2
Hydrazone Pigments (Formerly Called Azo Pigments)
Hydrazone pigments carry a hydrazone function (NHN) between two sp2-hybridized C atoms. Formerly, hydrazone pigments were called ‘azo pigments’, because they were believed to contain an azo group (NN).
However, in recent decades dozens of single-crystal structure analyses as well as spectroscopic investigations revealed that all commercial ‘azo pigments’ do actually not contain an azo group, but exist as hydrazones instead [1, 2]. Therefore, all commercial ‘azo pigments’ should be called ‘hydrazone pigments’ (Figure 2.1).

Figure 2.1 Molecular structure of Pigment Yellow 74 as determined by single-crystal X-ray analysis [3], clearly proving the hydrazone form. The ellipsoids represent the anisotropic thermal parameters. Colour code: C grey, N blue, O red and H white. All hydrogen atom positions have been determined and refined without constraints. Drawing made with Mercury [3a].
The hydrazone form is found in all types of azo pigments: in pigments based on acetoacetic arylides (a), in pigments based on β-naphthol and its derivatives (b), as well as in pyrazolone pigments (c). Hence the pigments are actually hydrazones of 2,3-diketobutyric amides (a), of 1,2-naphthoquinone (b) and of pyrazolonedione (c):



All benzimidazolone pigments, dihydrazone pigments (formerly called ‘disazo pigments’), dihydrazone condensation pigments (formerly called ‘disazo condensation pigments’) and laked pigments also exhibit the tautomeric hydrazone form instead of the azo form in the solid state [4]. The only ‘real’ azo pigments are the naphthalene sulfonic acid pigment lakes P.R.66 and P.R.67 (Section 2.7.4), which are unable to form tautomeric hydrazone species as they do not contain a hydroxy group in the ortho-position to the azo group.
The formation of the hydrazone form may be explained by the fact that a NN double bond is less stable than a NHNC< fragment. For pigments based on acetoacetic arylides (a) and pyrazolones (c) the hydrazone form is also preferred as a result of an increased conjugation of the π-systems, and a higher number of hydrogen bonds. For β-naphthol and Naphthol AS pigments (b) the formation of the hydrazone tautomer causes a partial loss of aromaticity in the naphthalene moiety, but obviously this does not have a major impact on the stability.
In solution, spectroscopic investigations and quantum-mechanical calculations show that there may be an equilibrium between azo form and the hydrazone form, depending on the substitution pattern, the solvent and the temperature [2].
For two non-commercial β-naphthol pigments with a strongly electron-donating substituent on the phenyl ring, an equilibrium between the tautomeric azo and hydrazone forms (about 80 : 20) was found in the solid state [5]. In this case the azo form is stabilized by mesomerism:

Because of their high solubility these compounds are not commercial pigments.
For all hydrazone pigments, according to the demand for insolubility in water and the commonly used organic solvents, substituents attached to the alkyl, aryl, or heteroaryl groups should not contain sulfo (SO3H) groups or long-chain alkyl groups which render the colourant soluble.
Monohydrazone and dihydrazone pigments contain one or two hydrazone functions, respectively. Compounds with more than two hydrazone groups (tris, tetra,…, polyhydrazones), however, have failed to gain commercial recognition as pigments.
History
The first important event in the history of hydrazone pigments was the discovery of the diazotization reaction by P. Gries in 1858. In 1875, Caro and Witt synthesized chrysoidine, the first azo dye, through a reaction sequence of diazotization and coupling (Section 2.2); a technique that continues to be used today.
Nomenclature
Owing to the complexity of their chemical names, hydrazone pigments are rarely referred to by IUPAC or Chemical Abstracts nomenclature. Practical considerations make it more convenient to classify these compounds according to the nature of the coupling reaction which leads to pigment formation (Section 2.2.2). This system defines the constitution of a pigment by the starting materials for the coupling reaction, the diazo component D and the coupling component C; and by the direction of the coupling (→). The formation of a monohydrazone pigment is thus represented by:
while dihydrazone formation obeys the equation:
This principle is equally accepted in the technical literature.
Although hydrazone pigments exhibit a wide range of colours covering essentially the entire visible spectrum, the blue and green representatives have no commercial significance because of missing brilliance. These shades are made available almost exclusively by phthalocyanine, triarylcarbonium and indanthrone pigments (Sections 3.1, 3.7.3.2 and 4.1). Among the hues produced by hydrazone pigments are all shades of yellow, orange, red, bordeaux, carmine and brown. Within these colour ranges almost all possible hues combined with the demanded properties can be provided together with the necessary properties.
The reaction sequence of diazotization and coupling is the basic reaction of the hydrazone pigment industry. Alternative routes to the formation of hydrazone or azo groups are sometimes used in the production of azo dyes, but they are less commonly employed for hydrazone pigments. Economical production methods make hydrazone pigments by far the largest fraction of organic pigments on the market today. Not only are the starting materials easily accessible (Section 2.1), but the hydrazone group is formed in a coupling reaction that is easily performed on a commercial scale, usually in an aqueous medium.
2.1 Starting Materials
Hydrazone pigments are typically formed by a reaction sequence of diazotization and coupling, involving a primary aromatic amine, which is referred to as a diazo component, and a nucleophilic aromatic or aliphatic compound with active methylene groups as a coupling component [6–8].
2.1.1 Diazo Components
The diazotization reaction typically involves an aromatic amine, such as a mono-, di- or trisubstituted aniline, as a diazo component. Coupling components are also frequently based on aniline or its derivatives. The following examples are thus important diazo components:

Another family of technically important diazo components for pigment formation includes a series of aromatic diamino compounds, primarily 3,3′-dichlorobenzidine, and to a lesser extent 3,3′-dimethoxybenzidine (o-dianisidine), 3,3′-dimethylbenzidine (tolidine) and 2,2′,5,5′-tetrachlorobenzidine:

Polycyclic amines, such as α-aminoanthraquinone, and heterocyclic amines, such as aminophthalimide, aminobenzazoles or aminoquinazolines are less interesting coupling components for hydrazone pigments.
Aromatic aminosulfonic acids, which play a major role in connection with pigment lakes, are produced by sulfonating the corresponding nitro compound and then reducing it to an aminosulfonic acid. An alternative technique, known as baking process, involves exposing an amine/dihydrosulfate to a temperature of 200–300 °C to effect rearrangement to p-aminosulfonic acid. Ortho-sulfonation prevails if the para position is occupied. In contrast to sulfonation techniques with sulfuric acid, this method avoids wastewater contamination with sulfuric acid.

The synthesis of an aromatic amine generally starts from the corresponding nitro compound. Nitration and subsequent reduction are key reactions in the synthesis of intermediates for hydrazone pigments.
Aromatic nitro compounds are obtained by nitrating appropriately substituted benzene derivatives with nitric acid. This reagent can be employed in a more or less concentrated form and is often used in combination with concentrated sulfuric acid as so-called mixed acid. This medium traps water and also has the advantage of serving as a ‘diluting agent’; in other words, as an agent to stabilize the temperature–time curve. It is also a very effective solvent for the nitro compound. Aromatic compounds with free amino functions can be nitrated in the presence of a considerable excess of sulfuric acid (formation of amino sulfates).
Additional substituents may be introduced into the aromatic nucleus by halogenation, oxidation, or nucleophilic replacement. The aromatic halogen is ‘activated’ by electronegative substituents (electron acceptors) in the ortho and para positions. -NO2, -COOH or -CO-alkyl groups are effective electron acceptors. These convert the chlorine atom into a leaving group, which can be replaced by a suitable nucleophilic group, such as -OCH3, -OC2H5 or -NH2.
2.1.1.1 Reduction Methods
There are a few methods of reducing nitro functions to amino groups which are of considerable technical importance:
- Catalytic hydrogenation:
Catalytic hydrogenation with molecular hydrogen is by far the most favoured option. It is carried out at temperatures between 20 and 120 °C and at pressures between 10 and 100 bar in 2–10 m3 tanks, which are pressure and shock resistant. Catalysts typically contain nickel, which may in some cases be replaced by a precious metal, such as palladium or platinum.
- Reduction with iron:
The traditional technique of reducing nitro compounds with iron powder in dilute acid (Béchamps–Brimmeyr reduction) continues to be used for nitro compounds that are adversely affected by the catalytic reduction method with hydrogen. The list of examples includes aromatic nitro compounds carrying halogen substituents, especially if these are attached in ortho or para position to the nitro group. The solution containing only a small amount of acid (such as acetic acid) is almost neutral and allows iron to precipitate as Fe3O4.
- Reduction with zinc in an alkaline medium:
4,4′-Diaminodiphenyl derivatives can be obtained from appropriately substituted nitrobenzenes by a rather dated process of alkaline reduction with zinc powder/sodium hydroxide solution, which affords hydrazobenzene. A more recent method uses a catalytic reduction process with hydrogen and specifically deactivated catalysts of precious metals. Subsequent acid-catalysed rearrangement with hydrochloric acid yields the hydrochloride of the target diamine:

- Reduction with sodium hydrogen sulfide or sodium sulfide:
Selective reduction (e.g. partial reduction of one of two nitro groups) is carried out with an alkali sulfide, such as sodium hydrogen sulfide NaHS (‘sodium sulfhydrate’) or sodium sulfide Na2S, in an aqueous or alcoholic solution. Azo groups are not affected by this method. The reaction converts sodium hydrogen sulfide or sodium sulfide mainly into sodium thiosulfate.
Example:

- Transfer hydrogenation with hydrazine:
The method is applied for sensitive aromatic nitro compounds, as for example for o/p-substituted nitrobenzenes. The reduction proceeds with hydrazine/precious metal (platinum) catalysts.
2.1.2 Coupling Compounds
The technically most significant groups of coupling compounds are:
- Compounds containing activated methylene groups of the type:

especially acetoacetic arylides:

- 2-Hydroxynaphthalene (β-Naphthol) and its 3-carboxylic acid derivatives:

- Pyrazolone derivatives (Section 2.1.2.3).
2.1.2.1 Acetoacetic Anilides
Compounds based on the general structure:
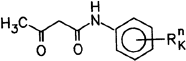
are produced by reacting acetoacetic ester or diketene with aromatic or heteroaromatic amines based on the structure:

The reaction may proceed in water, acetic acid, or any other organic solvent or mixture that is inert to diketene. This group also includes bifunctional coupling components, which are produced from 1,4-diaminobenzene or 4,4′-diaminodiphenyl derivatives:

For the name ‘Naphtol AS-G’ see footnote of Table 2.1.
Table 2.1 Important Naphtol AS derivativesa) as coupling components for hydrazone pigments.

