7
Pyrometallurgical Processes for Recycling Waste Electrical and Electronic Equipment
Jean-Philippe Harvey1, Mohamed Khalil2, and Jamal Chaouki2
1Polytechnique Montréal, Centre for Research in Computational Thermochemistry (CRCT), Department of Chemical Engineering, C.P. 6079, succ. Centre-Ville, Montreal, Quebec H3C 3A7, Canada
2Polytechnique Montréal, Process Engineering Advanced Research Lab (PEARL), Department of Chemical Engineering, C.P. 6079, succ. Centre-Ville, Montreal, Quebec H3C 3A7, Canada
7.1 Introduction
In the last few decades, the high demand for emerging electronic technologies combined with the short life-in-service of obsolete appliances has led to an exponential rise in waste electrical and electronic equipment (WEEE), which resulted in severe environmental issues: when incinerated, WEEE releases greenhouse gases and toxic emissions; when disposed of in landfills, it contaminates groundwater (Needhidasan et al., 2014). These terrible environmental consequences directly impact developing countries such as Ghana, the world’s largest e-waste landfill (Zvezdin et al. 2020; Álvarez-de-los-Mozos et al., 2020; Vaccari et al. 2019). The United Nations (UN) reported that over 50 million metric tonnes (Mt) of WEEE were produced worldwide in 2019 and that this volume is expected to increase by over 70 million metric tonnes (Mt) over the next few years (Franzolin 2020; Adusei et al. 2020; Aboughaly and Gabbar 2020).
Pyrometallurgy is one of the most viable extractive metallurgy techniques for recycling high volumes of WEEE (Ma 2019b; Willner et al. 2014). One significant advantage of many pyrometallurgical processes is the generation of high-temperature liquid phases (such as matte in the case of copper smelting and slag in the case of lead smelting) which are perfect reactive media to melt/dissolve WEEE. Pyrometallurgical processes often involve the simultaneous presence of multiple liquid solutions such as slag, matte, metallic melt, and molten salt in which valuable metals and impurities can be partitioned and eventually discarded (in the case of impurities) (Espinosa et al. 2015; Anindya et al. 2013).
Pyrometallurgy includes many extractive metallurgical operations such as smelting (performed in high-temperature furnaces [Hageluken, 2006; Theo 1998]) and refining (which includes chemical and electrochemical processes [Rudnik and Bayaraa 2016]). High-temperature electrochemical processes use electrical work to produce metals from the reduction of metallic cations present in electrolytes (such as molten salts [Flandinet et al., 2012]) and can also be considered in this branch of extractive metallurgy.
In terms of recovered metals and sustainability practices, pyrometallurgical processes significantly enhance recycling efficiency as they operate at high temperatures and involve liquid reactive media. In addition to copper and precious metals, modern smelters are integrated into the recycling route of other valuable metals such as selenium, cadmium, tellurium, and nickel (Ma 2019a). Another positive outcome of such operations is the energy savings from the exothermic plastic waste oxidation/combustion during the smelting operations. Pyrometallurgical processes, therefore, close many metal loops such as for iron (via basic oxygen furnace and electric arc furnace), copper and lead (via smelting operations), as well as aluminum (via tilting or stationary melting furnaces). They are considered an essential part of the recycling chain. This chapter describes these processes to recover metals from WEEE, such as waste printed circuit boards (WPCBs). Furthermore, we discuss their advantages, limitations, and associated challenges.
7.2 Printed Circuit Boards
Printed circuit boards (PCBs) are the most valuable and common component of consumer electronics (i.e. one of the most essential categories of WEEE). They constitute approximately 3 wt% of WEEE (Zhu et al. (2020); Chatterjee (2012); Luda (2011)). Their recycling has recently drawn considerable attention from both an economic and an environmental perspective (Ippolito et al. 2021; Wang et al. 2021a, b; Rao et al. 2021; Tanísalí et al., 2021). The presence of many valuable elements, which can be recovered (i.e. the concept of urban mining), combined with their scarcity in the earth’s crust is at the heart of this waste valorization. On the other hand, the toxic nature of many of their components (which may contain lead, chromium, and brominated compounds) is a critical aspect to consider when designing eco-friendly recycling processes.
Each PCB type has its own overall chemical composition, as well as mechanical and physicochemical properties. This leads to recycling challenges as each WPCB type will react differently to the unit operations of a given process (such as shredding, leaching, and smelting) Charles et al. (2020) presented an elemental mapping of the valuable metals that can be recovered from PCB (see Figure 7.1). It includes copper, gold, silver, palladium, yttrium, cobalt, antimony, and iridium, just to identify a few. More specifically, a typical PCB consists of approximately 70 wt% of plastics and other materials such as brominated flame retardants (BFRs), ceramics, and metals (Charitopoulou et al. 2020; Jandric et al. 2020).
Copper (20 wt%), solders alloys made of tin and lead (5 wt%), iron (3 wt%), as well as precious metals (about 200 ppm of gold, 800 ppm of silver, and 100 ppm palladium) represent the most common metals to be recovered in these waste materials. As seen in the previous chapters of this book, the wide variety of metallic elements to be recovered and the unique chemical nature of PCBs naturally lead to the design of many distinct recycling processes, including physical separation, hydrometallurgical, biometallurgical, and pyrometallurgical processes (Moosakazemi et al. 2020; Cui and Anderson 2020; Harikrushnan et al. 2016). We explore this in detail in this pyrometallurgical processes chapter.

Figure 7.1 Map identifying the rare and precious metals distribution in a PCB.
Source: Charles et al. (2020).
From an open access article permitting unrestricted use of the original work.
7.3 Pyrometallurgical Processes
A pyrometallurgical process involves using ores, concentrates, or waste materials to produce valuable metals ultimately. In the primary metal production route, these metals are in one of their oxidation states due to the ore genesis conditions (i.e. oxidizing conditions). Therefore, a metal production will require a reduction step, which is often performed using a carbon-based reducing agent such as coke or methane. In the case of WEEE, it is interesting to note that these metals are already in their fully reduced state, which has to be considered when designing an optimal recycling strategy. Moreover, basic thermodynamic considerations justify the use of high temperatures in most pyrometallurgical processes since carboreduction is energetically favored at high temperatures for many metals. Also, high-temperature liquid phases are often formed in these processes as they act as excellent reactive media. Another important consideration that promotes primary metal pyrometallurgical processes to recycle WEEE is that they can already process concentrates that naturally contain most of these metallic elements to be recovered. Therefore, these pyrometallurgical technologies can naturally process WEEE without any significant modification. A perfect example of such good process compatibility is the smelting of copper sulfide concentrate (such as chalcopyrite, CuFeS2) in the Noranda process, which can integrate up to about 14 wt% of shredded electronic waste in its feed as reported by Cui and Zhang (2008). In this case, smelting operations performed between 1200 and 1300 °C, which are designed to remove sulfur and iron from the system via the gas and the slag phase, respectively, are used to produce liquid copper, which also entrained noble metals (such as gold, silver, and platinum-group metals – PGMs). They are collected in the low-temperature anode mud of the electrolytic refining process, which is required to obtain pure copper that qualifies for electrical applications (Vegliò and Birloaga, 2018).
7.3.1 Smelting
The initial steps in the processing of e-wastes generally involve their breaking and shredding followed by some physical separation operations (such as magnetic separation, eddy current separation, air current separation, and corona electrostatic separation as reported by Zhang and Xu (2016)) to sort different fractions, which have distinct chemistry. The nonferrous metallic-rich fraction is then processed in one of the available smelting technologies. It mainly contains common base metals such as copper, nickel, tin, zinc, lead, and aluminum, and some residual ferrous components, and plastics (which cannot be removed entirely from the physical separation steps). Depending on the major base metal (i.e. Cu or Pb), the metallic fraction can be processed in either a copper or a lead smelter, which acts as a recycling unit to recover these elements as well as noble metals (gold, silver, and platinum-group metals) following further hydrometallurgical and electrochemical processes. Most smelters also simultaneously process important fractions of sulfide concentrates, which explain the formation of a matte phase (sulfur-rich liquid) and an oxide-rich slag phase (induced by the oxygen blowing and the addition of fluxes) along with a metallic phase at some point during the process. The evolution of an SO2-rich gaseous phase, which is collected to produce sulfuric acid, is also typical for such smelting operations. Further details on the copper (Cu)- and lead (Pb)-smelting strategies used to recycle e-wastes are presented in the Sections 7.3.1.1, 7.3.1.2, and 7.3.1.3.
7.3.1.1 Copper-Smelting Processes – Sulfide Route
The general principle of copper smelting is to remove sulfur and iron (i.e. the two main impurities of copper sulfide ores) using oxygen. Figure 7.2 shows the predominance diagram for the CuFeS-SiO2-O2 system at a temperature of 1200 °C. Silica is added to the system (using a 2 : 1 CuFeS2:SiO2 molar ratio) to produce a slag phase upon ferrous oxide (FeO) formation during the O2-enriched air blowing. At the beginning of the smelting operation, a (Cu,Fe)-rich matte and solid silica are present in the reactor. As oxygen is introduced into the system, it generates SO2(g) and ferrous oxide. The obtention of the adequate SiO2:FeO ratio leads to the formation of fayalite slag. When the (Cu-rich matte + slag) meta-equilibrium is reached (gray zone #1 on Figure 7.2), the smelting process is completed. The copper matte smelting can be summarized using the following overall reaction:

