, gaseous LPG is heavier than air and spreads over the ground. The volume of gaseous LPG at ambient temperature and atmospheric pressure exceeds that of liquid LPG by a factor of approximately 250. Because liquid LPG is stored under pressure, liquid LPG flashes when it is exposed to atmospheric pressure. The explosion happened when the tank burst. The volume enlargement by both flashing and combustion caused a sudden pressure increase. See also Sections 9.2 and 9.3.
A Longitudinal Crack
See Figure 9.4. Let D be the tank diameter, L its length, δ its wall thickness, and p the tank pressure. The force that tries to detach a front from the cylindrical part of the tank is 2(π/4)D 2 p. The force that keeps the front and the cylindrical part together is σ 1πDδ, where σ 1 is the longitudinal material stress. When the tank remains unimpaired, we can equate these two forces. It then follows:


Figure 9.4 A longitudinal crack.
The force that tries to pull the upper horizontal part of the hull from the lower horizontal part is 2DLp. The force that keeps the upper and the lower parts together is σ 22Lδ. When the tank remains unimpaired, we can equate these two forces. It then follows:
Thus, σ 2, the tangential material stress, exceeds σ 1, the longitudinal material stress, by a factor of two. This fact explains the observation that the tank burst longitudinally.
Remarks
It is safer to transport LPG by rail or by ship than by road transport. If rail transport is planned, it is, whenever possible, better to use rail transport not passing through built‐up areas.
The diameter of the fireball has been estimated at 40 m. The center of the fireball has been estimated at 40 m above ground level [6].
The filling station was surrounded by pastures. The nearest built‐up area was at a distance of 500 m. Internal parts of the tank traveled 125 m. As we now know it could be advisable to have a distance between LPG filling stations and built‐up areas of at least 400 m.
The fire‐brigade acted professionally by blocking the highway, stopping train traffic, and starting with extinguishing activities after a BLEVE occurred.
9.5 Los Alfaques in Spain in 1978
Event
A tank‐lorry loaded with 43 m3 of liquid propylene rode on a regional road near the small Spanish town San Carlos de la Rápita on July 11, 1978. It got off the road at 14.35 h due to a bursting tire when it passed the camping Los Alfaques, crossed a ditch, hit a pylon, and broke through the low stone wall of the camping. The accident caused the bursting of the tank and the flashing propylene gave rise to a cold BLEVE. The explosion took the lives of 216 people at the camping. More than 200 people were wounded. There was much material damage.
Detailed Description of the Event
See Figure 9.5. A tank‐lorry with a single‐hull tank was on its way from Tarragona to Puertollano. The distance between these two towns is 700 km. San Carlos de la Rápita is 90 km south of Tarragona. The tank‐lorry had loaded liquid propylene at the harbor of Tarragona. Propylene is the raw material for the manufacture of polypropylene, a plastic. The tank‐lorry could have covered the first 250 km on the highway between Tarragona and Sevilla. However, to avoid having to pay toll, the driver had been instructed to use the regional road. The boiling point of propylene at atmospheric pressure is – 47.70 °C. Thus, it can be stored as a liquid at ambient temperature under pressure. So, when the tank of the tank‐lorry burst, propylene was a flashing inflammable liquid and gave rise to a BLEVE. The background and the violence of a BLEVE have been discussed in Section 9.2. The explosion may have been ignited by the hot motor of the tank‐lorry. Note that the rip up of the tank was caused by a collision and not by a fire. It was a cold BLEVE.

Figure 9.5 Aerial view of the camping Los Alfaques.
There were 700–800 people present at the camping at the time of the accident. The camping was situated between the regional road and the Mediterranean. The explosion and the following fire hit approximately two thirds of the area, whereas one third remained almost unaffected. The explosion caused fire and subsequently fragmentation of the tank. Fragments were thrown away and one part of the tank crossed the regional road, traveled approximately 300 m, and hit the wall of a restaurant.
The Flashing of Propylene
The flashing of propylene has been discussed in Section 9.2. All liquid propylene in the tank was exposed to the atmosphere when the tank ripped up due to mechanical impact. More than one third of the liquid evaporated almost instantaneously and mixed with air. The evaporating propylene will also have entrained liquid droplets.