|
|||||
| Naphtol AS derivatives | C.I. azoic coupling compound number, C.I. constitution number | R2 | R3 | R4 | R5 |
| Naphtol AS | 2, 37505 | H | H | H | H |
| Naphtol AS-D | 18, 37520 | CH3 | H | H | H |
| Naphtol AS-OL | 20, 37530 | OCH3 | H | H | H |
| Naphtol AS-PH | 14, 37558 | OC2H5 | H | H | H |
| Naphtol AS-BS | 17, 37515 | H | NO2 | H | H |
| Naphtol AS-E | 10, 37510 | H | H | Cl | H |
| Naphtol AS-RL | 11, 37535 | H | H | OCH3 | H |
| Naphtol AS-VL | 30, 37559 | H | H | OC2H5 | H |
| Naphtol AS-MX | 29, 37527 | CH3 | H | CH3 | H |
| Naphtol AS-KB | 21, 37526 | CH3 | H | H | Cl |
| Naphtol AS-CA | 34, 37531 | OCH3 | H | H | Cl |
| Naphtol AS-BG | 19, 37545 | OCH3 | H | H | OCH3 |
| Naphtol AS-ITR | 12, 37550 | OCH3 | H | OCH3 | Cl |
| Naphtol AS-LC | 23, 37555 | OCH3 | H | Cl | OCH3 |
| a) There are two spellings of ‘Naphthol AS’: ‘Naphtol AS’ is a trade name of the former Hoechst AG, now Clariant. Other products are assigned ‘Naphthol AS’. A few ‘Naphthol Yellow pigments’ are also considered members of the ‘Naphthol AS’ series, although these pigments actually do not contain a naphthol moiety, but are based on acetoacetic arylides. These are internationally known as ‘Naphtol AS-G’, ‘Naphtol AS-IRG’ and so on. ‘Naphtol AS-IRG’, for instance, is acetoacetic-2,5-methoxy-4-chloroanilide. | |||||
The bisacetoacetylated 1,4-diaminobenzene (top) is also called DAEP (from the German ‘Diacetessig-p-phenylendiamin’).
2.1.2.2 β-Naphthol and its Derivatives
2-Hydroxynaphthalene (β-naphthol) is obtained from naphthalene. The reaction sequence includes sulfonation of the starting material at 150–160 °C and subsequent alkaline baking of the intermediate sodium naphthalene-2-sulfonate with sodium hydroxide at 300–320 °C for 6 to 8 h. After the reaction mixture has been allowed to cool, it is dissolved in water, sodium sulfite is removed by filtration, and the basic solution is neutralized with sulfuric acid. The liquid crude naphthol begins to precipitate at about pH 8. It is separated from the aqueous solution and purified by vacuum distillation:

2-Hydroxy-3-naphthoic acid (‘BONA’, ‘BON’ or ‘BONS’) is prepared by heating the sodium salt of 2-hydroxynaphthalene with carbon dioxide in a pressure chamber at 240–250 °C at a pressure of 15 bar (Kolbe synthesis). The reaction mixture is continuously agitated. The remaining 2-naphthol is separated and recycled:

The formation of the technically important 2′-hydroxy-3′-naphthoylanilines (Naphthol AS derivatives) is accomplished primarily by a condensation reaction between 2-hydroxy-3-naphthoic acid and an aromatic amine in the presence of phosphorus trichloride at 70–80 °C. Appropriate reaction media are organic solvents, such as toluene or xylene. In stoichiometric terms, one mole of 2-hydroxy-3-naphthoic acid reacts with 0.4–0.5 moles of phosphorus trichloride. The solution is allowed to cool to room temperature, then neutralized with a sodium carbonate solution, and the Naphthol AS derivative is isolated by filtration. Mechanistically, the reaction is thought to proceed via the phosphoazo compound (11):

An alternative is to react 2-hydroxy-3-naphthoic acid with thionyl chloride to form the naphthoyl chloride. Condensation with the aromatic amine is then typically carried out in the presence of a tertiary organic base. Table 2.1 lists the most significant Naphthol AS derivatives.
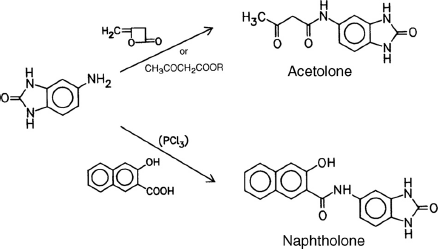
Likewise, heterocyclic coupling components are derived from a heterocyclic amine which is reacted with diketene, an acetoacetic ester, or 2-hydroxy-3-naphthoic acid:
2.1.2.3 Pyrazolone Derivatives
Derivatives of pyrazol-5-one are important heterocyclic coupling components. They are based on the following general structure:

The pyrazolone ring system is accessible through ring closure by the condensation of 1,3-diketo compounds with hydrazine derivatives.
Methods of synthesizing pyrazolone focus especially on ring closure by reaction of acetoacetic ester with phenyl- or p-tolylhydrazine, affording water and ethanol as by-products:

2.1.3 Important Intermediates
The list of important diazo components for the diazotization reaction includes aniline and several aniline derivatives, diamino diphenyl derivatives, and aromatic aminosulfonic acids. Important coupling components are acetoacetic arylides, pyrazolones, β-naphthol, 2-hydroxy-3-naphthoic acid and their aniline derivatives.
The following list includes starting materials that have found their way to large-scale production and application in the hydrazone pigment industry.
Important intermediates for hydrazone pigments are:
- aniline
- p-toluidine
- m-xylidine
- 2,5-dichloroaniline
- 4-chloro-2-nitroaniline
- 4-methyl-2-nitroaniline
- 4-chloro-2,5-dimethoxyaniline
- 3,3′-dichlorobenzidine
- 4-aminotoluene-2-sulfonic acid
- 2-chloro-5-aminotoluene-4-sulfonic acid
- acetoacetanilide
- acetoacet-o-methoxyanilide
- acetoacet-m-xylidide
- acetoacet-4-chloro-2,5-dimethoxyanilide
- β-naphthol.
Other common intermediates for hydrazone pigment production are 2,4-dinitroaniline, acetoacet-o-chloroanilide, acetoacet-o-toluidide, phenyl- and p-tolyl-methylpyrazolone, 2-hydroxy-3-naphthoic acid, Naphtol AS and its derivatives, and 2-chloro-4-aminotoluene-5-sulfonic acid.
2.2 Synthesis of Hydrazone Pigments
The production of hydrazone pigments relies almost exclusively on the azo coupling reaction [9, 10] to afford the hydrazone group. Diazotization of an aromatic amine yields a diazonium compound, which subsequently reacts with a coupling component (‘coupling’).
Generally speaking, the coupling reaction links an aromatic amine to a nucleophilic partner RH (coupling component); the amine is treated with a nitrosyl source XNO to form an azo compound. This sequence is expressed by the following reaction:

The resulting azo compound tautomerizes to yield the hydrazone pigment.
The reaction proceeds via two main steps: a diazotization reaction, which is the basis of diazonium salt formation, followed by azo coupling, which is responsible for the formation of the hydrazone compound.
2.2.1 Diazotization
Diazotization is the reaction of a primary aromatic amine with a nitrosating agent, such as sodium nitrite; or, to a lesser extent, with nitrosylsulfuric acid NOSO4H, nitrous gases, or organic nitrites in an aqueous acidic solution at a temperature between 0 and 5 °C, converting the amine into its diazonium salt:

The diazotization reaction was discovered in 1858 by Peter Griess. Following a suggestion by Kolbe, he treated picramic acid (2-amino-4,6-dinitrophenol) with nitrous gases in an ethanol solution in an attempt to replace the amino function by an OH group. Kolbe, who had been the first to carry out this substitution on p-aminobenzoic acid, worked at elevated temperature, so that the formation of the intermediate diazonium compound escaped his attention. Griess not only worked at low temperature, but diazopicramic acid also has the advantage of being relatively stable, which facilitated its detection as an intermediate. Griess described it as a ‘diazo’ compound, erroneously assuming that he had substituted two hydrogen atoms on the benzene ring by nitrogen.
On an industrial scale, diazotization reactions are carried out by dissolving the aromatic amine in hydrochloric or sulfuric acid. Despite the fact that 2 equivalents of acid per equivalent of amino group should theoretically suffice, as much as 2.5–3 equivalents per amino function are actually required to ensure complete diazonium salt formation. One equivalent of an aqueous sodium nitrite solution is added to the resulting mixture at 0–5 °C. The exothermic nature of the reaction, combined with the heat sensitivity of most diazonium salts, makes it necessary to provide cooling, usually by direct addition of ice.
Amines of low basicity require a higher acid concentration to avoid the formation of diazoamino compounds, which do not couple:

Diazoamino compounds occur only in the trans configuration. Their formation may be prevented by adding a small excess of nitrite during or after diazotization.
Nitrosylsulfuric acid is employed for amines of very low basicity; for example those with more than one electronegative function. Compounds such as di- and trinitroanilines, halogenated nitroanilines, and tetrahalogenanilines will not react under more moderate conditions. If necessary, these amines can also be diazotized after dissolving them in a mixture of glacial acetic acid and concentrated hydrochloric acid.
Aromatic diamines can be diazotized twice to produce bisdiazonium compounds (bisdiazotization).
2.2.1.1 Diazotization Mechanism
Contrary to earlier assumptions, the aryl ammonium ion is not able to undergo diazotization since the crucial step in the diazotization mechanism is the electrophilic nitrosation of the free amino group in the free-base primary aromatic amine 12:

Formation of the diazonium ion 14 proceeds via the diazo hydroxide 13:

The diazotization reaction requires an excess of acid; formation of the active nitrosating agent XNO proceeds via the underlying equilibrium:

The reaction mechanism is reviewed in more detail by Zollinger [10].
2.2.1.2 Methods of Diazotization
The following methods are currently used to produce hydrazone pigments on an industrial scale. The choice depends on the basicity and on the solubility of the individual product.
2.2.1.2.1 Direct Diazotization
An aqueous sodium nitrite solution is added to a cold solution or suspension of the primary aromatic amine in an excess of hydrochloric or sulfuric acid. A temperature of 0–5 °C is maintained by adding ice directly to the reaction mixture.
2.2.1.2.2 Indirect Diazotization Method
This method is particularly useful for aromatic aminocarboxylic and aminosulfonic acids, which are often only sparingly soluble in dilute acid. The amino compound is dissolved in water or in weak alkali and combined with a stoichiometric amount of sodium nitrite, upon which the resulting solution is poured into a mixture of acid and ice. Alternatively, the process may be reversed by pouring the acid into the amine–nitrite mixture.
2.2.1.2.3 Diazotization of Weakly Basic Amines
Amines of very low basicity undergo diazotization after being dissolved in concentrated sulfuric acid. The nitrosating agent is provided by nitrosylsulfuric acid, which is either purchased commercially or easily prepared by dissolving solid sodium nitrite in concentrated sulfuric acid.
Sulfuric acid as a reaction medium may be replaced by glacial acetic acid or by a mixture of glacial acetic acid/nitrosylsulfuric acid. In the former case, half-concentrated hydrochloric acid is added, and diazotization proceeds in an aqueous sodium nitrite solution. The combination of glacial acetic acid/nitrosylsulfuric acid is a particularly useful medium for the bisdiazotization of 1,2-, 1,3- or 1,4-diaminobenzenes (phenylenediamines).
2.2.1.2.4 Diazotization in Organic Solvents
Amines that are considerably or entirely insoluble in water are dissolved in glacial acetic acid or in other organic media, possibly mixed with water; for example alcohols or aprotic solvents. Addition of acid is followed by a typical diazotization reaction in an aqueous sodium nitrite solution. Other possible sources of the nitrosating species NO include nitrosylsulfuric acid, nitrosyl chloride, alkyl nitrite and nitrous gases.
The outcome of a diazotization reaction is largely controlled by the temperature, the pH and the concentration of the medium in which the reaction is carried out. Reactions involving sparingly soluble amines are also dependent on physical parameters, such as distribution and particle size, as well as on the possible presence of emulsifiers or dispersion agents.
A newer diazotization technique using masked diazonium compounds is described in connection with the different coupling methods (Section 2.2.2.1).
In aqueous media, most diazonium salts are only stable at low temperature. Heating frequently leads to decomposition; as a result, nitrogen and the corresponding phenol are formed. The stability of a diazonium compound is a function of the substitution pattern of the aromatic ring system. Electronegative (electron withdrawing) substituents (electron acceptors), such as halogens or nitro functions, render their host structure more sensitive to decomposition than do electron donors such as -CH3, -OCH3, -OC2H5. Some amines can even be diazotized up to a temperature of 50 °C. Other factors that affect the stability of a diazonium compound are visible light or heavy metal ions.
On an industrial scale, diazotization reactions are chiefly carried out in cast iron kettles that are lined with brick or rubber as a protection against acid. Wooden vats also continue to be used.
In most cases, diazonium salts are unstable in the dry state and are sensitive to heat and impact. Since isolation is not necessary for hydrazone pigment production, the diazonium compound is coupled with the coupling component as it is formed in solution or suspension.
2.2.2 Coupling
A coupling reaction is an electrophilic substitution of the diazonium compound with a nucleophilic partner (coupling component RH):

The azo compound subsequently undergoes tautomerization and forms the hydrazone compound.
Considering the electrophilic nature of the reaction mechanism, suitable coupling components for the synthesis of hydrazone pigments should carry a nucleophilic centre on the aromatic ring system (naphthols) or should be enolizable compounds with reactive methylene functions. Naphthols enter the reaction as naphtholates; compounds with reactive methylene groups participate as enolates.
The free acid that is produced in a coupling reaction according to the above equation makes it necessary to add bases or buffers to the reaction mixture to maintain a constant pH and to optimize the outcome of the coupling process. Highly basic solutions do not permit coupling because they shift the underlying equilibrium and thus convert the diazonium compound into a trans (‘anti’) diazotate ion, which does not couple:

Phenols, naphthols and enols therefore couple best in the weakly acidic to weakly basic pH range. To avoid the necessity of permanent pH correction, buffers such as sodium acetate, sodium phosphate, magnesium oxide, calcium carbonate, sodium or potassium hydrogen carbonate, or sodium or potassium carbonate are employed, unless a dilute (3–6%) sodium hydroxide solution is constantly added during the coupling reaction. Substituents showing −I/−R effects (electron acceptors) on the aromatic ring system of the diazo compound generally increase the reactivity. The same substituents, however, have an adverse effect on the coupling reaction if they are located in the aromatic (anilide) moiety of the coupling component. The reverse is also true; namely, +I/+R groups (electron donors) in this part of the molecule enhance the reaction.
This principle is exemplified by the following sequence of substituted anilines used as diazo compounds, whose coupling energy decreases with an increasingly electron-donating substitution pattern on the aniline skeleton:
polynitroanilines > nitrochloroanilines > nitroanilines > chloroanilines > anilinesulfonic acids > aniline > anisidines > aminophenols.
As mentioned above, the nature of the coupling mechanism, which is an electrophilic substitution reaction, makes the carbon atom with the highest electron density the most likely coupling location. This explains why hydroxy or amino functions (although the latter do not play a major role in pigment chemistry) direct the attack of the diazonium ion exclusively to the ortho or para position of an aromatic system. Blocking both these positions by substituents other than hydrogen precludes the reaction entirely or effects expulsion of one of the substituents. There is no other option; the meta position never participates in a coupling reaction.
Naphthalene derivatives as coupling components generally couple better than their benzene analogues; the latter only play a minor role in pigment chemistry.
The pH of the reaction medium is not the only parameter to determine the outcome of an azo coupling reaction. As mentioned above, most diazonium salts decompose at elevated temperature:

This undesirable effect largely compromises the advantage that increased temperature has in accelerating a coupling reaction. Rather than risking decomposition of the diazonium compound by elevating the reaction temperature, it is therefore much more useful to increase the pH or the concentrations of the reactants to enhance the rate of a coupling reaction.
Several bases, with pyridine heading the list, act as proton acceptors in the electrophilic coupling reaction. Their contribution is particularly useful if voluminous substituents exist in ortho or peri position relative to the coupling location of the intermediate; they may also facilitate coupling with diazonium ions of low electrophilicity (e.g. diazophenols).
Coupling components for hydrazone pigments are generally almost insoluble in water itself, but phenols and enols dissolve rapidly as phenolates and enolates in aqueous alkali solutions:

The remaining difficulty of working with a solution that is too basic for a coupling reaction can be avoided by carefully adding acid and thus precipitating the coupling component from the alkaline solution. A dispersing agent may be useful to produce an aqueous suspension with fine particle sizes, which couples easily with the diazonium compound.
2.2.2.1 Coupling Techniques
The following methods play a role in the commercial production of hydrazone pigments:
2.2.2.1.1 Direct Coupling
The coupling component is dissolved in an alkaline solution and, after adding a clarifying agent and possibly charcoal to the solution, it is filtered through a sparkler filter or a filter press.
The solution is then transferred into the coupling vessel equipped with a mechanical stirrer and, possibly in the presence of a surfactant, precipitated with acetic acid, hydrochloric acid or phosphoric acid. The coupling component may also be precipitated ‘indirectly’; that is, the appropriate mixture of acid and emulsifier is filled into the kettle first and the alkaline solution of the coupling component is then added gradually to the clear solution by gravity flow. The clarified solution of the diazonium compound is then introduced into or onto this coupling suspension.
Precipitating the coupling component with acetic acid or phosphoric acid often automatically provides the buffer that is necessary to maintain a certain pH throughout the coupling reaction. Otherwise, buffers such as sodium acetate, sodium phosphate or calcium carbonate (‘chalk coupling’) must be added.
2.2.2.1.2 Indirect Coupling
The clarified acidic diazonium salt solution is first transferred to a coupling vat equipped with a mechanical agitator, and the clarified alkaline coupling component solution is then added above or under the surface of the diazonium salt solution. Constant agitation is essential.
2.2.2.1.3 ‘Pendulum’ Technique
If a coupling reaction is carried out in the absence of a buffer system, a constant pH is maintained throughout the coupling process by continuously adding dilute sodium hydroxide solution (‘pendulum solution’) to the reaction mixture.
2.2.2.1.4 Organic Solvents as Coupling Media
Starting materials that are only sparingly soluble in water may require solvents that are either partially or entirely organic. Diazotization can either be carried out as usual with an aqueous sodium nitrite solution, or alternatively with nitrosylsulfuric acid or an organic nitrite. Appropriate solvents must be stable to the reactants. Examples include aromatic hydrocarbons, chlorohydrocarbons, glycol ethers, nitriles, esters, and dipolar aprotic solvents, such as dimethylformamide, dimethyl sulfone, tetramethylene sulfone, tetramethylurea and N-methylpyrrolidone.
A special type of azo bridge formation is observed in aprotic polar media (solvents with a dielectric constant below 15). In these solvents, the reaction proceeds via an aprotic diazotization–coupling mechanism [11], which unites both diazotization and coupling in one step. The process involves adding a volatile alkyl nitrite to a slightly acidic suspension of a combined solution of diazo and coupling component. Organic solvents have the double advantage of being almost completely accessible to recycling and of producing only very small amounts of contaminants that affect the wastewater.
2.2.2.1.5 Coupling with ‘Masked’ Diazonium Compound
Coupling reactions in organic solvents are occasionally carried out with ‘masked’ diazonium compounds; for example, with special diazonium moieties that are incorporated into a larger organic structure [12], for instance in a diazoamino compound (15) or a benzotriazinone (16):

The diazonium compound itself is liberated by adding a strong organic acid, such as halogenated acetic acid, after the coupling component has been introduced into the reaction mixture:

Hydrazone pigments are difficult to purify, because they emerge from the manufacturing process as almost insoluble substances. The purity of the starting materials is therefore of major concern to the producer. However, contamination is not the only factor to influence the outcome of pigment synthesis: the following factors have a much greater impact on pigment production than on the synthesis of dyes:
- coupling technique, for example sequence and rate of reactant addition;
- concentrations of the reactants;
- temperature of the reaction mixture;
- choice of organic solvent added;
- technical and operational parameters: shape and size of the reaction vats and the agitator, as well as the speed of agitation.
2.2.3 Finishing
Azo dyes differ greatly from hydrazone pigments in the form in which they emerge from the manufacturing process. Dyes are precipitated from the (aqueous) solution by salts. Little labour is involved in washing, drying, and finally standardizing a dye formulation: auxiliary agents and solid diluents are added to the product, which is now ready to be sold. Hydrazone pigments, on the other hand, emerge from the synthesis in the form of extremely small, insoluble particles (primary crystallites), which require aftertreatment or finishing. Physical properties, such as crystal shape, crystal size, and crystal quality, as well as particle size distribution, must be optimized to reach the desired quality. The properties of the primary crystals obviously depend on the pigment itself and can therefore be gauged by adjusting the parameters of the coupling reaction, such as temperature and pH.
More or less extensive finishing is generally necessary to prepare a crude hydrazone pigment for technical application. Washing and drying the crude pigment presscake directly on formation has a detrimental effect on the product. The primary particles may associate to form agglomerates and aggregates. As a consequence, the resulting hard particles will be difficult to disperse, affording a hard grained pigment with poor tinctorial strength. At this stage, it is not possible to convert the material into a useful pigment by milling.
Combining the physical parameters of the crystals to the advantage of pigment performance is a prerequisite to developing optimum application properties. Thermal treatment is the most important process on the way to this target.
Heating the crude pigment suspension or the salt-free pigment presscake in water and/or organic solvents improves the quality of the crystals. Presscakes are prewashed with water to remove salt, isolated, and mixed with water and/or solvent. This finishing process reduces the portion of extremely fine particles, which are the primary source of agglomeration, so that the particle size distribution narrows. Particularly insoluble pigments are finished in an organic medium, such as alcohol, glacial acetic acid, chlorobenzene, o-dichlorobenzene, pyridine, or dimethylformamide at 80–150 °C. Extensive thermal treatment appreciably enlarges the particle size.
A finishing process for organic pigments superior to solvent finish in terms of safety, environmental compatibility and resource consumption has been found using liquid or supercritical carbon dioxide [13].
The particle size distribution thus shifts towards larger sizes, which not only improves the rheological properties but frequently also effects an increase in opacity.
It is also possible to optimize the application properties of a pigment by adding appropriate auxiliaries with different chemical structures to the reaction medium in order to influence the surface structure of the resulting pigment particles. This process is known as surface treatment. Rosin or other resins, for instance, added to the reaction mixture during or immediately after coupling, will inhibit crystal growth, resulting in transparent pigments with fine particle sizes. Features such as these are often required in pigment application.
Preparation with aliphatic amines, on the other hand, may promote side reactions, converting portions of a pigment into compounds that are somewhat soluble in toluene. Toluene is the most important solvent for publication gravure printing inks. This preparative method reduces the viscosity of the printing ink. The pigment is thus partially converted into a soluble azomethine (Schiff's base), which is formed by reaction between the acetoacetic arylide and an aliphatic amine [14]:

Dispersion is the most important prerequisite for the technical application of a pigment. Pigment preparation typically comprises the following steps: Hydrazone pigment synthesis – drying (aggregation and agglomeration) – milling – combining the pigment with its application medium (dispersion). This sequence is often uneconomical, and it is useful to find ways to shorten it.
The last step – pigment dispersion – may be facilitated by adding appropriate agents to the reaction mixture before agglomeration can occur. These agents can either be chemically identical or be part of the medium of application. There are polymer dispersions, for instance, that may even be added during or after coupling. Pigment preparations which are produced via this route can often be distributed in the application medium (i.e. plastics) without extensive dispersion.
Finishing not only improves the application properties of a pigment, such as hue, tinctorial strength, brilliance, transparency/hiding power, dispersibility, and flow behaviour, but also considerably enhances its lightfastness and weatherfastness and its solvent and migration resistance.
2.2.4 Filtration, Drying and Milling
Following manufacture and possibly further processing, the pigment is separated from the suspension and dried. Both processes can be carried out either continuously or by batch operation, depending on the tonnage of the product.
Plate-and-frame filter presses are particularly suited to batch operations. Modern frames are made of polymers (mainly polypropylene), which have replaced wood as a building material. Large amounts of products, on the other hand, are filtered routinely via continuous-belt filters or rotary drum-type filters. Following the filtration step, inorganic salts are removed by washing with water.
Before being dried, the resulting pigment presscake may be flushed in a paste mixer. Since drying operations are traditionally slow, lasting from about 10 h to as long as 2 days, it is advantageous to increase the surface area within a pigment presscake to accelerate the drying process. This can be effected by mechanically chopping the presscake on the drying trays or more often by granulating the material by means of an extruder.
Batch drying is usually carried out in a steam-heated drying oven with a circulating air stream. Depending on the heat sensitivity of the pigment, vacuum-operated drying ovens may also be used for batch processes. Continuous-drying operations, on the other hand, are carried out with belt dryers or spray dryers. Belt dryers typically operate on the principle of hot air being blown through a tunnel in counter-direction relative to a running metal belt, which transports the pigment presscake. Parameters such as temperature and duration are variable.
In a spray dryer, the aqueous pigment paste passes through a rotating disk or nozzle into a cone-shaped spray chamber fed with hot air. The dried pigment powder trickles through a grate at the bottom.
Various mills are available for the pulverizing of pigment particles. To ensure optimum properties, the best type of mill for a given pigment is determined by a pilot experiment. Great care should be taken to avoid excessive pulverization, which might lead to reagglomeration of the primary particles and thus has a detrimental effect on the application properties of a pigment.
Before being milled on an industrial scale, each pigment has to pass a test concerning its sensitivity to dust explosion. There are standardized milling regulations for each hazardous class.
2.2.5 Hydrazone Pigment Synthesis by Continuous Operation
Several publications describe continuous techniques used to manufacture azo colourants, especially hydrazone pigments (for a selection see Reference [15]).
The widespread interest in continuous methods is readily explained by the expected advantages:
- product standardization through uniform reaction conditions;
- increased productivity compared to batch processes;
- improved methods of process and quality control.
In contrast to azo dyes, whose colouristic properties are almost exclusively defined by their chemical structure, the property of a hydrazone pigment depends largely on the physical characteristics of its particles. The continuous process of pigment synthesis is therefore designed to afford a product that already satisfies the commercial specifications. In other words, in a continuous operation, the ultimate performance of the material is determined by the coupling process. Converting a traditional batch technique into a continuous operation adds an extra degree of complexity: in the form of aqueous suspensions, diazo compounds sometimes and coupling components generally react under coupling conditions. It is therefore often difficult to maintain standardized conditions throughout such a heterogeneous reaction.
The following factors determine the outcome of hydrazone pigment production by continuous process:
- coupling rate,
- pH,
- temperature,
- concentration of the diazonium compound,
- impurities,
- form in which the coupling component precipitates,
- surfactants,
- rate of crystal nucleus formation,
- rate of crystal growth.
The first five of these parameters apply to all continuous azo-coupling reactions, while the last four pertain to hydrazone pigments only.
The overall process can be related to two operations: diazotization and coupling. Out of the vast number of patents concerning continuous methods of hydrazone pigment formation, the following examples have been chosen.
2.2.5.1 Diazotization by Continuous Technique
The most important side reaction during diazotization may lead to the formation of the diazoamino compound (Section 2.2.1). The diazonium compound may combine with unreacted amine to form the side product (Section 2.2.1). This effect is particularly prominent if it arises from a change of concentration. Insufficient nitrite prevents the free amine from immediately converting into the diazonium salt. It is possible to reduce this undesirable side reaction by maintaining an excess of nitrite during the diazotization reaction and thus enhancing the formation of diazonium compound over the side product. This provides an important method of process control. Obviously, the amount of formed diazoamino compound is largely determined by the reactivity of the diazonium salt. Weakly basic amines afford the side product much more readily than do more reactive amines (those with electron donating substituents).
Diazotization by continuous process may, for instance, be carried out as follows [16]: The three components (the aqueous amine suspension or its salt in aqueous mineral acid, a nitrite solution, and mineral acid) are transferred simultaneously into a diazotization vessel. For adequate process control, a portion of the reaction mixture is diverted between the storage tank and the diazotization vessel. It enters the diazotization vessel after remaining in an analyser only for about ⅓ to ¼ of the time required for the main stream in the diazotization vessel. The analyser is responsible for maintaining constant reactant concentrations throughout the coupling process. Any change to the excess of nitrite initially provided in the storage tank is continuously recorded and controlled through variations of the redox potential or the polarization voltage (polarography) within the analyser. The azo coupling process is thus operating at continuously constant concentrations. Figure 2.2 outlines the principle of this particular example of indirect diazotization by continuous process.