Figure 7.2 CuFeS2-SiO2-O2 predominance diagram (using a 2 : 1 CuFeS2:SiO2 molar ratio) at 1200 °C calculated using the FactSage software. The arrow shows the evolution of the partial pressure of O2 as air is blown in the system.
In theory, it is thermodynamically possible to identify operating conditions that lead to the direct production of metallic copper called direct-to-copper smelting by further oxidizing the matte (see the grey zone #2 in Figure 7.2). However, these operating conditions lead to significant copper oxidation and solid magnetite formation (spinel). Other fluxes such as CaO need to be used to lower the liquidus temperature of the slag. More importantly, copper is transferred to the slag phase. To make the process economically viable, the copper-rich slag phase needs to be treated with coke to recover the valuable metal. The Cu-rich matte is then converted into liquid copper using a similar strategy.
The primary production of copper from sulfides is performed via one of the following smelting technologies (Schlesinger et al. (2011c)):
- Submerged tuyere smelting: Oxygen-enriched air is the primary reactant, which is blown into the liquid matte via submerged tuyeres to produce SO2 and FeO (which is subsequently fluxed into a fayalite slag synthetically produced by adding silica). The concentrate can be injected into the system via either the submerged tuyere (in which case the concentrate needs to be dry and has to meet specific particle size requirements) or from the top of the reactor as in the Noranda technology. The latter feeding strategy is more suitable for charging a scrap of various sizes into the smelter. Silica is also added to the system to flux the oxidized iron.
- Flash smelting: This technology values a solid (concentrate) -gas (O2-enriched air) combustion-like reaction to promote the simultaneous formation of liquid matte and slag droplets, which eventually reach the matte–slag pool at the bottom of the reactor. This two-phase liquid system needs to settle in the furnace to promote efficient liquid–liquid phase separation and transfer of the copper from the slag phase to the matte. One drawback of this approach is that the concentrate feed needs to be calcined and being injected as fine particles.
- Vertical-lance smelting (ISASMELT): This technology developed in Australia is based on the use of a submerged gas-cooled lance, which blows a mixture of (oxygen-enriched + natural gas) blast into the liquid matte (see Figure 7.3). The vertical reactor is fed from the top and can tolerate various feed sizes, which ease the introduction of scrap. Magnetite, which is formed by the matte’s oxidation, is a key phase in this process. Firstly, it protects the lance from the matte as it solidifies around it. It also plays a central role in the production of SO2(g) as it reacts with the matte phase via a solid–liquid reaction. Alvear Flores et al. (2014) presented an exhaustive study about the importance of this compact technology for recycling. It is also a central unit operation of the Umicore process detailed by Hageluken (2006).
- Direct-to-copper smelting: As explained previously, the general idea behind this smelting strategy is to severely oxidize the matte until it produces metallic copper. According to Schlesinger et al. (2011a), about 25 wt% of Cu is inevitably transferred to the slag phase. It will be recovered by reducing the copper oxide in the slag using coke.

Figure 7.3 Umicore’s IsaSmelt furnace.
Source: Bakker et al. (2011).

Figure 7.4 Partitioning of WPCBs during the Cu-smelting operations.
Copper smelters clearly offer an opportunity to partition valuable metallic elements and other impurities that constitute WPCBs in various phases that form during the process (such as the matte, the slag, the metallic melt, and the exhaust gas-dust). One fundamental aspect to understand here is the fate of each element and contaminant under these smelting operating conditions. Figure 7.4 presents a schematic representation of the partitioning of various elements/component present in WPCBs. The first group called plastics is incinerated in situ. The products of this incineration are transferred to the gas phase. The second group called volatile metals includes zinc, lead, arsenic, bismuth, tellurium, and selenium. Apart from tellurium and selenium (which end up in the liquid copper matte as they are chalcogen elements), all these elements are mostly transferred to the SO2-rich exhaust gas. This is potentially problematic as they may exceed regulated concentrations when released to the atmosphere and become a danger for the environment. The third group is associated to the presence of glass fibers in WPCBs. It contains oxide components like Al2O3, SiO2, and CaO. These oxide components are easily fluxed in the slag phase during smelting. The last group is called low-vapor-pressure metals. It is subdivided into two categories: (i) the noble metals relative to iron (such as Cu, Ag, Au, and Pt), which will be carried in the Cu-rich liquid metal, and (ii) the reactive metals (such as Al, Ti, Ni, Co, and Fe), which will mostly be oxidized and transferred to the slag phase. A converting step is performed on the copper matte to produce the blister copper, which is refined using natural gas to remove the dissolved monatomic oxygen.
Finally, it is to be noted that many authors (Khaliq et al. (2014); Cui and Zhang (2008); Ma (2019a); Zhang and Xu (2016)) have reviewed the principal industrial e-waste recycling practices involving copper-smelting unit operations. Their studies reported the Noranda process in Canada, the Aurubis’s Kayser recycling system in Germany, the Boliden Ronnskar Smelter in Sweden, and the Umicore’s precious metal refining process in Belgium (see the process flowsheet in Figure 7.5). It is to be noted that the smelting operations at Boliden and Umicore are integrated into a much larger process scheme that also involves lead-smelting operations, which will be explained in the Section 7.3.1.3.

Figure 7.5 Flowsheet of Hoboken integrated smelter and refinery plant of Umicore.
Source: Tuncuk et al. (2012).
7.3.1.2 Copper-Smelting Processes – Secondary Smelters
There are two types of secondary smelters to process copper-rich scrap, i.e. the metal smelters and the black-copper smelters (Schlesinger et al. (2011b)). Tiltable reverberatory furnaces are used to process Cu-rich (high-grade) scrap. For low-grade scrap, a more elaborate strategy (involving a reduction step using coke followed by an O2(g) oxidizing refining step) is required. Low-grade scrap often includes shredded automobile products and dross coming from the decopperizing of lead bullion, which both contain lead. Therefore, the typical furnaces associated to the production of black copper (such as the Kaldo furnace and top submerged lance reactors like ISASMELT and AUSMELT) are often found in lead extraction operations.
7.3.1.3 Lead-Smelting Processes
Lead, like copper, is a chalcophile element which is naturally found in association with sulfur in different minerals. Lead sulfide ores such as galena (PbS) typically contain other minerals that are rich in zinc, copper, and iron (in the form of sulfates and sulfides) as well as precious metals, notably silver (Ayres et al. 2003). E-wastes such as lead-acid batteries are therefore fully compatible with this ore type as they simultaneously contain metallic lead, PbO2, and PbSO4 (Arnout et al. 2011). They can be mixed with the sulfide concentrate feeds.
Laubertova et al. (2017) presented an overview of the lead-smelting technologies. Two main routes can be used to process lead sulfide concentrates and lead-rich scrap, i.e. (i) the traditional sintering–smelting in blast furnaces (for Zn-lean concentrate) or using Imperial Smelting furnaces (for Zn-rich concentrate), and (ii) the direct smelting approach. Grant (2001) reported many direct lead-smelting approaches such as the Kaldo furnace (top-blown rotary converter), the QSL kiln, the ISASMELT and AUSMELT vertical reactor with submerged lance, and the Kivcet process. Contrary to conventional copper-smelting operations, which always require large amounts of concentrate to operate, several authors report that many lead-smelting operations run almost exclusively using scrap feed. This is explained by the high lead fraction that contains specific waste materials (such as lead-acid batteries) when compared to the WPCBs, which have smaller fractions of metallic components.
- Sintering–smelting: In this approach, the concentrate is first roasted using air to convert the sulfide into an oxide via the following reaction:

This reaction is important as lead oxide can be easily reduced to metallic lead by a carbon-based reducing agent (which is not the case for lead sulfide). Lead oxide is not the only phase in the sintered product. It also contains melilite, spinel, calcium silicate sulfate, as well as copper sulfide (Zhao 2013). Next, the lead oxide sinters are reduced in the blast furnace using coke. Silica and lime are also charged in the blast furnace from the top (Watt et al. (2018)). The tuyeres at the bottom of the reactor blast O2-enriched air, which react with the excess coke to produce CO(g) via the Boudouard reaction. This carbon-monoxide-rich gas will act as the main reducing agent for this reduction process (Vanparys et al. (2020)):

