References
[1] Leite FL, et al. Nanoneurobiophysics: new challenges for diagnosis and therapy of neurologic disorders. Nanomedicine. 2015;10(23):3417–3419.
[2] O’malley P. Nanopharmacology for the future-think small. Clin. Nurse Spec. 2010;24(3):123–124.
[3] Zhang Y. Nanopharmacology. Acta. Pharmacologica. Sinica. 2006;27:444–1444.
[4] Sharma HS, Sharma A. Neurotoxicity of engineered nanoparticles from metals. CNS Neurol. Disord. Drug Targets. 2012;11(1):65–80.
[5] Sharma HS, Sharma A. Nanowired drug delivery for neuroprotection in central nervous system injuries: modulation by environmental temperature, intoxication of nanoparticles, and comorbidity factors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4(2):184–203.
[6] H.S. Sharmaet al., Nanoparticles influence pathophysiology of spinal cord injury and repair, in: H.S. Sharma, (Ed.). Nanoneuroscience and Nanoneuropharmacology, vol.180, 2009, pp. 155–180 (Progress in brain research).
[7] Sharma HS. Nanoneuroscience and nanoneuropharmacology preface. Nanoneurosci. Nanoneuropharmacol. 2009;180:VII–VII10.
[8] Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010;385(1–2):113–142.
[9] Lal R, Ramachandran S, Arnsdorf MF. Multidimensional atomic force microscopy: a versatile novel technology for nanopharmacology research. AAPS J. 2010;12(4):716–728.
[10] Silva GA. Neuroscience nanotechnology: progress, opportunities and challenges. Nat. Rev. Neurosci. 2006;7(1):65–74.
[11] Barenholz Y. Nanomedicine: shake up the drug containers. Nat. Nanotechnol. 2012;7(8):483–484.
[12] Sharma HS. Nano neuro science: emerging concepts on nanoneurotoxicity and nanoneuroprotection. Nanomedicine. 2007;2(6):753–758.
[13] Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys. Rev. Lett. 1986;56(9):930–933.
[14] Garcia R, Perez R. Dynamic atomic force microscopy methods. Surf. Sci. Rep. 2002;47(6–8):197–301.
[15] Shenai MB, et al. A novel MEA/AFM platform for measurement of real-time, nanometric morphological alterations of electrically stimulated neuroblastoma cells. IEEE Trans. Nanobiosci. 2004;3(2):111–117.
[16] Noy A, Vezenov DV, Lieber CM. Chemical force microscopy. Annu. Rev. Mater. Sci. 1997;27:381–421.
[17] Ito T, Grabowska I, Ibrahim S. Chemical-force microscopy for materials characterization. TrAC Trends Anal. Chem. 2010;29(3):225–233.
[18] Maciaszek JL, et al. Topography of native SK channels revealed by force nanoscopy in living neurons. J. Neurosci. 2012;32(33):11435–11440.
[19] Loginova YF, et al. Biodistribution and stability of CdSe core quantum dots in mouse digestive tract following per os administration: advantages of double polymer/silica coated nanocrystals. Biochem. Biophys. Res. Commun. 2012;419(1):54–59.
[20] Zhou J, Yang Y, Zhang CY. Toward biocompatible semiconductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem. Rev. 2015;115(21):11669–11717.
[21] Donaldson K, et al. Nanotoxicology. Occup. Environ. Med. 2004;61(9):727–728.
[22] Marsh JN, et al. Molecular imaging with targeted perfluorocarbon nanoparticles: quantification of the concentration dependence of contrast enhancement for binding to sparse cellular epitopes. Ultrasound Med. Biol. 2007;33(6):950–958.
[23] Hoshino A, Hanada S, Yamamoto K. Toxicity of nanocrystal quantum dots: the relevance of surface modifications. Arch. Toxicol. 2011;85(7):707–720.
[24] Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006;114(2):165–172.
[25] Prasad BR, et al. Effects of long-term exposure of gelatinated and non-gelatinated cadmium telluride quantum dots on differentiated PC12 cells. J. Nanobiotechnol. 2012;10:4.
[26] Smith JD, et al. The use of quantum dots for analysis of chick CAM vasculature. Microvas. Res. 2007;73(2):75–83.
[27] Kim S, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004;22(1):93–97.
[28] Onoshima D, Yukawa H, Baba Y. Multifunctional quantum dots-based cancer diagnostics and stem cell therapeutics for regenerative medicine. Adv. Drug Deliv. Rev. 2015;95:2–14.
[29] Larson DR, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300(5624):1434–1436.