Figure 2.2 Diagram showing a continuous diazotization process (see text for details).
A storage tank (1) is used to combine the amine (2) with water (3) and nitrite (4). This make-up vessel (1) should contain approximately 90% of the theoretically required amount of nitrite. The milled amine suspension is transported into the reaction vessel (7) by a pump (5). Milling the amine in a roll mill, a carborundum mill or a ball mill (6) to reduce the particle size prior to the reaction is particularly essential for species with coarse particle sizes. Additional nitrite then enters the reaction vessel (7) through a valve (8). A 2% excess of nitrite is maintained throughout the reaction (7). An analyser (9) is responsible for precise nitrite addition. Electrochemical data continuously collected by a redox potentiometer, a voltmeter, or a polarograph (10) connected to the analyser (9) indicate the nitrite concentration in the amine suspension or solution leaving the reaction vessel (7) at any given time. In case of demand, the analyser transfers additional nitrite through the valve (8) into the reaction vessel (7). As soon as the desired excess is re-established within the monitor (10), the valve (8) interrupts the nitrite transfer. The reaction mixture is typically represented by only a small portion of the entire mass flow (11) that is diverted via the analyser. The main portion of the amine suspension leaving the reaction vessel (7) is pumped (12) directly into the diazotization vat (13). To ensure a quantitative diazotization procedure, mineral acid (14) is transferred into the analyser (9) and the diazotization vat (13).
2.2.5.2 Coupling by Continuous Process
There are basically two variations to the continuous-coupling technique [17]:
- reaction in a homogeneous medium, which means the coupling component is dissolved;
- reaction in a heterogeneous medium, using a suspension of the coupling component, which is obtained by precipitation.
The kinetics of the coupling mechanism include a number of sometimes very fast and competitive side reactions. The following steps, for instance, proceed simultaneously as a separately prepared diazonium salt solution is combined with an initially dissolved coupling component:
- the coupling reaction itself,
- decomposition of the diazonium salt,
- precipitation of the originally dissolved coupling component under the reaction conditions.
The manufacturer, whose main concern is to maintain a prevalence of the coupling reaction over the two others, that is, a sufficiently fast coupling rate, achieves his end by combining the reactants at the exact moment of interaction (turbulence). Suitable equipment, such as a mixing nozzle or a static mixing tube, is therefore indispensable for a continuous operation; but it is on the other hand quite successful in helping to avoid side reactions.
The particle size of the resulting pigment can only be influenced to a limited extent by adjusting the reaction parameters, because the decisive factor is the ratio of the rate of formation of the crystal nucleus to the rate of crystal growth.
Another variety of the continuous-coupling technique operates by transporting the coupling component suspension as a laminar flow upwards inside a vertical reaction tube. Portions of the diazonium compound, dissolved in an acidic aqueous medium, are added through appropriately located inlets in the walls of the reaction tube. The concentration of the added solution decreases as the reaction mixture flows upward and is designed to synchronize the uppermost inlet for the diazonium salt solution with the stoichiometric end point of the coupling reaction.
2.2.5.3 Process Control
Reliable measuring techniques [18] and appropriate process control are the basic elements of successful hydrazone pigment synthesis not only by the continuous process. These are the parameters that are responsible for maintaining constant reaction conditions: flow rate, pH, temperature and the concentrations of the reactants, before and after the point of mixing itself.
Potentiometric methods (e.g. with Pt/Hg2Cl2 or Au/Hg2Cl2 electrodes) are particularly useful to monitor both the diazotization and the coupling reaction. Polarography (determining the polarization voltage) indicates changes of the nitrite content during diazotization. It is also possible to measure the amount of nitrous gases escaping into the air above the agitated and therefore constantly renewed surface of the liquid [19].
On a cost/performance basis, large-scale pigment manufacture by the continuous operation as compared to batch operation remains uneconomical as far as technical considerations are concerned. The difficulty of attaining starting materials with a standardized quality and maintaining constant coupling rates adds to the complexity of process control (e.g. stability of the potentiometric electrode systems). These are clearly factors in favour of batch techniques, which remain the rule throughout the hydrazone pigment industry.
The dominant role of the batch operation over the continuous process throughout the pigment industry is somewhat in contrast to the patent literature, which includes numerous proposals for complete hydrazone pigment manufacture by the continuous process as well as descriptions of the partial steps, such as the diazotization or the coupling reaction.
2.2.6 Production Units for Hydrazone Pigment Manufacture by Batch Operation
A typical production unit consists of an acid-proof diazotization kettle (‘diazotizer’), a dissolution vessel to dissolve the coupling component in its medium, and a reaction vessel with an agitator, in which the coupling reaction is carried out. Typical vessel capacities are 20–80 m3, corresponding to batches of 0.5–2.5 t of hydrazone pigment.
Clarifying filters or clarifying presses are installed between diazotization kettle and dissolution tank and the reaction vessel. The crude pigment slurry from the coupling vessel is filtered off in a filter press; and a pressure vessel equipped with an agitator (for thermal aftertreatment) connected to a filter press completes the processing unit for the synthesis.
Drying and milling the resulting wet pigment presscakes, possibly preceded by extrusion or granulation, finally affords the desired pigment powder. Drying may be carried out either by a continuous process on a conveyor belt or as a batch operation in a convection oven.
The flow chart in Figure 2.3 outlines the typical sequence of hydrazone pigment synthesis, including essential equipment.
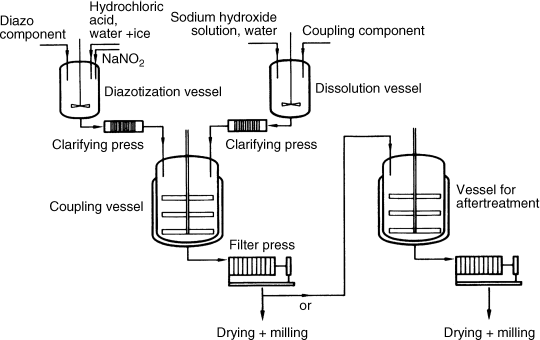
Figure 2.3 Diagram showing the equipment used to manufacture a hydrazone pigment.
The coupling component, usually in an alkaline solution, is clarified by mechanical and adsorptive methods in clarifying filters or presses and then charged into a ‘coupling vessel’. Acids, and possibly also surfactants, are added to precipitate the material. In the ‘diazo kettle’, the aromatic amine is dissolved in aqueous acid and then diazotized with aqueous sodium nitrite. After the coupling component slurry has been clarified by filtration, the diazonium salt solution is slowly transferred above or below the surface of the coupling component. It is also possible to reverse the sequence in which the reactants are combined, or to add them simultaneously to a buffer solution in the coupling vessel. Further treatment, such as precipitation (metal salt formation), metal complexation or even thermal aftertreatment may be carried out advantageously in the same vessel.
The solid hydrazone pigment is then separated by filtration, washed and dried immediately. It is also possible to reslurry the pigment presscake in another agitation vessel in order to prepare it for thermal aftertreatment. Milling follows drying.
Depending on the technical requirements such as corrosion resistance, pressure and temperature stability, industrial scale hydrazone pigment synthesis is carried out in appropriate equipment. Suitable materials include cast iron, stainless steel, steel lined with rubber, acid-proof brick, enamel, synthetic resins supported by glass fibre, and wood.
The most widely used material for diazotization, dissolution, and the coupling vessels is rubber-lined steel.
The rubber lining resists temperatures up to 100 °C for some time (about 1 h) without suffering damage; however, contact with organic solvents must be avoided. Recent developments include coupling vessels made of synthetic resins on a fibre glass structure, which have the multiple advantage of being very light, low-cost materials which for repair are easily accessible. Moreover, these vessels are fast to hydrochloric acid and resist temperatures up to 100 °C. Traditional wooden vats also continue to be used, which is not as surprising as it seems, considering the fact that they are corrosion resistant in aqueous media and are economically installed and repaired. Stainless steel is the material of choice for reactions in neutral or alkaline media or in organic solvents, as well as in autoclaves. Notably, however, the advantage of stainless steel is compromised by the fact that it will corrode if the vessel or any other part of the production unit is exposed to mineral acid. Thus, autoclaves made of this material cannot be used for reactions in slightly acidic media or salt solutions; chloride ions are particularly destructive. Such operations are therefore carried out in enamel lined pressure vessels, which in turn are not resistant to alkali, but are easy to clean. Stainless steel autoclaves lined with rubber or brick are also suitable for acidic reaction media; agitators, heating coils, and thermometer jackets are made of a nickel alloy (e.g. Hastelloy) with a varying content of Mo, Cr, Mn, Cu, Si, Fe and C. Brick lined vessels are primarily used in large-scale production, where the cost of installation and repair exceeds the advantage of enamel lining.
Reaction mixtures are transferred from one piece of equipment to another by pumping, particularly if the production unit includes wooden vats or vessels made of synthetic resins, which may not be pressurized. Steel vessels, on the other hand, allow transport by air or nitrogen pressure.
2.3 Monohydrazone Yellow and Orange Pigments (Formerly Called Monoazo Yellow and Orange Pigments)
The synthetic route to monohydrazone yellow pigments involves the coupling of a diazotized substituted aniline with a coupling component containing an active methylene moiety.
Hydrazone pigments were essentially unknown until the end of the nineteenth century, when coupling reactions were first carried out with β-naphthol as a coupling component. However, since the range of available colours was somewhat restricted at the time, it was not possible to manufacture shades of yellow beyond that of Dinitroaniline Orange (2,4-dinitroaniline → β-naphthol).
The discovery of acetoacetic arylides (N-acetoacetanilide = CH3-CO-CH2-CO-NH-Ph) as coupling components has therefore considerably broadened the scope of organic pigments in general. The resulting yellow monohydrazone pigments were discovered by Meister Lucius & Brüning in Germany (later: Hoechst AG) in 1909 [20] and entered the market in 1910 under the trade name of ‘Hansa Yellows'.
The synthetic pathway to such compounds had originally been suggested by the observation that 1,3-diketo compounds couple with diazonium salts to form yellow dyes, which was published as early as 1897. At present, acetoacetic arylides are not the only 1,3-diketo compounds to be used in hydrazone pigment synthesis. Introducing the pyrazolone-(5)-ring skeleton provides a convenient method of synthesizing structures in which the acetoacetyl aniline forms a heterocycle:

In 1884, H.J. Ziegler first used pyrazolones as coupling components. In an attempt to find a new dye by synthesizing a coloured osazone from phenyl hydrazine-4-sulfonic acid and dioxo tartaric acid, he obtained yellow tartrazine by condensation:

Ziegler not only confirmed the chemical structure of his product but also described the tautomeric structures of the hydrazone (17) and the azo form (18). He then proceeded to prepare tartrazine by coupling diazotized p-sulfanilic acid with 1-sulfophenyl-3-carboxy-pyrazol-5-one. The discovery of the pyrazolone skeleton as a coupling component, which improved the versatility of the method, led to the production of numerous monohydrazone pigments and dihydrazone pigments. However, the production of pyrazolone-based monohydrazone pigments (monohydrazone orange pigments) has declined largely in favour of the corresponding dihydrazone pigments.
Apart from pyrazolones, only a few other heterocycles are used as coupling components for diazonium salts. The list includes barbituric acid, which is used for the synthesis of P.O.64 (Section 4.3) and of the nickel complex pigment P.Y.150 (Section 4.2), and 2,4-dihydroxyquinoline, which is used as a starting material for the nickel complex pigment P.Gr.10 (Section 4.2):

2.3.1 Chemistry, Manufacture and Crystal Structures
2.3.1.1 Non-laked Monohydrazone Yellow and Orange Pigments
Most non-laked monohydrazone yellow and orange pigments are derivatives of the following structure:
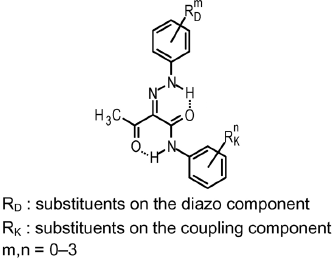
RD and RK represent substituents such as CH3, OCH3, Cl, NO2, CF3.
In some cases the coupling component may also be a pyrazolone derivative, leading to pigments with the following structure:

The customary method of preparation involves diazotizing a substituted aniline with an aqueous sodium nitrite solution at low temperature (0–5 °C). Coupling with acetoacetic arylide is carried out in a weakly acidic solution (pH 4–5). The resulting pigment suspension is heated shortly to 70–80 °C and then filtered. Ionic (salt) impurities are removed by washing the presscake with water, after which the product is dried at 60–80 °C. Controlling the particle size is essential. Powders with fine particle sizes are produced by adding appropriate agents, such as dispersing or emulsifying agents, to the reaction mixture before or during coupling. Coarse particle sizes (monohydrazone yellow or orange pigments), on the other hand, are attained by thermal aftertreatment. Heating either the crude pigment suspension or the isolated and washed pigment presscake to a temperature above 80 °C, possibly under pressure, affords the desired particle size.
The diazo component is typically a substituted aromatic amine which carries either an NO2 group or, less frequently, OCH3, Cl, or CH3 in the ortho position relative to the amino function. Several studies propose methods to improve the solvent and migration resistance of monohydrazone yellow and orange pigments, which is normally somewhat poor. Introducing carbonamide or sulfonamide groups into the diazo compound and/or the coupling compound provides a convenient method of modifying the chemical structure. The results were as follows:

Only a few of these species, however, have gained commercial significance.
A more traditional approach consists of introducing sulfonic acid substituents into yellow monohydrazone compounds. These can simply be precipitated, that is, converted into lakes by salt formation, especially with calcium salts, and thus afford the more solvent and migration resistant monohydrazone yellow pigment lakes.
Crystal structures have been determined for many monohydrazone yellow and orange pigments [21–28]. All compounds exclusively exhibit the tautomeric hydrazone form. The acetoacetylamino fragment and the adjacent phenylhydrazone group form a large, conjugated system, which is always planar or close to planar. Similarly, in pyrazolone pigments the pyrazolone moiety forms a large planar (or nearly planar) system with the phenylhydrazone group. In both series, the phenyl ring of the coupling component can be coplanar as well, or can be rotated out of the plane by up to about 30°, depending on the molecular packing. The two NH groups of the hydrazone and the CONH fragments form intramolecular hydrogen bonds NH![]() OC. These hydrogen bonds become bifurcated, if the adjacent phenyl rings carry a substituent with lone pairs, for example OCH3 or NO2, in the ortho-position (see molecular structure of P.Y.74, Figure 2.1).
OC. These hydrogen bonds become bifurcated, if the adjacent phenyl rings carry a substituent with lone pairs, for example OCH3 or NO2, in the ortho-position (see molecular structure of P.Y.74, Figure 2.1).
The phenyl ring is always orientated in such a way that the substituents RD or RK which are in ortho-position to the NH group are located cis to the H atom of the NH group; a trans conformation would lead to a negative steric interaction between the substituent and the NNC or NCO groups, respectively.
In all pigments the molecules are quite densely packed (Figure 2.4 and 2.5). The molecules are connected by strong van der Waals interactions, supported by Coulomb interactions. The NH groups of the phenylhydrazone and the acetoacetylamino moieties participate in intramolecular hydrogen bonds only. Intermolecular hydrogen bonds are only formed, if at least one of the phenyl rings carries an additional NH group (e.g. with SO2NHPh or CONH2 as substituent).