The liquid lead (called lead bullion) obtained from this operation is collected at the bottom of the furnace along with a slag and a matte phase. In many cases, arsenic is present in the charge and will form speiss (i.e. iron arsenide) upon the reaction with iron. Figure 7.6 taken from the work of CHAIDEZ-FELIX et al. (2014) shows the phase assemblage obtained from a lab-scale lead blast furnace experiment at 1573 K. It consists of an equilibrium between lead bullion, speiss, matte, and slag. In the Umicore process presented in Figure 7.5, the lead bullion obtained from the blast furnace is sent to a refinery unit, the matte is sent to a copper smelter, while the speiss is processed in the Umicore Plant in Olen. The Imperial Smelting process works similar to the blast furnace and is used to process zinc-rich concentrates.
- Direction smelting: In this approach, the sulfide concentrate is directly reduced by coke in a furnace. Slag-forming fluxes are also added to the reactor. The following reactions simultaneously take place:




Figure 7.6 (a) longitudinal section of the solidified sample: crucible is at the bottom, followed by the bullion region, an interface zone (Cu−Sb alloy, speiss and matte) and the slag at the top of the sample. (b) magnification of the interface zone. The slag phase appears in this figure as dark globules and the lead bullion as a light zone. (c) Cu−Sb dendritic precipitation in the bulk of the bullion phase.
Source: Taken from Chaidez-Felix et al. (2014)). Reprinted with permission from Transactions of Nonferrous Metals Society of China 2014.
As explained by Grant (2001), the direct smelting of lead sulfide concentrate to obtain a low-sulfur lead bullion and a low-lead slag in a single step is difficult to implement in practice. The PbS-O2-S2-(SiO2)0.4(FeO)0.3(CaO)0.1 predominance diagram calculated at 1473 K and 1 atm presented in Figure 7.7 validates this affirmation.
Figure 7.7 shows that the sulfur removal of the system performed via the O2-enriched air blowing may provide the desired (liquid lead + slag) equilibrium but inadequate compositions (see the red star on Figure 7.7). In this zone, the liquid lead activity of about 0.97 implies that it dissolved a significant amount of oxygen. Moreover, Figure 7.7b shows that the liquid PbO activity is high in this zone, which would lead to important lead losses in the slag. Therefore, the oxidation alone is not enough to smelt the concentrate. The addition of coke into the system allows a significant lowering of the oxygen chemical potential. This leads to an important reduction of the Pb losses to the slag phase (see the star in the bottom left corner in Figure 7.7b). The reduction step is also not sufficient by itself as it cannot fully control the system’s sulfur chemical potential. This explains why a two-stage strategy – oxidation followed by reduction – is required (Grant, 2001). Finally, it is to be mentioned that some direct smelting furnaces can process concentrate-free feeds. Laubertova et al. (2017) reported the example of a shaft furnace battery recovery process that uses battery scrap, coke, iron scrap, and reusable slag to produce lead bullion.

Figure 7.7 PbS-O2-S2-(SiO2)0.4(FeO)0.3(CaO)0.1 predominance diagram generated at 1473 K and 1 atm calculated using the FactSage software. Panel (a) shows iso-activities of pure liquid lead, while panel (b) shows iso-activities of pure liquid PbO.
7.3.1.4 Advantages and Limitations of Smelting Processes
We conclude this section by highlighting the advantages and limitation of the smelting processes to recycle electronic waste.
Advantages
One major advantage of using smelting technologies to recycle waste electronics is that they were initially designed to deal with several elements’ simultaneous presence in the concentrates they process. Most primary metal-smelting operations have to deal with: (i) the presence of undesired silicates/oxide compounds, which are removed via the formation of a slag phase, (ii) the presence of reactive metals and other reactive compounds such as plastics and sulfides, which can be oxidized using O2-enriched air and then transferred to the slag phase or the exhaust gas of the process; (iii) the possibility to reduce valuable oxide components using carbon-based reducing agents; (iv) the production of metallic melts, which are then refined and tapped. Therefore, these smelting processes can tolerate the variable nature of the overall chemical composition of waste electronics. It is of prime importance to select the primary smelting process that matches the waste’s major metallic component. The integration of scrap into primary metal processes is especially important for electronic wastes since their metallic fraction is not always high. Therefore, these recycling operations benefit from the formation of liquid phases during the primary smelting operations. The other advantages can be listed as follows:
- Most primary metal-smelting processes have been exploited for decades and optimized to recover all valuable metals. The perfect example is copper’s primary production to process copper sulfides in Quebec (i.e. the Noranda process). Apart from copper production, the other metallurgical operations that have been appended to this process (such as the electro-refining of copper) have allowed recovering noble metals such as silver, gold, platinum, and palladium as chalcogen metalloids such as tellurium and selenium.
- Many chemical reactions involved in the smelting operations are exothermic, which lower these processes’ overall energetic requirements. The in situ exothermic incineration of plastics can also be viewed as a positive outcome.
- Smelters are typically large reactors with high productivity, which allows the daily processing of large volumes of electronic waste.
- Many primary smelters are already available to process electronic waste, which lowers the investments required to recycle them.
Limitations
The major limitation of primary smelting operations to process electronic waste is their relatively low selectivity. As an example, noble metals and chalcogen metalloids are alloyed with copper at the end of the smelting, converting, and refining process. Their recovery requires subsequent hydrometallurgical and electrorefining operations. Other limitations include:
- The high replacement cost of refractory and other critical components. The liquid phases that are formed during smelting are chemically aggressive and wear many critical components of the vessel.
- The initial investments to build such processes when not available.
- The need, in many cases, to simultaneously process concentrates when recycling electronic waste to ensure a proper operation of the smelter.
Finally, it is to be mentioned that the sustainability of the primary metal-smelting processes to recycle electronic waste will need to be analyzed in the future, especially in the context of the establishment of an authentic circular economy. The reduction of harmful gaseous compounds such as dioxins and brominated compounds via improved scrubbing operations is another important aspect that will need to be addressed in the future to make these smelting operations more sustainable.
7.3.2 Electrochemical Processes
As mentioned in the introduction of Section 7.3 of this chapter, one interesting characteristic of electronic waste is several metals’ simultaneous presence in their fully reduced state. This contrasts with the oxidized states they naturally adopt in ores. The reduction operations required to extract metals are energy-intensive, especially for reactive elements like aluminum, magnesium, and lithium. Therefore, there is the potential for huge energy savings to be made if one can design a process that accounts for this feed specificity. Electrochemical processes fall into this opportunistic category. Su (2020) presented a review of the different electrochemical methods that exist to recycle solid and liquid waste (see Figure 7.8). It includes electrolysis, electrodialysis, electrocoagulation, and electroflotation. In this chapter, we only focus on electrolytic processes.
In a nutshell, an electrolytic process requires an electrical system constituted of a conductive anode and cathode, and an electrolyte (which can host metallic cations), and a power source. It is also important that this electrolyte possess a good ionic conductivity via highly mobile ions. Electrical work is transferred to the system to overcome the positive Gibbs free energy variation associated with the overall oxidoreduction reaction that leads to the production of metal (as well as the energy associated with anodic and cathodic overpotentials). The anode hosts the oxidation reaction (which typically leads to the production of O2, Cl2, or CO2), while the cathode hosts the metallic reduction (the metal to be recovered is collected at the cathode). In electrorefining operations (such as in electrolytic refining of copper), the anodic reaction is the oxidation of the metal itself. As it will be presented in this section, the important selectivity of electrolytic processes (linked to the reduction potential uniqueness of each metallic ion) makes them interesting candidates for electronic waste recycling operations in the future. Electrowinning and electrorefining technologies can be divided into low- and high-temperature processes.

Figure 7.8 An overview of select electrochemical approaches for waste recycling and revalorization.
Source: Su (2020). Reprinted with permission from The Electrochemical Society 2020.
7.3.2.1 High-Temperature Electrolysis
In extractive metallurgy, many reactive metals can be produced using high-temperature electrolysis. This is typically performed in molten salts such as chlorides, fluorides, and chlorofluorides. Excellent review work on the subject is presented by Yan and Fray (2010). A famous example of high-temperature electrolysis is the production of aluminum via the Hall–Heroult process, which uses cryolite (Na3AlF6) as the electrolyte. Magnesium and lithium can also be commercially produced via high-temperature electrolysis using molten chloride electrolytes. One significant advantage of using chloride systems is that they melt at relatively low temperatures when compared to oxide-equivalent systems. It is also possible to design a molten chloride electrolyte that is not too volatile and has good ionic conductivity. Fluoride salts can dissolve oxides, which is a major advantage in primary metal production (e.g. alumina in cryolite). However, fluoride melts tend to be more aggressive and are liquid in higher temperature ranges.
In the context of recycling electronic waste, the use of high-temperature electrolysis would normally require a chlorination step to oxidize their metallic components. This step could be done via a roasting strategy. During the molten chloride electrolysis, Cl2(g) is evolved at the anode and can be recycled in the chlorination/roasting step or used in situ to chlorinate metallic components. The main environmental concern with this process is the accidental release of chlorine in the atmosphere as a result of a process failure. The material selection to building the equipment for handling chlorination operations is also critical and challenging. Applications of molten chloride electrolysis to recover rare-earth elements from electronic waste were recently reviewed by Xi et al. (2020). They reported Abbasalizadeh et al. (2016)’s work in which AlCl3 is used as the chlorination agent to transfer Nd and Dy (via an exchange reaction) into the eutectic LiCl-KCl-NaCl molten chloride. Nd and Dy’s transfer in the salt is possible as their respective chloride (NdCl3 and DyCl3) are more thermodynamically stable than AlCl3.