Remarks
We see here a worst‐case scenario. The accident happened in July and there were many campers on the camping. The driver drove on a regional road. The tank‐lorry got off the road at the location of the camping. The tank‐lorry had been filled with the maximum volume of 43 m3 liquid propylene. The collision of the tank‐lorry caused the bursting of the tank so that the full amount of liquid propylene was immediately available. The ignition source, i.e. the hot motor of the tank‐lorry, was directly available.
It would have been better if the tank‐lorry had used the highway between Tarragona and Valencia.
The location of the camping along a regional road was not ideal. It is safer to transport liquid propylene by rail or by ship than by road transport. If rail transport is planned, it is advisable to use rail transport not passing through built‐up areas.
There were small containers containing liquid butane at the camping. Many of these containers exploded after the BLEVE occurred.
The tank of the tank‐lorry had a single hull. It would have been better if the tank would have had a double hull. However, it is possible that a double hull would not have been able to contain the propylene after the tank‐lorry got off the road.
9.6 Viareggio in Italy in 2009
Event
A freight‐train consisting of an engine and 14 wagons filled with LPG passed the railway station of Viareggio in Italy on June 29, 2009, shortly before midnight when the shaft of one of the foremost wagons broke. The breakage caused the derailment of the first five or six wagons. The first wagon, directly behind the engine, fell over and hit a small metal pole. That created a hole of approximately 15 cm in the hull [7]. See Figure 9.6. LPG, an inflammable flashing liquid, quickly leaked from the wagon through this hole. Gaseous LPG/air mixture reached a built‐up area next to the railway yard. Several vapor/air explosions were ignited in houses, causing the death of 22 people.

Figure 9.6 The damaged LPG wagon at Viareggio. The photograph shows the hole and the pole.
Source: Courtesy of Gelling Publishing, Nieuwerkerk aan den IJssel, The Netherlands.
Additional Facts
There was, for a relatively short time, a fire around the derailed wagons. However, the derailed wagons did not burst and a BLEVE did not occur. There were, at the location of the derailment, six railway tracks in parallel. The train rode at one side of the railway yard. The gaseous LPG/air mixture crossed the railway yard to reach the built‐up area. The specific mass of gaseous LPG exceeds the air specific mass by a factor of 1.5–2. Thus, gaseous LPG spreads over the ground.
Remarks
The accident described was a serious accident. However, the consequences could have been even more serious if a fire had been ignited, reached a wagon, and one or more BLEVEs had occurred. LPG leaked from one wagon only.
It would be wise to avoid rail transport of LPG through town centers.
9.7 A Narrow Escape at Tilburg in The Netherlands in 2015
Event
A passenger‐train, with a velocity of 45 km h−1, collided head‐on with the last wagon of a stationary freight‐train at Tilburg in The Netherlands on March 6, 2015 [8]. See Figure 9.7. The last wagon contained 52.5 t of 1,3‐butadiene, a flashing inflammable liquid. Personal damage did not occur. The collision caused damage to both the wagon containing 1,3‐butadiene and the passenger‐train. A small leakage of 1,3‐butadiene occurred; however, the escaping material did not ignite. The contents of the wagon could be transferred to a different wagon.

Figure 9.7 Situation after the collision between the wagon and the passenger‐train.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.
Detailed Description of the Event
The freight‐train was on its way from the Chemelot industrial area in the Dutch province Limburg in the southern part of The Netherlands to the shunting‐yard Kijfhoek at Zwijndrecht in The Netherlands. Zwijndrecht is approximately 20 km east of Rotterdam. The train consisted of one engine and 35 wagons. Six wagons were loaded and the others were empty. The train had made a stop at the railway‐yard Tilburg‐Goederen (Tilburg‐Goods) between the railway stations Tilburg and Tilburg‐Universiteit (Tilburg‐University) to enable the replacement of the engine‐driver. However, the freight‐train was too long for the track on which the train made the stop. The train's length had been increased after the information concerning the train's length had been passed on to the railway company. The freight‐train had passed two switches just before it entered the track on which the train came to a halt. On both switches, the freight‐train deviated from its original direction. See Figure 9.8. These two switches remained in the position giving access to the track on which the freight‐train stood because of the freight‐train's length. For the interlocking system, the freight‐train had not yet completely passed the two switches.