Figure 2.4 Pigment Yellow 1 [21]. Space-filling model of one layer. Colour code: C dark grey, H white, N blue and O red. All drawings of crystal structures were made with the program SCHAKAL [123].
The molecular arrangements exhibit a wide variety. The list includes layer with structures, wavy layers (Figure 2.6), herringbone packings (Figure 2.7), structures in which the molecules of neighbouring stocks have different orientations (Figure 2.8), layers with steps (Figure 2.9), structures built from strongly bended molecules (Figure 2.10) and also structures consisting of almost perfectly planar layers (Figures 2.11 and 2.12). The crystal structure strongly depends on the substitution pattern and on the polymorphic form. Subtle changes in the substitution pattern or in the process conditions may have a drastic influence on the arrangement of the molecules in the crystal and, therefore, on the properties of the pigment.

Figure 2.5 Pigment Yellow 1; view perpendicular to the layers of molecules.

Figure 2.6 Pigment Yellow 1. Wavy layer structure. View along the layers, showing 4 × 4 molecules.

Figure 2.7 Herringbone packing of molecules in Pigment Yellow 6 [22].

Figure 2.8 Molecular packing in Pigment Yellow 65 [23], showing 12 molecules. Molecules of neighbouring stocks have different orientations.

Figure 2.9 Molecular packing (layer with steps) in Pigment Yellow 74 [24].

Figure 2.10 Molecular structure of Pigment Yellow 97 [25]. The SO2NHPh fragment is strongly bent out of the molecular plane. Intermolecular hydrogen bonding between the sulfonamide groups in neighbouring molecules is observed. Colour code: C dark grey, H white, N blue, O red, S yellow and Cl green.

Figure 2.11 Crystal structure of P.Y.10 [26]. Layer structure. View perpendicular to the layer of molecules.

Figure 2.12 Crystal structure of P.Y.10, built from planar layers. View along the layers.
2.3.1.2 Monohydrazone Yellow Pigment Lakes
Monohydrazone yellow pigment lakes are basically synthesized by introducing acidic groups into the diazo or coupling component and by precipitating the product as an insoluble salt. Usually the precipitation is carried out with salt solutions of calcium, barium, strontium or manganese.
Although the term ‘lake' was originally used for dyes which were absorbed onto alumina hydrate (aluminium hydroxide) leading to complex aluminium salts (see P.R.83, a nonhydrazone compound, Scheme 3.14, section 3.7.2), it is nowadays generally used for the insoluble metal salt pigments. In Europe these pigments are also known as ‘toners', but since this term is differently used elsewhere, metal salt pigments are called ‘lakes' throughout this book. In practice, only those species which carry a sulfonic acid function on the diazo component are commercially important. The list includes derivatives of the following structure:

Several commercially available yellow monohydrazone pigment lakes are based on a pyrazolone sulfonic acid derivative as a coupling component. An example is the aluminium tartrazine lake, listed in the Colour Index as Pigment Yellow 100, 19140:1:
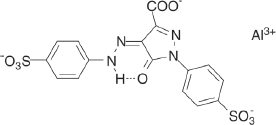
Other examples [29] include yellow pyrazolone monohydrazone pigment lakes based on the general structure:

The first crystal structures of laked yellow pigments were published only in 2009, that is, about 40 years after the first structures of non-laked yellow pigments. The reason is to be sought in the low solubility of laked pigments in all organic solvents, which hinders the growth of single crystals suitable for X-ray structure analysis. Single crystals could only be grown for the β-phase of P.Y.183. The crystal structures of the α-phase of P.Y.183 as well as of the α- and β-phases of P.Y.191 were determined from X-ray powder diffraction data [30, 31]. The α-phase of P.Y.191, which is the usual commercial phase, is a monohydrate, that is, the lattice contains one molecule of water per pigment molecule. The structure contains two symmetrically independent Ca2+ ions. One of them is coordinated to two water molecules and four sulfonate groups (from four different pigment molecules). The other Ca2+ ion does not coordinate to water molecules, but to six sulfonate groups (Figure 2.13). Ca2+ ions and sulfonate groups form a two-dimensional coordination network (Figure 2.14). These polar layers, which also contain the water molecules, are separated by non-polar layers consisting of the C/N/H skeleton of the molecules. The Cl atoms are accommodated within these non-polar layers. The polar layer is held together by Coulomb interactions and hydrogen bonds, where as in the non-polar layer the molecules are mainly connected by van der Waals interactions (Figure 2.13).

Figure 2.13 Crystal structure of α-P.Y.191. The α-phase of P.Y.183 is isostructural to α-P.Y.191.

Figure 2.14 Two-dimensional coordination network formed by Ca2+ ions and sulfonate groups in the α-phases of P.Y.191 and P.Y.183. Colour code: Ca light grey, O red, S yellow and H white.
The α-phase of P.Y.183, also a monohydrate, is isostructural to the α-phase of P.Y.191.
Similar double-layer structures are also found in the β-phases of P.Y.191 and P.Y.183. Both phases are trihydrates. The Ca2+ ions are coordinated to three water molecules and four sulfonate groups (Figure 2.15). However, the structures are different: The Ca2+ ions and sulfonate groups form a chain network for β-P.Y.191, but a double-chain network for β-P.Y.183 (Figure 2.16).

Figure 2.15 Crystal structures of the β-phases of P.Y.191 (a) and of P.Y.183 (b).

Figure 2.16 Coordination network of Ca2+ ions and sulfonate groups in the β-phases of P.Y.191 (chain (a)) and of P.Y.183 (double chain (b)).
Thermal gravimetric investigations of P.Y.191 and P.Y.183 reveal that upon heating the β phases (trihydrates) release two water molecules and convert to the α phases (monohydrates). In the monohydrates the water molecules are bound very strongly. The α phases are stable up to temperatures of more than 250 °C(!) before they slowly release the final water molecule and form anhydrates. The anhydrates are stable up to at least 400 °C. Subsequent cooling to room temperature in air again yields the α phases. Interestingly, the hydrates of P.Y.183 and P.Y.191 are thermally more stable than their inorganic counterpart CaSO4·2H2O (gypsum). CaSO4·2H2O releases 1.5 water molecules upon heating. The resulting hemihydrate is stable only up to 160 °C before it converts into the anhydrate. Hence the dehydration temperature is about 100 °C lower than of the organic sulfonates P.Y.183 and P.Y.191. The strong binding of the water molecules in the α-phases of P.Y.183 and P.Y.191 explains why these pigments can be used for plastics colouration, although they are hydrates.
2.3.2 Properties
2.3.2.1 Non-laked Monohydrazone Yellow and Orange Pigments
Monohydrazone yellow and orange pigments provide a range of colours from intensely greenish to very reddish yellow or yellowish orange shades. Monohydrazone pigments based on pyrazolone as the coupling component are reddish yellow; commercially, however, they have largely been displaced by other products. Most of the industrially significant representatives of this class of pigments exhibit a tinctorial strength that is only about half of that of the diarylide yellow pigments covering the same range of colours (Section 2.4.1.2); there is only one monohydrazone yellow pigment that is strong enough to compete with comparable diarylide yellow pigments.
Monohydrazone yellow pigments exhibit good lightfastness and durability in full shades and close to deep shades. To optimize the pigment performance for a certain target application, physical parameters, such as particle size or crystallinity, may be adjusted accordingly. However, such techniques do not open up new areas of use.
The fact that monohydrazone yellow and orange pigments are not completely insoluble in organic solvents frequently affects their performance in certain vehicles, resulting in poor migration resistance, that is, a trend towards bleeding and blooming, as well as a tendency to recrystallize. This obviously restricts the use of such pigments in a variety of areas and in several application materials.
Although it is sometimes possible to considerably improve the fastness properties of a monohydrazone yellow pigment by introducing carbonamide groups or sulfonamide functions into the molecule, the applicability of a pigment is not likely to extend basically beyond its original scope.
Although monohydrazone yellow pigments are low-cost materials, this advantage is compromised by their limited performance in application, which is a function of their chemical constitution.
2.3.2.2 Monohydrazone Yellow Pigment Lakes
Introducing acidic substituents into the basic structure of a typical monohydrazone yellow pigment makes it possible to convert the material into a lake by salt formation. This improves the application properties of a pigment compared to its non-laked counterpart. Such pigments exhibit particularly good migration resistance and heat stability, making them useful colourants for plastics.
2.3.3 Application
The largest fraction of monohydrazone yellow pigments today are used throughout the air drying paints and emulsion paints industry. Recommended types with large pigment particles show only a slight tendency to recrystallize in most application media used by the paint industry: this slight recrystallization is generally tolerated. Monohydrazone yellow pigments, especially Pigment Yellow 1 and Pigment Yellow 3, are used routinely in air drying paints for their excellent lightfastness and durability in full shades. However, the application performance of these types deteriorates upon white reduction with TiO2. The lightfastness of a particular type of P.Y.1, for instance, which in full shades equals step 7–8 on the Blue Scale, decreases to step 5–6 upon 1:5 reduction with TiO2 and will be reduced to as little as 4–5 at 1:60 TiO2. Excellent hiding power and clean, brilliant full and medium shades make some of these pigments suitable to replace Chrome Yellow in chromium free pigmentation. Good rheology makes it possible to prepare comparatively highly pigmented formulations without affecting the flow behaviour of the paint. Although this class of organic pigments does not typically lack opacity, a high monohydrazone yellow pigment concentration will enhance the hiding power of the material in question even more.
Monohydrazone yellow and orange pigments are easily dispersed in most media. Aided by a dissolver, several types can even be worked into long-chain alkyd resin systems.
Poor solvent resistance and a tendency to bloom considerably restrict the use of monohydrazone yellow pigments. As the fastness requirements for pigments in industrial paints have become increasingly stringent, monohydrazone yellow pigments are only used in special cases. The ultimate decision is made on the basis of the concentration limits beyond which blooming occurs in a given system (Section 1.6.3.1). Such coatings are stable to blooming, but they still bleed.
As mentioned above, regular monohydrazone yellow pigments may be substituted with sulfonamide groups to improve their fastness to organic solvents. Products such as Pigment Yellow 97 thus become eligible for application in stoving enamels. These products do not bloom under the typical processing conditions and may even resist bleeding if they are cured at moderate temperature.
Strong migration tendencies practically exclude monohydrazone yellow and orange pigments from being used for the mass colouration of plastics: they bleed and bloom considerably in most polymer systems. Again, Pigment Yellow 97 is an exception which can be used under certain conditions in PVC pastes. A few selected types are applied to a limited extent in urea-formaldehyde resins, also known as thermosetting plastics.
The printing inks field is the primary user of certain monohydrazone yellow pigments, especially in areas for which the routinely used diarylide yellow pigments are not sufficiently lightfast; such as for posters and packaging and partially for wallpaper. In the field of printing inks today they are primarily used for the packaging of printing inks.
However, problems may arise through the solvents which are commonly used to formulate speciality packaging gravure inks (Section 1.8.1.2). Depending on the processing conditions and especially on the method of dispersion, pigments may recrystallize in these media. Tinctorially strong, transparent types are particularly sensitive to recrystallization, which not only reduces the tinctorial strength but also opacifies the ink. Using inks based on water or water/alcohol will circumvent the problem. Their low thermostability and migration fastness prevent these pigments from being useful for metal deco printing.
In the printing inks field, monohydrazone yellow and orange pigments rank next to the much stronger and solvent resistant diarylide yellow pigments. Monohydrazone yellow and orange pigments, however, reign supreme where lightfastness is a concern. This is also true for other media.
The paint, printing ink, and plastics industries are not the only users; monohydrazone yellow and orange pigments have gained recognition in a host of applications. Several types – sometimes sold as pigment preparations – are used in the office article sector, where they lend colour to felt-tip pen inks, drawing inks, coloured pencils, wax crayons, watercolours and so on. They are also used as colourants for wood stains, plywood, veneered wood, shoe polish, floor polish, fertilizers, match heads, and in the cosmetics industry, where they are used, for instance, to colour soaps. These pigments also lend themselves to textile printing and for paper spread-coating and mass colouration of paper.
2.3.4 Commercially Available Monohydrazone Yellow and Orange Pigments
2.3.4.1 General
The oldest monohydrazone yellow pigment is Pigment Yellow 1, which continues to be produced and shipped on a large scale. Its discovery and introduction in 1910 was followed by the development of various related pigments of this type, not all of which have turned into a commercial success. A few, such as pyrazolone-based Pigment Yellow 10, are still produced by some regional companies and are targeted for smaller niche applications. Others, such as P.Y.74 are produced and sold world-wide in large amounts.
Tables 2.2 and 2.3 list the currently available non-laked monohydrazone yellow and orange pigments. Most of them carry a nitro substituent in the diazonium component, usually in ortho position relative to the hydrazone group. The more migration-resistant Pigment Yellow 97 lacks a nitro group in its molecule. Seven monohydrazone yellow pigments are based on 2-nitro-4-chloroaniline or derivatives as a diazo component.
Table 2.2 Non-laked monohydrazone yellow and orange pigments.