Figure 7.9 Flowsheet of the RE recovery from NdFeB scrap using molten chlorides.
Source: Hua et al. (2014).
Interestingly, this chlorination strategy will not transfer iron and boron in the molten chloride since their chlorides are less stable than AlCl3. As a result, the electrodeposited rare-earth metals are free of iron. Another strategy to chlorinate the rare-earth elements from magnet scrap is to use a molten MgCl2-KCl bath (Hua et al. (2014)). In this case, the electrolysis leads to an Mg-Nd alloy’s electrodeposition, which is inevitable because of the small reduction potential differences between these two elements. The proposed flowsheet of Hua et al. (2014) to process NdFeB scrap is presented in Figure 7.9.
7.3.2.2 Low-Temperature Electrolysis
Even though low-temperature electrolytic operations using aqueous solution should not be considered as pyrometallurgical processes, their technological importance in refining primary metals and in recycling e-waste motivated an overview of these processes in this section. Low-temperature electrolytic processes typically use sulfuric, hydrochloric, and boric acid solutions (Jin and Zhang (2020)) as well as ionic liquids. It is interesting to note that conventional hydrometallurgical processes to recover valuable metals from electronic waste use similar aqueous solution to perform the chemical leaching (Su 2020), i.e. the first step of the extraction. The subsequent purification and recovery steps in extractive hydrometallurgy are based on completely different strategies such as precipitation and cementation. Here are some recent examples of low-temperature electrolytic processes to recycle electronic waste.
- Copper recovery from e-waste: There is rich literature on the recovery of copper, which is the main metallic element contained in WPCBs using low-temperature electrolytic processes. In their work, Fogarasi et al. (2013) presented the two main electrolytic routes that can be taken to perform the copper recovery (Figure 7.10). In route A (sulfuric acid electrolyte), copper’s direct electrochemical oxidation is performed, followed by the electrodeposition of pure copper at the cathode. In route B, the Fe3+/Fe2+ redox pair is used to achieve a faster dissolution of copper (Cu). Fogarasi et al. (2013) reported that route A leads to the extraction of a 98% purity of copper for the energy consumption of 1.06 kWh per kg, while route B produces a 99% purity of copper energy consumption of 1.75 kWh per kg. Here are other examples of copper extraction based on similar processes.
Song et al. (2021) were able to electrochemically extract 95 wt% of the copper dissolved in a multimetal sulfuric acid leaching solution of waste liquid crystal display panels that contained indium, copper, aluminum, and molybdenum. Barragan et al. (2020) proposed a new strategy for the efficient recovery of highly pure copper and antimony (in the form of Sb2O3) from WPCB by leaching the waste in hydrochloric acid solution that also contained ferric chloride (to accelerate the copper dissolution), followed by a precipitation step and an electrowinning process. Zhang et al. (2018) proposed a greener ionic liquid electrolyte that contains N-butyl sulfonate pyridinium hydrosulfate, i.e. [BSO3HPy]HSO4, to replace H2SO4 in a slurry electrolytic system made of a graphite anode and a titanium cathode. The optimal replacement of 10% H2SO4 by this ionic liquid allowed to obtain a copper recovery of 90.94% with a purity of 81.69%. Finally, it is worth mentioning the technoeconomic assessment for the recovery of metals from WEEE reported by Diaz and Lister (2018), which compared the low-temperature electrolytic recycling technology to the black copper-smelting operations (which also include an electrolytic step to produce pure copper). As explained previously, the black copper-smelting route of low-grade scrap starts with its melting and subsequent reduction using coke. An oxidizing step is then performed to remove less noble elements like iron, lead, zinc, and tin. The more noble metals (Ni, Ag, Au, and platinum groups) are collected in the anode mud of the electrorefining process. In their study, Diaz and Lister (2018) noted a competitive alternative to process electronic waste via the low-temperature electrolytic recycling technology alone when compared to black copper smelting. A significantly lower capital investment can be achieved with the electrochemical process (i.e. 2.9 kg e-waste per dollar of capital investment) compared to the black copper-smelting process (i.e. 1.3 kg per dollar of capital investment). This is not a surprising conclusion and validates the conventional industrial practices that use leaching and electrolytic refining to extract copper from copper oxide ores. In this case, the main difference is the much higher quantity of impurities in the e-waste (compared to the oxide concentrate), which will require larger electrolyte purification units.

Figure 7.10 Block diagram of the copper recovery process from WPCBs by mediated electrochemical oxidation and cathodic deposition using sulfuric acid electrolyte (Route A) and hydrochloric-acid-containing FeCl3 electrolyte (Route B).
Source: Fogarasi et al. (2013).
- Noble metal and rare-earth recovery: The acidic sulfate electrolytic solution typically used to electrorefine copper is not strong enough to dissolve noble metals, which end up in the anode mud. Because of that, a stronger electrolyte would be required to extract them via a subsequent electrolytic process. Lister et al. (2014) reported such a two-stage electrorecycling strategy (Figure 7.11). In the first stage of the process, iron, nickel, tin, copper, silver, and rare-earth elements are dissolved in an acidic sulfate solution. Copper and silver are then plated at the cathode. Rare-earth elements dissolved in the solution could be potentially recovered at this stage by precipitation using sulfate double salts. In stage 2, pallidum and gold could be dissolved in an HCl solution (via the anodic production of Cl2) and finally plated at the cathode.
It is also to be noted that the reduction potential of copper and gold is significantly different (i.e. +0.34 V vs +1.83 V relative to the standard hydrogen electrode). Therefore, it is interesting to evaluate the possibility to electrodeposit each metal from a single electrolytic solution selectively. This is a particularly attractive strategy if one can find a more environment-friendly electrolyte than cyanide solution that can efficiently dissolve gold such as ammoniacal thiosulfate solutions. Kasper et al. (2018) studied the electrochemical behavior of gold and copper simultaneously present in such a solution. They performed electrowinning tests using graphite electrodes to conclude that it is feasible to use such an electrolyte to extract copper and gold at two distinct potentials. Unfortunately, the purity of the individual metal deposits was not reported in their study.

Figure 7.11 Proposed flow sheet for a mobile electronic recycling by Lister et al. (2014).
Source: Lister et al. (2014).
7.3.3 Other Pyrometallurgical Operations Used in Electronic Waste Recycling
7.3.3.1 Roasting
Roasting is a pyrometallurgical treatment that can be used during the recycling process of electronic waste. It consists of heating a system to a specific temperature in some reactants’ presence (which can be solid, liquid, or gas). Solid–gas reactions are often promoted in these reactors. In the case of electronic waste, Panda et al. (2020) recently proposed a low-temperature chlorination roasting step of pyrolyzed WPCB using ammonia chloride (NH4Cl) in air at temperatures between 200–325 °C (Figure 7.12).
The thermal decomposition of NH4Cl leads to the production of H2(g) and HCl(g). The latter gaseous compound acts as the chlorination agent via a chemical reaction of the following type (e.g. copper):
This approach provides a recovery of 93% for Cu and 100% for Ni, Zn, and Pb using a roasting temperature of 300 °C in the form of metallic chlorides. These metallic chlorides could in turn be processed using a molten chloride electrolytic method as detailed previously. Roasting strategies have also been used in the recycling of permanent magnets. Yoon et al. (2014) used oxidation roasting at 600 °C to convert Nd into Nd2O3, which was then processed via leaching.
7.3.3.2 Molten Salt Oxidation Treatment
Molten salt oxidation treatment is another pyrometallurgical strategy that can be used to recycle electronic waste. It relies on the introduction of electronic waste in a molten salt bath (which can be a eutectic carbonate or hydroxide mixture). As explained by Flandinet et al. (2012), these molten salts are specifically selected for their ability to dissolve solid undesirable e-waste components such as oxides, glasses, and plastics, as well as gaseous compounds (CO2 and halogenated compounds). It is also important to prevent the dissolution of metals in the molten salt to recover them more easily, it is not always desired to generate a more severe oxidizing environment (using air injection in the reactor for example) as it could ultimately lead to the transfer of valuable metals into the salt. In their work, Flandinet et al. (2012) used a eutectic KOH-NaOH molten salt at 300 °C to treat WPCBs. They were able to virtually recover all the metallic fraction of the WPCBs. This approach also prevented the release in the gas phase of most of the halogenated compounds, which were trapped in the molten salt.