[30] Bonoiu A, et al. MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res. 2009;1282:142–155.
[31] Basso AS, et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 2008;118(4):1532–1543.
[32] Re F, Gregori M, Masserini M. Nanotechnology for neurodegenerative disorders. Maturitas. 2012;73(1):45–51.
[33] Yang Z, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2010;6(3):427–441.
[34] Melita ED, Purcel G, Grumezescu AM. Carbon nanotubes for cancer therapy and neurodegenerative diseases. Rom. J. Morphol. Embryol. 2015;56(2):349–356.
[35] Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neuro degenerative disorders. Prog. Polym. Sci. 2007;32(8–9):1054–1082.
[36] Zhang LW, et al. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int. J. Toxicol. 2007;26(2):103–113.
[37] Sayes CM, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006;161(2):135–142.
[38] Hwang J, et al. Biofunctionalized carbon nanotubes as substrates for drug delivery and neural regeneration. Tissue Eng. Part A. 2015;21:S366–S1366.
[39] Vergoni AV, et al. Nanoparticles as drug delivery agents specific for CNS: in vivo biodistribution. Nanomed. Nanotechnol. Biol. Med. 2009;5(4):369–377.
[40] Cheung W, et al. DNA and carbon nanotubes as medicine. Adv. Drug Deliv. Rev. 2010;62(6):633–649.
[41] Keefer EW, et al. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 2008;3(7):434–439.
[42] Tosi G, et al. Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J. Controll. Release. 2007;122(1):1–9.
[43] Hu H, et al. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4(3):507–511.
[44] Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000;14(3):175–182.
[45] Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007;7(5):1123–1128.
[46] Matsumoto K, et al. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J. Biosci. Bioeng. 2007;103(3):216–220.
[47] Powell SK, et al. Neural cell response to multiple novel sites on laminin-1. J. Neurosci. Res. 2000;61(3):302–312.
[48] Brambilla D, et al. Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. Nanomed. Nanotechnol. Biol. Med. 2011;7(5):521–540.
[49] Dugan LL, et al. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA. 1997;94(17):9434–9439.
[50] Silva GA. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg. Neurol. 2005;63(4):301–306.
[51] Bar-Shir A, Engel Y, Gozin M. Synthesis and water solubility of adamantyl-OEG-fullerene hybrids. J. Org. Chem. 2005;70(7):2660–2666.
[52] Brown AP, et al. Evaluation of the fullerene compound DF-1 as a radiation protector. Radiat. Oncol. 2010;5:1–9.
[53] Daroczi B, et al. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin. Cancer Res. 2006;12(23):7086–7091.
[54] Lin HS, et al. Fullerenes as a new class of radioprotectors. Int. J. Radiat. Biol. 2001;77(2):235–239.
[55] Patlak M. Nanotechnology takes a new look at old drugs. J. Natl. Cancer Inst. 2010;102(23):1753–1755.
[56] Deda DK, Araki K. Nanotechnology, light and chemical action: an effective combination to kill cancer cells. J. Braz. Chem. Soc. 2015;26(12):2448–2470.
[57] Nance EA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012;4:149.
[58] Kanwar JR, et al. Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomed. Nanotechnol. Biol. Med. 2012;8(4):399–414.
[59] Modi G, et al. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog. Neurobiol. 2009;88(4):272–285.
[60] Abdelwahed W, et al. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006;58(15):1688–1713.
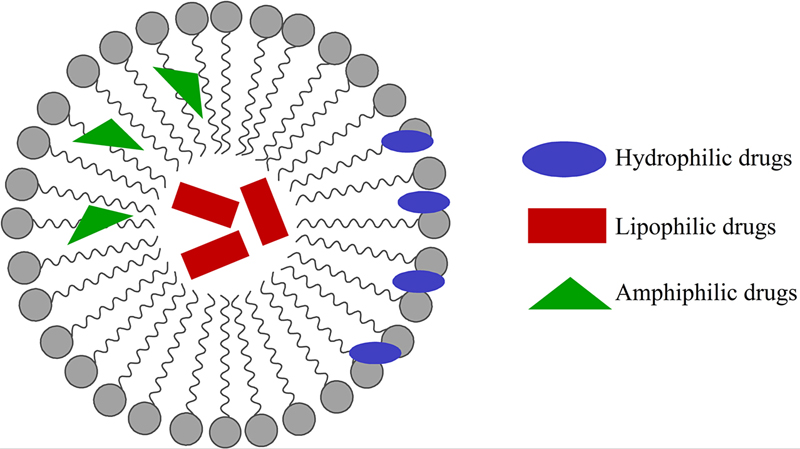
[61] Cevc G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004;56(5):675–711.