Figure 9.8 The passenger‐train passed a stop sign and hit the freight‐train on Track 912 B. A translation of the words in the figure follows. Text of the left‐hand side of the figure from top to bottom: Station Tilburg Universiteit, railway station Tilburg University; Perron, platform; Richting west (naar Breda en Kijfhoek), direction west (to Breda and Kijfhoek); Emplacement Tilburg Goederen, railway yard Tilburg goods. Text of the right‐hand side of the figure from top to bottom: Richting oost (naar station Tilburg, Eindhoven en Chemelot), direction east (to railway station Tilburg, Eindhoven and Chemelot); Goederentrein vanaf Chemelot, freight‐train from Chemelot; Goederentrein vanaf Kijfhoek, freight‐train from Kijfhoek; Reizigerstrein, passenger‐train; Botsing, collision.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.
Rail transport is safeguarded in The Netherlands. The Dutch acronym for the safeguarding system is ATB (“Automatische Treinbeïnvloeding,” that is, Automatic Train Control). The objective of the system is to prevent trains from passing stop signs (red signs). It has been mentioned in Section 3.2.3 that the original system has two shortcomings. One shortcoming is that ATB is not active if the train's speed is lower than 40 km h−1. In the course of the years, the original system has been improved in this aspect. However, the track on which the passenger‐train rode prior to the accident had not been improved. The driver of the passenger‐train missed a stop sign. ATB did not automatically bring the train to a halt because the train's velocity was 45 km h−1 and that velocity was not yet high enough to activate ATB. Thus, the passenger‐train passed the stop sign and two switches and ran into the freight‐train. The driver of the passenger‐train activated the brakes just before the collision; however, his train hit the other train while still having a velocity of approximately 45 km h−1.
Railroad stop signs were passed 169 times in The Netherlands in 2013, so the passing of the stop sign at Tilburg is, in the light of that fact, not exceptional. The passing of a stop sign hardly ever results in an accident in that country because the train's velocity is, in principle, lower than 40 km h−1.
The passenger‐train had been designed around 1964 and was not equipped with crash absorbers. Crash absorbers are parts that, in case of a collision, can absorb energy and thus prevent those parts, which are important for the safety of the passengers or the load, from being damaged. Figures 9.9 and 9.10 depict crash absorbers. The last wagon of the freight‐train had been equipped with crash absorbers. On the collision, first, the automatic coupling at the front of the passenger‐train hit the draw‐hook of the wagon. The automatic coupling was pushed in. Second, the front part of the passenger‐train hit the crash absorbers of the wagon. Due to height differences, only the upper parts of the crash absorbers were hit. This led the front part of the passenger‐train to climb up against the crash absorbers and to hit the dished end of the vessel containing 1,3‐butadiene. The crash absorbers were only slightly damaged and thus could only absorb a small amount of energy. See Figure 9.9. Figure 9.10 shows deformed crash absorbers. The latter crash absorbers are not related to the accident at Tilburg on March 6, 2015. The front part of the passenger‐train dented the dished end of the vessel containing 1,3‐butadiene. The vessel did not rip up; however, the lid of a manhole in the dished end started to leak slightly due to deformation of the dished end. The collision caused a 4‐m displacement of the wagon.

Figure 9.9 The crash absorbers of the wagon were hardly damaged.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.

Figure 9.10 This photograph shows damaged crash absorbers (the tubes) that have been damaged by a collision and have absorbed energy.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.
It is possible to equip crash absorbers with parts to prevent the other wagon or carriage to climb up against the crash absorbers. See Figure 9.11. It is also possible to equip wagons with vertical shields to protect the dished ends. The intention of the latter provision is to protect the vessel against a penetration by a sharp object. The wagon at Tilburg had not been equipped with these provisions. The installation of a provision to prevent the other wagon or carriage to climb up against the crash absorber or the installation of a vertical shield is obligatory in The Netherlands for wagons transporting a very toxic or pyrophoric liquid or a toxic gas. 1,3‐Butadiene does not belong to that category. The Netherlands follows in this respect the rules of the RID [9].

Figure 9.11 This photograph shows a provision to prevent a wagon or carriage to climb up against a crash absorber.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.