Figure 7.12 Flowsheet of the recycling process of pyrolyzed printed circuit boards using a roasting strategy.
Source: Panda et al. (2020). Reprinted with permission from Journal of Hazardous Materials 2020.
Lin et al. (2017) used a different approach based on the use of a molten (Li,Na,K)2CO3 eutectic carbonate salt reactor operated at temperatures between 550 and 700 °C. They also performed air injection to promote the oxidation reactions. Overall, they were able to recover 95% of the copper available in the WPCB even though they expected more copper oxidation (which should have lowered its recovery efficiency). Other molten salts have been studied in the literature, such as the NaOH-Na2CO3-NaNO3 salt (Liu et al. (2016)) and LiCl–KCl eutectic mixtures (see for example Riedewald and Sousa-Gallagher (2015)) (Figure 7.13).
7.3.3.3 Distillation
As discussed previously, some metals (like cadmium, zinc, and lead) contained in e-waste are more volatile than others (like copper and tin). One can take advantage of this property to separate and purify some metallic fractions by distillation. Figure 7.14 shows the evolution of the vapor pressure (expressed in log10(Patm)) of some pure metals calculated using the FactSage software. Lead chloride is also presented in Figure 7.14 to see the impact of chlorination on the lead volatility. The boiling point at 1 atm for Cd (766.5 °C), Zn (908.3 °C) and PbCl2 (950.8 °C) is also clearly visible in Figure 7.14 (i.e. the temperature at which the vapor pressure reaches 1 atm). A threshold vapor pressure of 10−6 atm is also drawn in Figure 7.14. It represents a typical vapor pressure above which volatilization becomes experimentally non-negligible in open systems.

Figure 7.13 Flowsheet of a molten NaOH-KOH eutectic salt oxidation process (a) to recover the metallic fraction of WPCb (b).
Source: Flandinet et al. (2012). Reprinted with permission from Journal of Hazardous Materials 2012.
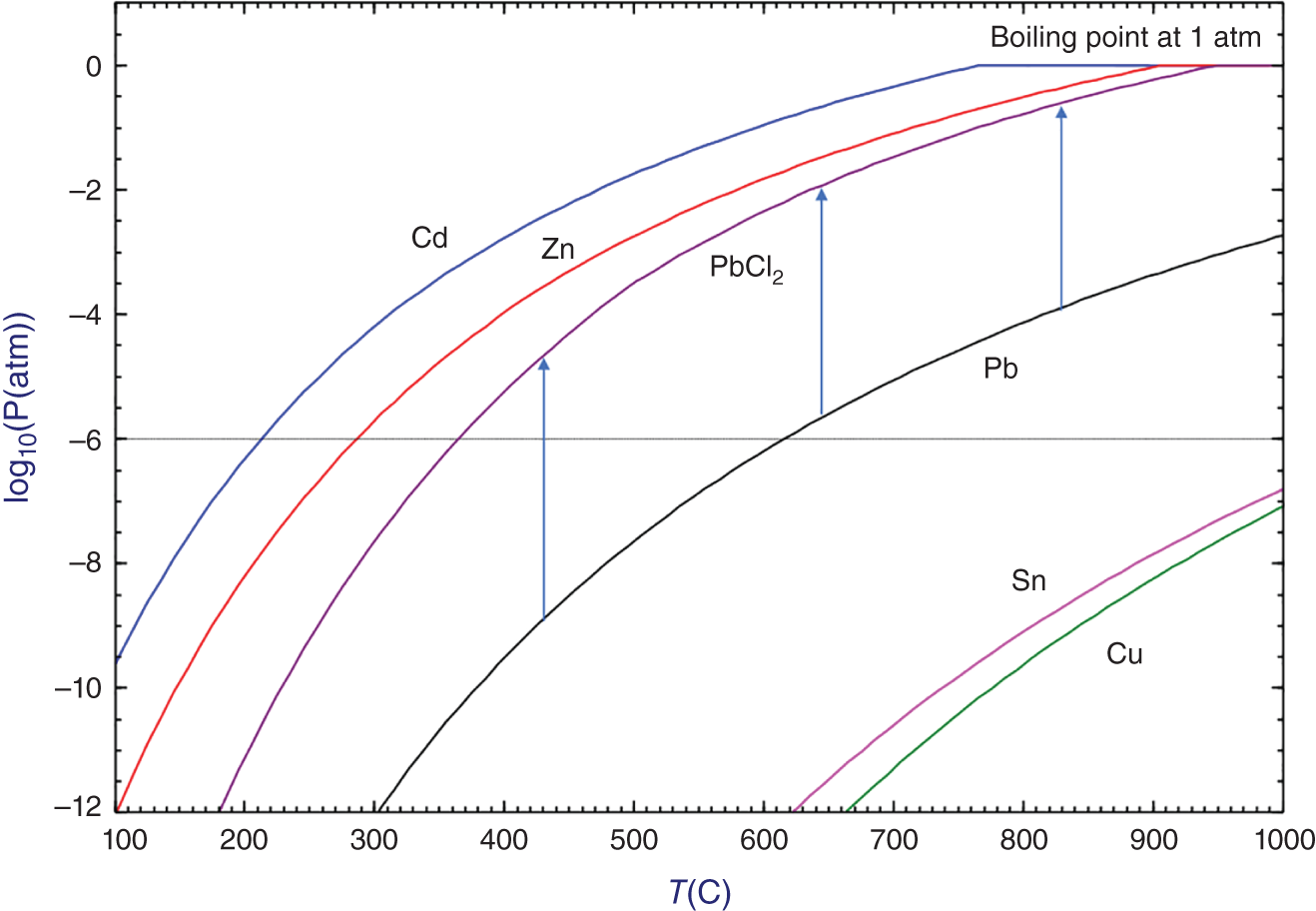
Figure 7.14 Evolution of the vapor pressure of different metallic elements as a function of temperature as calculated by the FactSage software.
Zhan and Xu (2008) explored the possibility to remove zinc from Cu-rich fractions via vacuum separation at 1123 K. The application of a vacuum lowers the total pressure of the system, which increases the volatility of all the metals at a given temperature. Using such an approach, they were able to purify copper particle obtained from WPCBs from and original purity of 90.68 wt% to a final purity of 99.84 wt% by volatilizing zinc. These authors presented a series of experimental studies on the purification of copper-rich particle using vacuum separation as reported by Ma (2019a).
7.3.3.4 Pyrolysis
Pyrolysis is another important recycling operation, which allows an efficient and more environment-friendly separation of organic and volatile components from the metallic fraction of WPCB. It is defined as the thermal degradation of organic materials under vacuum or inert conditions to produce value-added products such as oil, gas, and carbon black (Xiong et al. 2020; Jadhao et al. 2020; Huang and Lo 2020). The use of conventional or microwave pyrolysis in recycling electronic plastics such as high-impact polystyrene (HIPS) and acrylonitrilebutadiene styrene (ABS) widely emerged in the last decade due to the several advantages it offers: (i) it closes the loop of waste plastic stream, (ii) it produces value-added products at low operating costs and temperature, and (iii) it potentially prevents the release of toxic gases when compared to combustion and incineration processes(Czégény et al., 2012; Miskolczi et al. 2008).
The pioneering work of de Marco et al. (2008) provides fundamental data about the pyrolysis products of electronic wastes. According to these authors, the solids that remain after the pyrolysis are composed of metals and ceramics mixed with about 3–34 wt% of char-like carbonaceous compounds. The collected condensable gases produce a low-viscosity liquid composed of aromatic compounds (in the case of WPCB). The noncondensable pyrolysis gases are composed of light hydrocarbon molecules as well as significant amounts of CO and CO2. It is to be noted that one major challenge that limits the use of e-waste pyrolysis byproducts is the presence of halogenated flame retardants, mostly BFRs such as tetrabomobisphenol A (TBBPA). These brominated compounds contaminate the collected liquids and gaseous products by producing bromophenols and release toxic emissions such as hydrogen bromide (HBr) (Das et al. 2020; Ma and Kamo 2019).
Pyrolysis is typically an endothermic process that requires energy to proceed. Conventional pyrolysis methods of e-waste plastics or WPCB include fixed-bed reactors (Hall and Williams 2007), fluidized-bed reactors (Zhang et al., 2018; Guo et al. 2010), and rotating reactors (Ma et al. 2018). The performance of all these pyrolysis strategies is well documented in the literature. The use of microwave irradiation to perform the pyrolysis of WPCB is an interesting alternative to these conventional methods because of the following advantages (Huang et al., 2016): (i) it is a volumetric heating method (and therefore more energy efficient), (ii) it is a selective-heating method; (iii) it is a high-heating-rate method, and (iv) it is an easy-to-control (start/stop) method. Zhang et al. (2021) recently proposed a microwave-assisted catalytic process to pyrolyze WPCB.
Other scientific advances and progress in the field of e-waste pyrolysis in recent years mainly focus on three distinct aspects, which are:
- Fundamental description of the degradation mechanisms and kinetics of WPCBs: The reaction mechanisms and pathways for the degradation of WPCB and e-waste plastics (as well as their corresponding kinetics) have been extensively studied in the literature (see for example Yao et al. (2020);Alenezi and Al-Fadhli (2018); Quan et al. (2009); Cai and Shao-hong (2006)). Thermal analysis methods such as the thermogravimetric analysis (TGA) and the differential scanning calorimetry (DSC) have been used to identify reaction mechanisms at the laboratory-milligram scale. Larger-scale experiments typically performed in tubular furnaces are also frequently used in the literature to analyze and characterize the different pyrolysis products (i.e. the solid residue, the condensate, and the volatile fraction).
Studies on the pyrolysis pathways of WPCB reported that hydrogen bromide is produced during the initial step of the process, followed by the depolymerization of epoxy resins, random rupture, and reactions of free radical to form the different pyrolysis products (Gao et al. 2021; Gao and Xu 2019; Ma and Kamo, 2018). The effect of different process parameters (such as the heating rate, the carrier gas, and the operating temperature) on the degradation mechanisms and the composition of the pyrolysis products have also been reported by many authors (Diaz et al. 2018; Evangelopoulos et al. 2017; XIE et al. 2016).
Interestingly, the presence of copper is of prime importance during the pyrolysis of WPCB as it acts as a catalyst to promote some chemical reactions, especially in the presence of brominated compounds (Gao et al. 2021; Liu et al. 2018; Ma and Kamo 2018). More specifically, copper changes the pyrolysis mechanism of WPCB by promoting the conversion of organic bromides to Br2 and HBr, while reducing the apparent activation energy (Ea) of the overall pyrolysis reaction (Gao et al. 2021). Ma and Kamo (2018) also noted that the simultaneous addition of iron and nickel particles in a two-stage pyrolysis bed reactor leads to a synergistic effect on the product yields of WPCB. Their presence promotes the formation of phenol and other aromatic compounds, such as benzene and toluene.
- Recovery of metals from the solid residue: Pyrolysis of WPCB also proved to be a promising technique to efficiently separate the volatile organic fraction from the valuable metallic components. The solid residue that contains these valuable metals (along with oxides and carbonaceous compounds) is then processed via hydrometallurgical or electrometallurgical methods to selectively recover each metal. Jadhao et al. (2020) proposed a new approach to recover the metallic fraction of WPCB using a chemical-free green ultrasonication technology of the solid residue obtained after a mild-temperature pyrolysis process performed between 200 and 600 °C. Many other approaches have been proposed to improve individual metal recovery from solid pyrolysis residue. It includes: (i) low-temperature alkaline smelting coupled with liquid–liquid phase separation (Chen et al. (2020), (ii) combination of physical separation methods such as sieving, gravity separation, and magnetic separation (Wang et al. 2020), and (iii) the use of higher pyrolysis temperatures (i.e. 850 °C) to obtain distinct copper-rich, lead-tin-rich, and slag-rich residues to promote more efficient partitioning of rare-earth elements (Khanna et al. 2018).
- Capture and stabilization of brominated compounds: Copyrolysis of e-waste plastics or WPCB in the presence of alkali and alkaline earth-based additives (such as CaO and CaCO3) significantly enhance the quality of the pyrolytic liquid products (Gao and Xu 2019; Xie et al. 2017; Kumagai et al. 2017; Jung et al. 2012; Zuo et al., 2011; Bhaskar et al. 2004). These additives stabilize the hydrogen bromide emissions in the form of brominated salt compounds via a reaction such as the following:

As a result, bromine is not transferred to the gas phase, which prevents the contamination of the collected pyrolytic oil and gas. There are still several important scientific elements to understand regarding the identification and thermal stability of the brominated compounds formed during the pyrolysis, which may impact the recovery of valuable metals.
References
- Abbasalizadeh, A., Teng, L., Seetharaman, S. et al. (2016). Chapter 24 – Rare earth extraction from NdFeB magnets and rare earth oxides using aluminum chloride/fluoride molten salts. In: Rare Earths Industry (eds. I.B. De Lima and W.L. Filho), 357–373. Elsevier ISBN 9780128023280, https://doi.org/10.1016/B978-0-12-802328-0.00024-3.
- Aboughaly, M. and Gabbar, H.A. (2020). Recent technologies in electronic-waste management. In: E-waste Recycling and Management, 63–80. Cham: Springer.
- Adusei, A., Arko-Mensah, J., Dzodzomenyo, M. et al. (2020). Spatiality in health: the distribution of health conditions associated with electronic waste processing activities at Agbogbloshie, Accra. Annals of Global Health 86 (1): 31. https://doi.org/10.5334/aogh.2630.
- Alenezi, R.A. and Al-Fadhli, F.M. (2018). Thermal degradation kinetics of waste printed circuit boards. Chemical Engineering Research and Design 130: 87–94.
- Álvarez-de-los-Mozos, E., Rentería-Bilbao, A., and Díaz-Martín, F. (2020). WEEE recycling and circular economy assisted by collaborative robots. Applied Sciences 10 (14): 4800.
- Anindya, A., Swinbourne, D.R., Reuter, M.A., and Matusewicz, R.W. (2013). Distribution of elements between copper and FeOx–CaO–SiO2 slags during pyrometallurgical processing of WEEE: Part 1–Tin. Minerals Processing and Extractive Metallurgy 122 (3): 165–173.
- Arnout, S., Nagels, E., and Blanpain, B. (2011). Thermodynamics of lead recycling. In: Proceedings – European Metallurgical Conference, EMC 2011, vol. 2, 363–372.
- Ayres, R.U., Ayres, L.W., and Råde, I. (2003). Lead, zinc and other byproduct metals. In: The Life Cycle of Copper, Its Co-Products and Byproducts, 101–147. Dordrecht: Springer.
- Bakker, M.L., Nikolic, S., and Mackey, P.J. (2011). ISASMELT™ TSL – applications for nickel. Minerals Engineering 24 (7): 610–619.
- Barragan, J.A., Ponce de León, C., Alemán Castro, J.R. et al. (2020). Copper and antimony recovery from electronic waste by hydrometallurgical and electrochemical techniques. ACS Omega 5 (21): 12355–12363.
- Bhaskar, T., Uddin, A., Kaneko, J. et al. (2004). Pyrolysis of polypropylene/polyethylene/polystyrene and polyvinylchloride mixed plastics using CaCO3. Progress in Rubber, Plastics and Recycling Technology 20 (2): 163–170.
- Cai, G.G.C.L.Q. and Shao-hong, M.Z.P. (2006). Pyrolysis kinetics of waste print circuit board in vacuum condition. Journal of South China University of Technology 3.
- Chaidez-Felix, J., Romero-Serrano, A., Hernandez-Ramirez, A. et al. (2014). Effect of copper, sulfur, arsenic and antimony on silver distribution in phases of lead blast furnace. Transactions of Nonferrous Metals Society of China 24 (4): 1202–1209.
- Charitopoulou, M.A., Kalogiannis, K.G., Lappas, A.A., and Achilias, D.S. (2020). Novel trends in the thermo-chemical recycling of plastics from WEEE containing brominated flame retardants. Environmental Science and Pollution Research: 1–24. https://doi.org/10.1007/s11356-020-09932-5.
- Charles, R.G., Douglas, P., Dowling, M. et al. (2020). Towards increased recovery of critical raw materials from WEEE – evaluation of CRMs at a component level and pre-processing methods for interface optimisation with recovery processes. Resources, Conservation and Recycling 161: 104923.
- Chatterjee, S. (2012). Sustainable electronic waste management and recycling process. American Journal of Environmental Engineering 2 (1): 23–33.
- Chen, B., He, J., Sun, X. et al. (2020). Separating and recycling metal mixture of pyrolyzed waste printed circuit boards by a combined method. Waste Management 107: 113–120.
- Cui, H. and Anderson, C. (2020). Hydrometallurgical treatment of waste printed circuit boards: Bromine leaching. Metals 10 (4): 462.
- Cui, J. and Zhang, L. (2008). Metallurgical recovery of metals from electronic waste: a review. Journal of Hazardous Materials 158 (2): 228–256.
- Czégény, Z., Jakab, E., Blazsó, M. et al. (2012). Thermal decomposition of polymer mixtures of PVC, PET and ABS containing brominated flame retardant: formation of chlorinated and brominated organic compounds. Journal of Analytical and Applied Pyrolysis 96: 69–77.
- Das, P., Gabriel, J.C.P., Tay, C.Y., and Lee, J.M. (2020). Value-added products from thermochemical treatments of contaminated e-waste plastics. Chemosphere: 129409.
- De Marco, I., Caballero, B., Chomon, M. et al. (2008). Pyrolysis of electrical and electronic wastes. Journal of Analytical and Applied Pyrolysis 82 (2): 179–183.
- Diaz, L.A. and Lister, T.E. (2018). Economic evaluation of an electrochemical process for the recovery of metals from electronic waste. Waste Management 74: 384–392.
- Diaz, F., Flerus, B., Nagraj, S. et al. (2018). Comparative analysis about degradation mechanisms of printed circuit boards (PCBS) in slow and fast pyrolysis: the influence of heating speed. Journal of Sustainable Metallurgy 4 (2): 205–221.
- Espinosa, D.C.R., Moraes, V.T., and Tenorio, J.A.S. (2015). Pyrometallurgical processing. In: Electronic Waste. Topics in Mining, Metallurgy and Materials Engineering (eds. H. Veit and A. Moura Bernardes), 81–85. Cham: Springer https://doi.org/10.1007/978-3-319-15714-6_8.
- Evangelopoulos, P., Kantarelis, E., and Yang, W. (2017). Experimental investigation of pyrolysis of printed circuit boards for energy and materials recovery under nitrogen and steam atmosphere. Energy Procedia 105: 986–991.
- Flandinet, L., Tedjar, F., Ghetta, V., and Fouletier, J. (2012). Metals recovering from waste printed circuit boards (WPCBS) using molten salts. Journal of Hazardous Materials 213: 485–490.
- Flores, G.R.A., Nikolic, S., and Mackey, P.J. (2014). ISASMELT™ for the recycling of E-scrap and copper in the US case study example of a new compact recycling plant. JOM 66 (5): 823–832.
- Fogarasi, S., Imre-Lucaci, F., Ilea, P., and Imre-Lucaci, A. (2013). The environmental assessment of two new copper recovery processes from waste printed circuit boards. Journal of Cleaner Production 54: 264–269.
- Franzolin, C.J. (2020). Planned obsolescence resulting from electrical and electronic equipment: waste rights and brazil’s national solid waste policy. In: Sustainable Consumption (eds. A. Amaral Junior, L. Almeida and L. Klein Vieira), 463–477. Cham: Springer https://doi.org/10.1007/978-3-030-16985-5_25.
- Gao, R. and Xu, Z. (2019). Pyrolysis and utilization of nonmetal materials in waste printed circuit boards: debromination pyrolysis, temperature-controlled condensation, and synthesis of oil-based resin. Journal of Hazardous Materials 364: 1–10.