[62] Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58(14):1532–1555.
[63] Xiang TX, Anderson BD. Liposomal drug transport: a molecular perspective from molecular dynamics simulations in lipid bilayers. Adv. Drug Deliv. Rev. 2006;58(12–13):1357–1378.
[64] Subramanian S, et al. Nanosponges: a novel class of drug delivery system—review. J. Pharm. Pharm. Sci. 2012;15(1):103–111.
[65] Vinogradov SV, et al. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Controll. Release. 2005;107(1):143–157.
[66] Oishi M, et al. Endosomal release and intracellular delivery of anticancer drugs using pH-sensitive PEGylated nanogels. J. Mater. Chem. 2007;17(35):3720–3725.
[67] Teli MK, Mutalik S, Rajanikant GK. Nanotechnology and nanomedicine: going small means aiming big. Curr. Pharm. Des. 2010;16(16):1882–1892.
[68] Konan YN, Gurny R, Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2002;66(2):89–106.
[69] Schaffazick SR, et al. Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration. Quim. Nov. 2003;26(5):726–737.
[70] Wilson B, et al. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008;1200:159–168.
[71] Wilson B, et al. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008;70(1):75–84.
[72] Rao KS, et al. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29(33):4429–4438.
[73] Voon SH, et al. Vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small. 2014;10(24):4993–5013.
[74] Batrakova EV, et al. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm. Res. 1998;15(10):1525–1532.
[75] J. Kreuter. Application of nanoparticles for the delivery of drugs to the brain, In: A.G. Deboer (Ed.), Drug Transport(ers) and the Diseased Brain, vol. 1277, Elsevier Science Bv, Amsterdam, 2005, pp.85–94 (International Congress Series).
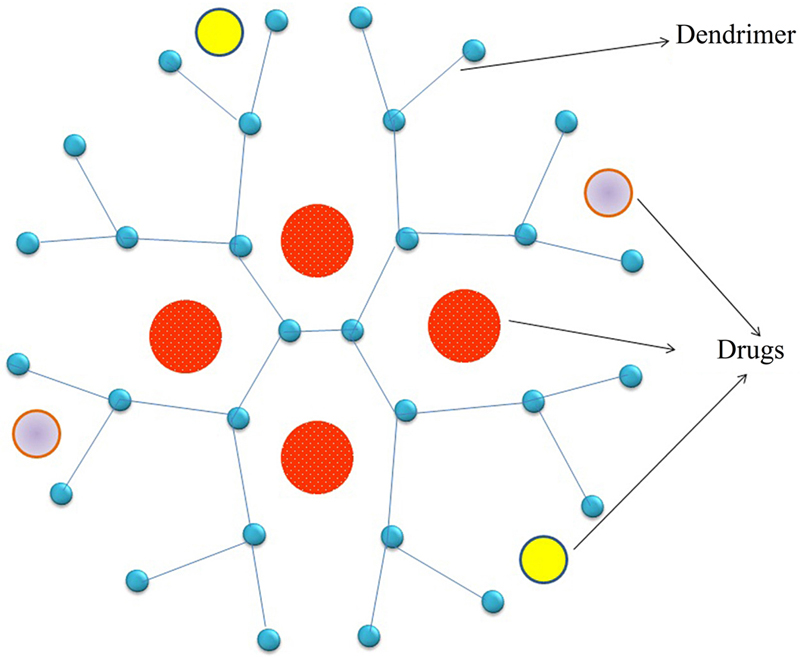
[76] Kannan S, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci. Transl. Med. 2012;4(130).
[77] Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014;39(2):268–307.
[78] Forstner P, et al. Cationic PAMAM dendrimers as pore-blocking binary toxin inhibitors. Biomacromolecules. 2014;15(7):2461–2474.
[79] Trotta F, et al. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014;11(6):931–941.
[80] Torne SJ, et al. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010;17(6):419–425.
[81] Alongi J, et al. Role of beta-cyclodextrin nanosponges in polypropylene photooxidation. Carbohydr. Polym. 2011;86(1):127–135.
[82] Swaminathan S, et al. Formulation of betacyclodextrin based nanosponges of itraconazole. J. Incl. Phenom. Macrocycl. Chem. 2007;57(1–4):89–94.
[83] Wiemer J, et al. Informatics united–exemplary studies combining medical informatics, neuroinformatics, and bioinformatics. Methods Inf. Med. 2003;42(2).