1,3‐Butadiene
1,3‐Butadiene can be polymerized to polybutadiene. It is an inflammable flashing liquid. Its boiling point at atmospheric pressure is −4.4 °C and the saturated vapor pressure at 25 °C is 2.7 bara. At a given temperature and pressure, its specific mass is 1.87 times greater than the air specific mass. So, as a gas, it spreads over the ground and does not rise.
The lower and upper explosion limits (LEL and UEL) at atmospheric pressure and 20 °C are, respectively, 1.4% and 16.3% by volume.
The aforementioned physical properties of 1,3‐butadiene illustrate that the accident at Tilburg could well have developed into a cold BLEVE. We see a narrow escape.
Remarks
The Dutch Safety Board recommends that the last wagon of a freight‐train should not transport a dangerous good. A further recommendation is to provide crash absorbers of all wagons transporting dangerous goods with parts to prevent a wagon or carriage hitting the wagon containing a dangerous good to climb up against their crash absorbers. Alternatively, to install vertical shields to protect dished ends.
Additional Observations Concerning the Train Accident at Tilburg
ATB and Switches
The damaged freight‐train normally rode on a track that is safeguarded by an improved version of ATB (ATB‐vv). It means that trains are automatically, irrespective of the train's velocity, brought to a halt if a stop sign is passed. However, the damaged freight‐train was not on a track safeguarded by ATB‐vv at the time of the collision.
Passing a switch and deviating from the original direction means running a risk. The freight‐train had passed two switches on which a deviation from the original direction occurred.
The diversion from the normal track and the passing of two switches with deviations from the original direction occurred to enable the replacement of the engine‐driver.
It is not optimum that, because of logistic or economic reasons, additional risks at the transport of dangerous goods are introduced.
The Train's Length
The information concerning the train's length passed on to the railway company was not correct.
The Responsibility of the Companies Transporting Dangerous Goods
The Dutch Safety Board recommends that the companies shipping dangerous goods check the way their goods are transported.
Narrow Escape
The collision caused a 4‐m displacement of the wagon and of the whole freight‐train. This was possible because only 6 of 35 wagons were loaded, and the wagons did not have their brakes activated. The brakes of the engine of the freight‐train had been activated. Thus, the freight‐train's displacement could absorb part of the collision energy. Such an energy absorption by the freight‐train would not have been possible if more wagons had been loaded and if the wagons would have had their brakes activated. More energy to rip up the wagon containing 1,3‐butadiene would then have been available.
Final Remarks
It has been agreed in The Netherlands to compile trains transporting dangerous goods such that the risk of a warm BLEVE is reduced. It means that a wagon containing an inflammable liquid, such as petrol, will not be next to a wagon containing an inflammable flashing liquid like LPG. The rationale is that if an inflammable liquid takes fire, a wagon next to the damaged wagon will be heated. If that wagon contains an inflammable flashing liquid, a warm BLEVE may develop. See Section 9.2.
9.8 Diemen in The Netherlands in 2014
Event
An explosion occurred in the basement of apartment building De Beukenhorst at Diemen in The Netherlands on September 4, 2014 [10]. Work concerning the renovation of an elevator was being carried out that day. A steel transfer line in the foundation of the building was considered a blind one. It was tried to remove the line with a crane. The personnel at the site did not know that a functioning natural gas line was inside the steel line. The attempts to remove the steel line caused, inside the building, damage to the natural gas line, thus allowing natural gas to flow freely into the building. The personnel outside the building did not notice the severe leakage. Explosive natural gas/air mixtures could form in spaces of the building and have subsequently been ignited. The explosion took the lives of two people and wounded 15 people. The material damage was substantial.
Detailed Description of the Event
The activities carried out on September 4, 2014 concerned the removal of the foundations of an elevator‐shaft and its entry. See Figure 9.12. The shaft itself had already been removed on that date. The work was carried out by three men, including a crane‐driver, working for a subcontractor. The crane‐driver, while removing rubble with the crane, noticed a steel line below ground level at approximately 15.00 h. The line ran perpendicular to the apartment building. The three men thereupon stopped working. The crane‐driver reported his finding to the general foreman and asked for instructions. The general foreman and the supervisor of the housing association checked the situation and then consulted drawings in the site hut. The drawings did not show the line. The general foreman then drew the conclusion that the line was a blind one and ordered the crane‐driver to remove the line. The crane‐driver detached the line from the foundation at the street side with the crane at approximately 15.15 h. The three men then noticed a yellow line inside the steel line, which they identified as a natural gas line. They reported their finding to the general foreman who again checked the situation. Those present at the site smelled natural gas, however, only faintly. They drew the conclusion that a small amount of gas was released by the steel line. The general foreman called the gas distributing company at 15.27 h to report the incident. The mention was classified as “urgent,” not as “very urgent.” The employee of the gas distributing company told the general foreman that a fitter would come to check the situation.