- Gao, R., Liu, B., Zhan, L. et al. (2021). Catalytic effect and mechanism of coexisting copper on conversion of organics during pyrolysis of waste printed circuit boards. Journal of Hazardous Materials 403: 123465.
- Grant, R. (2001). Lead production. In: Encyclopedia of Materials: Science and Technology (eds. K.J. Buschow, R.W. Cahn, M.C. Flemings, et al.), 4439–4442. Ox: Elsevier.
- Guo, Q., Yue, X., Wang, M., and Liu, Y. (2010). Pyrolysis of scrap printed circuit board plastic particles in a fluidized bed. Powder Technology 198 (3): 422–428.
- Hageluken, C. (2006). Recycling of electronic scrap at umicore’s integrated metals smelter and refinery. World of Metallurgy – ERZMETALL 59 (3): 152–161.
- Hall, W.J. and Williams, P.T. (2007). Separation and recovery of materials from scrap printed circuit boards. Resources, Conservation and Recycling 51 (3): 691–709.
- Harikrushnan, B., Shreyass, G., Hemant, G., and Pandimadevi, M. (2016). Recovery of metals from printed circuit boards (PCBS) using a combination of hydrometallurgical and biometallurgical processes. International Journal of Environmental Research 10 (4): 511–518.
- Hua, Z., Wang, J., Wang, L. et al. (2014). Selective extraction of rare earth elements from ndfeb scrap by molten chlorides. ACS Sustainable Chemistry & Engineering 2: 2536–2543.
- Huang, Y.-F. and Lo, S.-L. (2020). Energy recovery from waste printed circuit boards using microwave pyrolysis: product characteristics, reaction kinetics, and benefits. Environmental Science and Pollution Research 27 (34): 43274–43282.
- Huang, Y.-F., Chiueh, P.-T., and Lo, S.-L. (2016). A review on microwave pyrolysis of lignocellulosic biomass. Sustainable Environment Research 26 (3): 103–109.
- Ippolito, N.M., Medici, F., Pietrelli, L., and Piga, L. (2021). Effect of acid leaching pre-treatment on gold extraction from printed circuit boards of spent mobile phones. Materials 14 (2): 362.
- Jadhao, P.R., Ahmad, E., Pant, K., and Nigam, K. (2020). Environmentally friendly approach for the recovery of metallic fraction from waste printed circuit boards using pyrolysis and ultrasonication. Waste Management 118: 150–160.
- Jandric, A., Part, F., Fink, N. et al. (2020). Investigation of the heterogeneity of bromine in plastic components as an indicator for brominated flame retardants in waste electrical and electronic equipment with regard to recyclability. Journal of Hazardous Materials 390: 121899.
- Jin, W. and Zhang, Y. (2020). Economic evaluation of an electrochemical process for the recovery of metals from electronic waste. ACS Sustainable Chemistry & Engineering 8: 4693–4707.
- Jung, S.-H., Kim, S.-J., and Kim, J.-S. (2012). Fast pyrolysis of a waste fraction of high impact polystyrene (HIPS) containing brominated flame retardants in a fluidized bed reactor: the effects of various ca-based additives (CAO, CA(OH)2 and oyster shells) on the removal of bromine. Fuel 95: 514–520.
- Kasper, A.C., Veit, H.M., Garcıa-Gabaldon, M., and Herranz, V.P. (2018). Electrochemical study of gold recovery from ammoniacal thiosulfate, simulating the PCBS leaching of mobile phones. Electrochimica Acta 259: 500–509.
- Khaliq, A., Rhamdhani, M.A., Brooks, G., and Masood, S. (2014). Metal extraction processes for electronic waste and existing industrial routes: a review and Australian perspective. Resources 3 (1): 152–179.
- Khanna, R., Ellamparuthy, G., Cayumil, R. et al. (2018). Concentration of rare earth elements during high temperature pyrolysis of waste printed circuit boards. Waste Management 78: 602–610.
- Kumagai, S., Grause, G., Kameda, T., and Yoshioka, T. (2017). Thermal decomposition of tetrabromobisphenol – a containing printed circuit boards in the presence of calcium hydroxide. Journal of Material Cycles and Waste Management 19 (1): 282–293.
- Laubertova, M., Piroskova, J., and Dociova, S. (2017). The technology of lead production from waste. World of Metallurgy - ERZMETALL 70 (1): 47–54.
- Lin, C., Chi, Y., and Jin, Y. (2017). Experimental study on treating waste printed circuit boards by molten salt oxidation. Waste and Biomass Valorization 8 (7): 2523–2533.
- Lister, T.E., Wang, P., and Anderko, A. (2014). Recovery of critical and value metals from mobile electronics enabled by electrochemical processing. Hydrometallurgy 149: 228–237.
- Liu, J., Guo, X., Liu, Y. et al. (2016). Effects of alkali-salt fusion process on recovery of amphoteric metals from waste printed circuit boards. Minerals Processing and Extractive Metallurgy 125 (4): 211–215.
- Liu, W., Xu, J., Han, J. et al. (2018). Kinetic and mechanism studies on pyrolysis ofprinted circuit boards in the absence and presence of copper. ACS Sustainable Chemistry & Engineering 7 (2): 1879–1889.
- Luda, M.P. (2011). Recycling of printed circuit boards. In: Integrated Waste Management - Volume II (ed. S. Kumar). Inte-chOpen, https://doi.org/10.5772/17220. Available from: https://www.intechopen.com/books/integrated-waste-management-volume-ii/recycling-of-printed-circuit-boards.
- Ma, E. (2019a). Chapter 11 – recovery of waste printed circuit boards through pyrometallurgy. In: Electronic Waste Management and Treatment Technology (eds. M.N.V. Prasad and M. Vithanage), 247–267. Butterworth-Heinemann, ISBN 9780128161906, https://doi.org/10.1016/B978-0-12-816190-6.00011-X.
- Ma, E. (2019b). Recovery of waste printed circuit boards through pyrometallurgy. In: Electronic Waste Management and Treatment Technology, 247–267.
- Ma, C. and Kamo, T. (2018). Two-stage catalytic pyrolysis and debromination of printed circuit boards: effect of zero-valent fe and ni metals. Journal of Analytical and Applied Pyrolysis 134: 614–620.
- Ma, C. and Kamo, T. (2019). Enhanced debromination by Fe particles during the catalytic pyrolysis of non-metallic fractions of printed circuit boards over ZSM-5 and Ni/Sio2-Al2O3 catalyst. Journal of Analytical and Applied Pyrolysis 138: 170–177.
- Ma, H., Du, N., Lin, X. et al. (2018). Experimental study on the heat transfer characteristics of waste printed circuit boards pyrolysis. Science of the Total Environment 633: 264–270.
- Miskolczi, N., Hall, W.J., Angyal, A. et al. (2008). Production of oil with low organobromine content from the pyrolysis of flame retarded hips and ABS plastics. Journal of Analytical and Applied Pyrolysis 83 (1): 115–123.
- Moosakazemi, F., Ghassa, S., Soltani, F., and Mohammadi, M.R.T. (2020). Regeneration of sn-pb solder from waste printed circuit boards: a hydrometallurgical approach to treating waste with waste. Journal of Hazardous Materials 385: 121589.
- Needhidasan, S., Samuel, M., and Chidambaram, R. (2014). Electronic waste – an emerging threat to the environment of urban indi. Journal of Environmental Health Science and Engineering 12: 36.
- Panda, R., Jadhao, P.R., Pant, K.K. et al. (2020). Eco-friendly recovery of metals from waste mobile printed circuit boards using low temperature roasting. Journal of Hazardous Materials 395: 122642.
- Quan, C., Li, A., and Gao, N. (2009). Thermogravimetric analysis and kinetic study on large particles of printed circuit board wastes. Waste Management 29 (8): 2353–2360.