[84] S. Usui, Neuroinformatics research in vision, in: L. Rutkowski, J. Kacprzyk (Eds.), Neural Networks and Soft Computing, 2003 (Advances in Soft Computing).
[85] Usui S. Neuroinformatics research for vision science: NRV project. Biosystems. 2003;71(1–2):189–193.
[86] De Jong P, et al. Initial sequencing and analysis of the human genome (vol 409, pg 860, 2001). Nature. 2001;412(6846).
[87] Simpson AJG, et al. The genome sequence of the plant pathogen Xylella fastidiosa. Nature. 2000;406(6792):151–159.
[88] Bota M, Sporns O, Swanson LW. Neuroinformatics analysis of molecular expression patterns and neuron populations in gray matter regions: the rat BST as a rich exemplar. Brain Res. 2012;1450:174–193.
[89] Usui S, et al. Visiome environment: enterprise solution for neuroinformatics in vision science. Neurocomputing. 2004;58:1097-L1101.
[90] Usui S. Visiome: neuroinformatics research in vision project. Neural Netw. 2003;16(9):1293–1300.
[91] Wrobel A. The need of neuroinformatic approach in functional neurophysiology. Acta Neurobiol Exp. 2005;65(4):421–423.
[92] Klopper R. Future communications: mobile communications, cybernetics, neuroinformatics and beyond. Alternation. 2005;12(1a):121–144.
[93] Yamaji K, et al. Customizable neuroinformatics database system: XooNIps and its application to the pupil platform. Comput. Biol. Med. 2007;37(7):1036–1041.
[94] Usui S, et al. Keyword extraction, ranking, and organization for the neuroinformatics platform. Biosystems. 2007;88(3):334–342.
[95] Parekh R, Ascoli GA. Neuronal morphology goes digital: a research hub for cellular and system neuroscience. Neuron. 2013;77(6):1017–1038.
[96] Gillies A, Willshow D. Neuroinformatics and modeling of the basal ganglia: bridging pharmacology and physiology. Expert Rev. Med. Dev. 2007;4(5):663–672.
[97] Franze K, Guck J. The biophysics of neuronal growth. Rep. Prog. Phys. 2010;73(9).
[98] Iliev IG. Neurobiophysics. Encycl. Appl. Phys. 1994;11:297–322.
[99] S. Chiesa et al., Building an index of nanomedical resources: an automatic approach based on text mining, in: I. Lovrek, R.J. Howlett, et al. (Eds.), Knowledge-Based Intelligent Information and Engineering Systems, Pt 2, Proceedings, vol. 5178, 2008 (Lecture Notes in Artificial Intelligence).
[100] De la Iglesia D et al. Nanoinformatics: new challenges for biomedical informatics at the nano level: Studies in health technology and informatics, 150 Amsterdam: IOS Press BV; 2009: .
[101] Maojo V, et al. Nanoinformatics: developing new computing applications for nanomedicine. Computing. 2012;94(6):521–539.
[102] Maojo V, et al. Nanoinformatics and DNA-based computing: catalyzing nanomedicine. Pediatr. Res. 2010;67(5):481–489.
[103] Gonzalez-Nilo F, et al. Nanoinformatics: an emerging area of information technology at the intersection of bioinformatics, computational chemistry and nanobiotechnology. Biol. Res. 2011;44(1):43–51.
[104] V. L.-A., et al. Action GRID: assessing the impact of nanotechnology on biomedical informatics, AMIA Annu. Symp. Proc. 6 (2008) 1046.
[105] F. Martin-Sanchez et al., A primer in knowledge management for nanoinformatics in medicine, in: I. Lovrek, R.J. Howlett et al. (Eds.), Knowledge-Based Intelligent Information and Engineering Systems, Pt 2, Proceedings, vol. 5178, 2008 (Lecture notes in artificial intelligence).
[106] E. Jakobsson, M.D. Wang, L. Molnar, Bio-nano-info integration for personalized medicine, 2007.
[107] Gruber TR. Toward principles for the design of ontologies used for knowledge sharing. Int. J. Hum. Comput. Stud. 1995;43(5–6):907–928.
[108] De La Iglesia D, et al. International efforts in nanoinformatics research applied to nanomedicine. Methods Inf. Med. 2011;50(1):84–95.
[109] D.G. Thomas et al., Ontologies for cancer nanotechnology research, Embc: 2009 Annual International Conference of the Ieee Engineering in Medicine and Biology Society, vols. 1–20, 2009.
[110] Thomas DG, Pappu RV, Baker NA. NanoParticle Ontology for cancer nanotechnology research. J. Biomed. Inform. 2011;44(1):59–74.