Figure 9.12 Schematic representation of a gas connection at apartment building De Beukenhorst. A translation of the words in the figure follows: Lift, elevator; Buitengevel flatgebouw, outer front apartment building; Stijgleiding, ascending line; Liftput, elevator pit; Entrée, entrance; Aansluitleiding, connecting line; Doorvoerbuis, guiding line; Fundering, foundation.
Source: Courtesy of the Dutch Safety Board, The Hague, The Netherlands.
Then, the work was stopped and the crew waited for the fitter. The general foreman and the three men working for the subcontractor remained at the site. The supervisor of the housing association and one more employee of the same organization were also present. The site was not evacuated. Neither the fire‐brigade nor the police was informed.
Several residents smelled gas leakage: two of them reported this to the employees at the site and one resident called the housing association. The employee of the housing association present at the site entered the basement of the building. She told those present that she smelled gas strongly and heard the sound of escaping gas. The crane‐driver stood behind her in the doorway. A natural gas/air explosion occurred at 15.41 h and killed both the employee and the crane‐driver. Fifteen people were wounded. The general foreman, the supervisor of the housing association, and several residents were among the wounded. The explosion caused considerable damage to the building. Part of the building took fire.
Cause of the Explosion
The yellow natural gas line noticed by the personnel of the subcontractor transported natural gas from a main line to a number of apartments. The detachment of the steel line caused damage to the yellow line inside the basement of the building. A coupling broke loose and the natural gas could flow freely into the building. The lower explosion limit (LEL) of natural gas in air at atmospheric pressure and ambient temperature is 5.0% by volume. The upper explosion limit (UEL) is 16.0% by volume. Natural gas/air mixtures had formed in the basement of the building. Part of these mixtures contained between 5.0% and 16.0% by volume of natural gas in air. Such mixtures had been ignited.
Prologue of the Accident
See the timeline of the accident in Figure 9.13. The housing association possessing De Beukenhorst, built in 1974, decided to renovate the elevators of the building in 2012. It was necessary to upgrade the elevators. An important aspect was that new elevators should be larger than the existing ones. A contractor signed the order for the renovation on February 12, 2014. It would be necessary to dig into the ground because the new foundation would be larger than the existing foundation. It is, in such a case, obligatory in The Netherlands to collect information concerning the presence of cables and lines in the ground. The contractor informed a central organization in The Netherlands and asked for information on March 14, 2014. The information was received; however, the presence of a natural gas line between the main natural gas line and the apartment building at the location of the elevator‐shaft in question was not communicated. Nevertheless, that gas line was present in the spring of 2014. The contractor was instructed by the natural gas distributing company to coordinate his work with that company. There was a communication between the contractor and the natural gas distributing company on March 19, 2014. As stated, a mention of the work had been made on March 14, 2014. The information received thereupon had a validity of 20 days. The work had to be started within that period; otherwise a new mention would have to be made. The reason for this limited validity is that the situation might have changed after 20 days. A guideline “Preventing damage to cables and lines due to digging” is valid in The Netherlands. In line with this directive, the contractor dug test ditches to check exactly the position of cables and lines on April 25, 2014. The contractor then concluded that a main natural gas line had to be relocated to allow the building of the new, enlarged elevator‐shaft. The main natural gas line had been made of gray cast iron. The gas distributing company planned to replace that line in the fall of 2014. The reason for this replacement was that gray cast iron is vulnerable to vibrations and external loads. The plan was changed because of the renovation of the elevator‐shafts. First, the gas distributing company had the natural gas lines between the main line and the apartments replaced in the months April through June 2014. The line damaged on September 4, 2014 was replaced on June 23, 2014. Next, the company had the main line replaced between July 7, 2014 and August 27, 2014. The company informed the contractor that the renovation of the elevator‐shafts could be started after August 24, 2014. The contractor started the work shortly after that date. The contractor did not renew the digging mention. The earlier digging mention had lost its validity as more than 20 days had elapsed. Had he done so, information regarding the line damaged on September 4, 2014 would have been received. The reason for not renewing the mention is that the contractor thought he already possessed all relevant information because of his communications with the gas distributing company.