|
||||||||
| C.I. Name | C.I. Const. No. | Shade | ||||||
| P.Y.1 | 11680 | NO2 | CH3 | H | H | H | H | yellow |
| P.Y.2 | 11730 | NO2 | Cl | H | CH3 | CH3 | H | reddish yellow |
| P.Y.3 | 11710 | NO2 | Cl | H | Cl | H | H | very greenish yellow |
| P.Y.5 | 11660 | NO2 | H | H | H | H | H | very greenish yellow |
| P.Y.6 | 11670 | NO2 | Cl | H | H | H | H | yellow |
| P.Y.49 | 11765 | CH3 | Cl | H | OCH3 | Cl | OCH3 | greenish yellow |
| P.Y.65 | 11740 | NO2 | OCH3 | H | OCH3 | H | H | reddish yellow |
| P.Y.73 | 11738 | NO2 | Cl | H | OCH3 | H | H | yellow |
| P.Y.74 | 11741 | OCH3 | NO2 | H | OCH3 | H | H | greenish yellow |
| P.Y.75 | 11770 | NO2 | Cl | H | H | OC2H5 | H | reddish yellow |
| P.Y.97 | 11767 | OCH3 | SO2NH-C6H5 | OCH3 | OCH3 | Cl | OCH3 | yellow |
| P.Y.98 | 11727 | NO2 | Cl | H | CH3 | Cl | H | greenish yellow |
| P.Y.111 | 11745 | OCH3 | NO2 | H | OCH3 | H | Cl | greenish yellow |
| P.Y.116 | 11790 | Cl | CONH2 | H | H | NHCOCH3 | H | yellow [27] |
| P.Y.130 | 117699 | NO2 | Cl | H | H | OCH3 | H | reddish yellow |
| P.Y.203 | 117390 | NO2 | H | CH3 | OCH3 | H | H | yellow |
| P.O.1 | 11725 | NO2 | OCH3 | H | CH3 | H | H | very reddish yellow |
Table 2.3 Non-laked monohydrazone yellow and orange pigments based on diazo or coupling components that deviate from the general structure shown in Table 2.2.

|
||||
| C.I. Name | C.I. Constitution Number | DC | CC | Shade |
| P.Y.10 | 12710 |  |
PMP | reddish yellow |
| P.Y.60 | 12705 |  |
PMP | reddish yellow |
| P.Y.165 | a) | a) | a) | reddish yellow |
| P.Y.167 | 11737 |  |
 |
greenish yellow |
| P.O.6 | 12730 |  |
PMP | orange |
| a) P.Y.165 is identical with P.Y.10. | ||||
Acetoacetic-o-anisidide, a frequently used coupling component for monohydrazone yellow pigments, is generally considered one of the most important intermediates for the production of monohydrazone and dihydrazone yellow pigments worldwide.
The currently available salt type monohydrazone yellow pigments are mostly based on 2-nitroaniline-4-sulfonic acid as a diazo component and are mostly sold as calcium lakes (Table 2.4).
Table 2.4 Monohydrazone yellow pigment lakes.