- Rao, M.D., Singh, K.K., Morrison, C.A., and Love, J.B. (2021). Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. In: Separation and Purification Technology, vol. 263, ISSN 1383-5866, https://doi.org/10.1016/j.seppur.2021.118400, 118400. https://www.sciencedirect.com/science/article/pii/S1383586621001027.
- Riedewald, F. and Sousa-Gallagher, M. (2015). Novel waste printed circuit board recycling process with molten salt. Methods X 2: 100–106.
- Rudnik, E. and Bayaraa, E. (2016). Electrochemical dissolution of smelted low-grade electronic scraps in acid sulfate-chloride solutions. Hydrometallurgy 159: 110–119.
- Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G. (2011a). Chapter 10 – direct-to-copper flash smelting. In: Extractive Metallurgy of Copper, 5e (eds. M.E. Schlesinger, M.J. King, K.C. Sole and W.G. Davenport), 179–190. Oxford: Elsevier.
- Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G. (2011b). Chapter 19 – chemical metallurgyof copper recycling. In: Extractive Metallurgy of Copper, 5e (eds. M.E. Schlesinger, M.J. King, K.C. Sole and W.G. Davenport), 389–396. Oxford: Elsevier.
- Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G. (2011c). Chapter 5 – matte smelting fundamentals. In: Extractive Metallurgy of Copper, 5e (eds. M.E. Schlesinger, M.J. King, K.C. Sole and W.G. Davenport), 73–88. Oxford: Elsevier.
- Song, Q., Liu, Y., Zhang, L., and Xu, Z. (2021). Selective electrochemical extraction of copper from multi-metal e-waste leaching solution and its enhanced recovery mechanism. Journal of Hazardous Materials 407: 124799.
- Su, X. (2020). Electrochemical separations for metal recycling. Electrochemical Society Interface 29 (3): 55–61.
- Tanísalí, E., Ozer, M., and Burat, F. (2021). Precious metals recovery from waste printed circuit boards by gravity separation and leaching. Mineral Processing and Extractive Metallurgy Review 42 (1): 24–37.
- Theo, L. (1998, May). Integrated recycling of non-ferrous metals at Boliden Ltd. Ronnskar smelter. In: Proceedings of the 1998 IEEE International Symposium on Electronics and the Environment. ISEE-1998 (Cat. No. 98CH36145), 42–47. IEEE.
- Tuncuk, A., Stazi, V., Akcil, A. et al. (2012). Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Minerals Engineering 25 (1): 28–37.
- Vaccari, M., Vinti, G., Cesaro, A. et al. (2019). Weee treatment in developing countries: environmental pollution and health consequences – an overview. International Journal of Environmental Research and Public Health 16 (9): 1595.
- Vanparys, R., Brooks, G., Rhamdhani, M.A., and Crivits, T. (2020). Reduction of lead-rich slags with coke in the lead blast furnace. In: PbZn 2020: 9th International Symposium on Lead and Zinc Processing (eds. A. Siegmund, S. Alam, J. Grogan, et al.), 173–185. Cham: Springer International Publishing.
- Vegliò, F. and Birloaga, I. (2018). Waste Electrical and Electronic Equipment Recycling: Aqueous Recovery Methods. Woodhead Publishing.
- Wang, X., Jiao, F., Qin, W. et al. (2020). Combination of pyrolysis and physical separation to recover copper and tin from waste printed circuit boards. JOM 72 (9): 3179–3185.
- Wang, C., Sun, R., and Xing, B. (2021a). Copper recovery from waste printed circuit boards by flotation-leaching process optimized using response surface methodology. Journal of the Air & Waste Management Association.
- Wang, R., Zhang, C., Zhao, Y. et al. (2021b). Recycling gold from printed circuit boards gold-plated layer of waste mobile phones in “mild aqua regia” system. Journal of Cleaner Production 278: 123597.
- Watt, W., Hidayat, T., Shishin, D., and Jak, E. (2018). Advanced thermochemical fundamental and applied research to improve the integrity of the steel water jacketed furnace at port Pirie. In: Extraction 2018, 229–239. Cham: Springer.
- Willner, J., Fornalczyk, A., Cebulski, J., and Janiszewski, K. (2014). Preliminary studies on simultaneous recovery of precious metals from different waste materials by pyrometallurgical method. Archives of Metallurgy and Materials 59 (2) https://doi.org/10.2478/amm-2014-0136.
- Xi, X.-L., Feng, M., Zhang, L.-W., and Nie, Z.-R. (2020). Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. International Journal of Minerals, Metallurgy, and Materials 27: 1599–1617.
- Xie, Y., Sun, S., and Zeng, J. (2016). Effect of pyrolysis conditions on vacuum pyrolysis gaseous product from waste printed circuit board. Environmental Science & Technology: 02.
- Xie, Y., Sun, S., Liu, J. et al. (2017). The effect of additives on migration and 35 transformation of gaseous pollutants in the vacuum pyrolysis process of waste printed circuit boards. Waste Management & Research 35 (2): 190–199.
- Xiong, J., Yu, S., Wu, D. et al. (2020). Pyrolysis treatment of nonmetal fraction of waste printed circuit boards: focusing on the fate of bromine. Waste Management & Research 38 (11): 1251–1258.
- Yan, X.Y. and Fray, D.J. (2010). Molten salt electrolysis for sustainable metals extraction and materials processing: a review. In: Electrolysis: Theory, Types and Applications (eds. S. Kuai and J. Meng), 255–302. Nova Science.
- Yao, Z., Xiong, J., Yu, S. et al. (2020). Kinetic study on the slow pyrolysis of nonmetal fraction of waste printed circuit boards (NMF-WPCBS). Waste Management & Research 38 (8): 903–910.
- Yoon, H.-S., Kim, C.-J., Chung, K.W. et al. (2014). Leaching kinetics of neodymium in sulfuric acid from e-scrap of ndfeb permanent magnet. Korean Journal of Chemical Engineering 31: 706–711.
- Zhan, L. and Xu, Z. (2008). Application of vacuum metallurgy to separate pure metal from mixed metallic particles of crushed waste printed circuit board scraps. Environmental Science & Technology 42 (20): 7676–7681.
- Zhang, L. and Xu, Z. (2016). A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. Journal of Cleaner Production 127: 19–36.
- Zhang, G., He, Y., Feng, Y. et al. (2018). Recovery of residual metals from fine non-metallic fractions of waste printed circuit boards using a vibrated gas-solid fluidized bed. Separation and Purification Technology 207: 321–328.
- Zhang, T., Mao, X., Qu, J. et al. (2021). Microwave-assisted catalytic pyrolysis of waste printed circuit boards, and migration and distribution of bromine. Journal of Hazardous Materials 402: 123749.
- Zhao, B. (2013). Lead and zinc sintering, 165–199. Rijeka: Sintering applications. InTech.
- Zhu, X.-N., Zhang, Y.-K., Zhang, Y.-Q. et al. (2020). Flotation dynamics of metal and non-metal components in waste printed circuit boards. Journal of Hazardous Materials 392: 122322.
- Zuo, X., Damoah, L.N., Zhang, L. et al. (2011). Green pyrolysis of used printed wiring board powders. In: Recycling of Electronic Waste II (eds. L. Zhang and G.K. Krumdick). https://doi.org/10.1002/9781118086391.ch3.
- Zvezdin, A., Di Mauro, E., Rho, D. et al. (2020). En route toward sustainable organic electronics. MRS Energy & Sustainability 7 (16) https://doi.org/10.1557/mre.2020.16.