Figure 9.13 Timeline of the Diemen accident.
Observations
Damage to natural gas lines due to digging activities is a frequently occurring phenomenon in The Netherlands. For example, 5207 of these cases have been registered in that country in 2013. Approximately 75% of these cases concern damage to lines between main lines and houses, apartment buildings, offices, and so on. Damage to these lines may result in an accumulation of natural gas inside buildings and that is potentially dangerous.
An aspect was that digging in the ground was not a core activity for the contractor of the renovation of the elevator shaft.
A principal of building activities is, according to Dutch law, obliged to see to it that digging activities on behalf of his premises are carried out carefully. The housing association was the principal in this case. The Dutch Safety Board concludes that the principal could have done more to ensure that the contractor would collect all relevant information concerning underground natural gas lines.
The system to show all cables and lines to contractors making a digging mention in The Netherlands can be improved. The natural gas line in the foundation of the elevator‐shaft was not shown in the spring of 2014. The Dutch Safety Board recommends to improve that system.
The prologue of the accident shows that the contractor initially took steps and measures to deal carefully with natural gas lines. The renovation of the elevator was delayed by months because a main natural gas line had to be relocated.
The guideline “Preventing damage to cables and lines due to digging” prescribes to stop the work when an unknown line is met. The work should not be resumed until the purpose of the unknown line has been established.
The same guideline orders the evacuation of the site, removal of ignition sources, and calling the fire‐brigade or the police when a natural gas line has been damaged. The reason that these actions were not taken is that, outside the building, it did not appear that the situation called for these measures. The leakage was inside the building and was not noticed.
The operator of the gas distributing company receiving the call on the damage classified the situation as “urgent” and not as “very urgent.” The Dutch Safety Board recommends to improve the procedure on accepting damage reports.
References
- [1] Pietersen, C.M. (2009). After 25 Years – The Two Largest Industrial Disasters – LPG Disaster Mexico City – Bhopal Tragedy, 48. Nieuwerkerk aan den IJssel, The Netherlands: Gelling Publishing (in Dutch).
- [2] Pietersen, C.M. (2009). After 25 Years – The Two Largest Industrial Disasters – LPG Disaster Mexico City – Bhopal Tragedy, 17–22, 31–62, 117–123. Nieuwerkerk aan den IJssel, The Netherlands: Gelling Publishing (in Dutch).
- [3] Pietersen, C.M. (1988). Analysis of the LPG‐disaster in Mexico City. Journal of Hazardous Materials 20: 85–105.
- [4] Arturson, G. (1987). The tragedy of San Juanico – the most severe LPG disaster in history. Burns 13: 87–102.
- [5] Pietersen, C.M. (2009). After 25 Years – The Two Largest Industrial Disasters – LPG Disaster Mexico City – Bhopal Tragedy, 38. Nieuwerkerk aan den IJssel, The Netherlands: Gelling Publishing (in Dutch).
- [6] Steunenberg, C.F., Hoftijzer, G.W., and van der Schaaf, J.B.R. (1981). Investigation concerning an accident at Nijmegen at which a tank‐lorry was involved. Polytechnisch tijdschrift/procestechniek 36: 175–182. (in Dutch).
- [7] Pietersen, C.M. (2009). After 25 Years – The Two Largest Industrial Disasters – LPG Disaster Mexico City – Bhopal Tragedy, 169. Nieuwerkerk aan den IJssel, The Netherlands: Gelling Publishing (in Dutch).
- [8] Dutch Safety Board (2016). Risk Control at Rail Transport of Dangerous Goods, 1–102. The Hague, The Netherlands: Dutch Safety Board (in Dutch).
- [9] Convention relative aux transports internationaux ferroviaires (COTIF) (2011). Appendice C – Règlement concernant le transport international ferroviaire des marchandises dangereuses (RID), 1‐1–7‐19. France: COTIF (in French).
- [10] Dutch Safety Board (2015). Dangers of Gas Lines on Digging, 1–70. The Hague, The Netherlands: Dutch Safety Board (in Dutch).