|
|||||
| C.I. Name | C.I. Constitution Number | M | Shade | ||
| P.Y.61 | 13880 | H | H | Ca | greenish yellow |
| P.Y.62 | 13940 | CH3 | H | Ca | yellow |
| P.Y.133 | 139395 | H | H | Sr | greenish yellow |
| P.Y.168 | 13960 | Cl | H | Ca | greenish yellow |
| P.Y.169 | 13955 | H | OCH3 | Ca | reddish yellow |
| P.Y.205 | — | — | — | — | yellow |
| P.Y.206 | — | — | — | — | reddish yellow |
| Structures that deviate from the above-mentioned chemical constitutions: | |||||
| P.Y.100 | 19140:1 |  |
greenish yellow | ||
| P.Y.142 | — |  |
bright yellow | ||
| P.Y.183 | 18792 |  |
reddish yellow | ||
| P.Y.190 | 189785 |  |
yellow | ||
| P.Y.191 | 18795 |  |
reddish yellow | ||
| P.Y.191:1 | 18795 | Structure: see P.Y.191 | M2+ = |
reddish yellow | |
| P.Y.209 | — | 
|
reddish yellow [32] | ||
| P.Y.209:1 | — | Structure see P.Y.209 | Sr2+ | reddish yellow | |
| P.Y.212 | — | 
|
Sr2+ | reddish yellow [33] | |
2.3.4.2 Individual Non-laked Monohydrazone Yellow and Orange Pigments
2.3.4.2.1 Pigment Yellow 1
This compound has entered pigment history as ‘Hansa Yellow G'. The commercial varieties are products with comparatively coarse particle sizes and specific surface areas of approximately 8–30 m2 g−1. P.Y.1 provides good hiding power, it is used primarily in air drying paints as well as in packaging and textile printing.
Decreasing the particle size produces more transparent, more greenish and cleaner pigment types; the tinctorial strength frequently improves. This advantageous trend is compromised by a considerable decrease in lightfastness and resistance to organic solvents. In several application media, the tendency to recrystallize is also enhanced (Section 1.7.7). Pigment Yellow 1 types with very fine particle sizes lose the advantage of superior lightfastness compared to diarylide yellow pigments with comparable shades, whose solvent resistance is much better and whose tinctorial strength is almost twice as high as that of P.Y.1.
Although inferior fastness to organic solvents, involving unsatisfactory migration properties, excludes P.Y.1 from important areas of application, such as baking enamels, there are certain conditions under which it may be applied in such media. The user must in this case observe a certain concentration limit beyond which the pigment may bloom. The pigment is stable up to 140 °C.
For decades, P.Y.1 represented the standard source of yellow for all printing purposes. Today, however, P.Y.1 has been replaced in this field by the stronger diarylide yellow pigments.
The tinctorial strength of a printing ink is measured in terms of the pigment concentration in a standardized ink film that will produce a certain depth of shade in letterpress proof-prints. Depending on the type, inks made from P.Y.1 must contain about 8–11 wt% pigment to produce 1/3 SD films; while inks made from the similarly shaded diarylide yellow pigment P.Y.14 require only between 4% and 6% pigment. The lightfastness of such a printed P.Y.1 sample equals approximately step 5 on the Blue Scale, while the corresponding P.Y.14 specimen only reaches step 3. The corresponding values for 1/1 SD prints are 6–7 and 3 on the Blue Scale, respectively.
P.Y.1 prints are comparatively sensitive to most common organic solvents, such as esters, ketones, and aromatic hydrocarbons, but completely fast to alcohols and aliphatic hydrocarbons. The prints are also resistant to soap, alkali and acid.
In the printing ink industry, P.Y.1 is used especially to replace diarylide yellow pigments in areas where the latter are not sufficiently lightfast to meet the technical standards. This is especially true for the packaging printing industry, which particularly appreciates the good hiding power of most types of P.Y.1. Since they are sensitive to organic solvents, such grades are mainly used in alcohol-based nitrocellulose (NC) printing inks and in waterborne inks. Poor migration resistance and heat stability largely precludes the use of P.Y.1 in plastics.
In general, P.Y.1 is used extensively in various applications, including those generally mentioned in connection with monohydrazone yellow pigments. As a result of its good lightfastness, this pigment is particularly interesting for textile printing; however, its fastness to dry-cleaning solvents and to dry heat setting (fixation) is poor. P.Y.1 is easily dispersed in most media, irrespective of the dispersion equipment.
2.3.4.2.2 Pigment Yellow 2
This pigment has little impact on the market today and is only occasionally found in printing inks or in office articles, for instance in coloured pencils. Its shade is more reddish and its tinctorial strength superior to that of P.Y.1. The low specific surface area of the types which are still commercially available makes for good hiding in print. Solvent resistance and other fastness properties equal those of P.Y.1; P.Y.2 is only slightly less lightfast than P.Y.1.
2.3.4.2.3 Pigment Yellow 3
P.Y.3 grades produce a pure, much greener yellow than the Pigment Yellow 1 types. Pigment Yellow 3 can easily be combined with blue pigments to produce shades of green; it may also be used to modify the hue of a green pigment, such as Copper Phthalocyanine Green. The commercial types exhibit low specific surface areas and confer good hiding power on paints, coatings and prints.
P.Y.3 is even less stable to most organic solvents than P.Y.1; it migrates in baking enamels and is applied in this context only rarely and at a pigment concentration below the concentration limit above which blooming occurs (Section 1.6.3.1). As a comparison, the concentration limit beyond which P.Y.3 blooms in a certain urea/alkyd resin paint at a temperature of 120 °C (30 min) is 1%; at 140 °C, the limit increases to 2.5%. Higher temperatures always cause blooming, irrespective of the concentration. Any pigment that is to be applied in a baking enamel should be submitted to a test run in the specific material. There are commercially available grades which recrystallize considerably less in alkyd resin paints.
P.Y.3 is completely water, acid and alkali resistant. Alkali fastness is a major prerequisite for several applications, especially in aqueous media.
The pigment exhibits excellent lightfastness and durability; its performance in this respect is superior to that of P.Y.1, especially if it is reduced with titanium dioxide. Compared to P.Y.98, which exhibits a similar hue but is much stronger, P.Y.3 grades have the advantage of being more reasonable in terms of cost but less fast to solvents and migration. In air drying paints, for instance, medium white reductions of P.Y.3 are somewhat less lightfast but more durable than comparable P.Y.98 grades.
P.Y.3 and P.Y.1 are used in similar applications. The paint industry employs these pigments in air drying, cellulose nitrate, polyurethane, acid hardening and similar paints. P.Y.3 may lend colour to emulsion paints, and its full and medium shades even comply with the industrial standards for exterior house paints. In the printing inks field, the pigment is primarily used in various packaging inks. P.Y.3 also plays a role in textile printing, office articles, artists' paints, and several special purposes. It is, however, not used in plastics; a certain exception is the colouration of unsaturated polyester resins, whose hardening process by peroxide is not influenced by P.Y.3 and in which the pigment exhibits good lightfastness.
Single crystal data and X-ray powder patterns were reported by Whitaker [34, 35].
2.3.4.2.4 Pigment Yellow 5
Over the last few decades, commercial recognition of Pigment Yellow 5 has decreased considerably. Two crystal modifications of the pigment are available [36]; both show practically identical shades. P.Y.5 is a greenish yellow pigment, applied mainly in printing inks and occasionally also in air drying systems. Although its hue is considerably redder than that of P.Y.3, its application properties parallel that of P.Y.3. It is slightly less lightfast and in this respect similar to the more reddish P.Y.1.
2.3.4.2.5 Pigment Yellow 6
This representative is only of limited regional importance. Its shade is a medium yellow, which places it in a competitive position with several similar colourants of the same class, which it also resembles in its fastness properties.
Crystal data are described by A. Whitaker [22, 37].
2.3.4.2.6 Pigment Yellow 10
P.Y.10 provides clean, reddish yellow hues. Its very good lightfastness makes it almost as fast as P.Y.97. However, its solvent fastness is poor and in this respect inferior to most other monohydrazone yellow pigments; it shares this deficiency with P.O.1. Its migration resistance is also poor and considerably less satisfactory than that of the slightly greener P.Y.65. In the face of increasingly stringent application requirements, P.Y.10 has almost vanished from the European market in recent years. Originally, it was used in special printing inks and in air drying paints. In the USA, it is still applied in traffic marking paints.
2.3.4.2.7 Pigment Yellow 49
This very lightfast pigment has a clean greenish yellow shade and is used for viscose spin dyeing and for the mass colouration of viscose foils, sponges, and so on. It is commercially available in the form of aqueous pigment preparations, in which the pigment is predispersed. In 1/1 to 1/3 SD, the lightfastness equals step 8, in 1/12 SD it reaches a value of 7–8 on the Blue Scale. The pigment performs excellently on textiles, but its fastness to wet and dry crocking is not always satisfactory.
2.3.4.2.8 Pigment Yellow 60
This colourant is produced in the USA, where it also plays a minor role on the market. Its shade is a reddish yellow. P.Y.60 is used in trade sales paints and in emulsion paints. Poor resistance to organic solvents, lack of overcoating fastnesss, and a lightfastness that deteriorates rapidly with the degree of reduction with TiO2 are reasons for the limited commercial interest in this pigment. Crystal data have been published by A. Whitaker, who described it already as a hydrazone pigment [38].
2.3.4.2.9 Pigment Yellow 65
Pigment Yellow 65 provides reddish yellow shades. Although its importance as a colourant on the European and Asian market has diminished over the last years, there is still some demand for it in the USA. The types which are still commercially available consist of pigments with comparatively coarse particle sizes and with specific surface areas of approximately 6–20 m2 g−1. The pigment thus provides excellent hiding power. There is some resemblance between Pigment Yellow 65 and P.Y.74 types with high hiding power in that they perform equally well in terms of solvent resistance, recrystallization fastness in solvent containing media, lightfastness, and durability. Tinctorially, however, P.Y.65 is considerably inferior, which precludes its application in printing inks. Opaque varieties of the slightly more greenish diarylide yellow pigment P.Y.83, for instance, are more than three times as strong as P.Y.65, yet provide similar lightfastness. Media in which P.Y.65 grades are applied include air drying and emulsion paints. Combinations of the positional isomers P.Y.65 and 74 are also commercially available. Crystal data have been reported by A. Whitaker [23, 39].
2.3.4.2.10 Pigment Yellow 73
Depending on particle size distribution and the application medium, this pigment more or less bears some resemblance to P.Y.1 as far as shade and tinctorial strength are concerned. At equal tinctorial strength, however, P.Y.73 is more lightfast and durable. Compared to P.Y.1, it is also considerably more insoluble in various organic solvents, such as aliphatic and aromatic hydrocarbons, and thus also less prone to recrystallize. This facilitates its incorporation into solvent containing media, such as air drying systems and emulsion paints, as well as in nitrocellulose systems and so on throughout the printing inks field. Compared to P.Y.1, Pigment Yellow 73 grades generally afford more transparent prints and are also somewhat redder than the P.Y.74 types with fine grains, which provide a tinctorial strength which is about twice as high. P.Y.73 representatives are more lightfast and durable than the tinctorially similar dihydrazone yellow pigments, which are also very stable to solvents.
2.3.4.2.11 Pigment Yellow 74
P.Y.74, a commercial pigment of considerable significance, is used primarily in the printing ink and paint industries. Its greenish yellow shades are somewhere between those of P.Y.3 and P.Y.1. The pigment is considerably stronger than and superior to all comparable monohydrazone yellow pigments. P.Y.74 grades with very different particle sizes are commercially available. Varieties with fine particle sizes, that is, pigments with specific surface areas between approximately 30 and 70 m2 g−1, are used primarily in the printing ink industry. They provide brilliant prints, which are brighter and more transparent than those of other monohydrazone yellow pigments. In terms of tinctorial strength, these P.Y.74 varieties reach the level of the similarly coloured dihydrazone yellow pigments, such as the slightly redder P.Y.12. The pigment concentration that is necessary to produce a 1/3 SD P.Y.12 proof-print is 4.5%, compared to 4.2% in the case of P.Y.74. In letterpress application, P.Y.74 has a yellow hue, which corresponds to the DIN 16508 standard for letterpress; in offset printing, it matches not only the DIN 16509 standard in the yellow section of the DIN Scale for process colour printing (Section 1.8.1.1), but also the standard yellow on the Kodak scale. On the European Scale (Section 1.8.1.1), P.Y.74 is somewhat more greenish than the standard yellow. Its shade can be adjusted with suitable reddish yellow pigments.
The lightfastness of types with fine particle sizes exceeds that of colouristically similar dihydrazone yellow pigments (P.Y.12) by 2 to 3 steps on the Blue Scale. This explains why types with fine particle sizes are preferred where excellent lightfastness is required, such as in packaging printing. P.Y.74 is not different from other yellow pigments of this class in that it performs poorly in solvents. The prints are not very suitable for calendering and are not colourfast to sterilization. As typical monohydrazone yellow pigments, P.Y.74 specimens are fast to alkali, acid and soap. Other aspects of their fastness in print, such as butter resistance and fastness to the solvent mixture described in DIN 16 524 (Section 1.6.2.1), are not convincing. However, like other pigments of their class, P.Y.74 varieties with comparatively fine particle sizes are also used in the paint industry, where they lend colour to air drying and emulsion paints. Excellent tinctorial strength, especially in pastel shades, that is in white reductions, make P.Y.74 more advantageous on a cost/performance basis than the somewhat redder P.Y.1. A strong tendency to bloom, however, precludes the use of P.Y.74 in baking enamels.
For some years, the paint industry in particular has focussed increasingly on P.Y.74 types with very low specific surface areas between about 12 and 20 m2 g−1 and correspondingly very large particle sizes. Such materials exhibit good hiding power and are particularly interesting as replacements for Chrome Yellows in air drying systems. As a result of favourable pigment rheology, P.Y.74 concentrations in paints can be increased to even improve the hiding power without affecting the rheology, that is, the flow properties, the ease of processing, or the brilliance of the product (Section 1.7.8). Compared to transparent types with fine particle sizes, the more opaque varieties are less brilliant, more reddish, and more lightfast and durable. They are also faster to various organic solvents; but their tendency to migrate is similar. The crystal structure was determined by Whitaker [40] and more accurately by Paulus [24].
2.3.4.2.12 Pigment Yellow 75
Although it is sold in the USA, this pigment has failed to gain commercial importance. Its hue is a reddish yellow, much redder than that of P.Y.74 but greener than that of P.Y.65. In full shade and in white reductions, it resembles the shade of the pigment which is produced by mixed coupling between the two, or plain mixtures of the positional isomers, which are also marketed in the USA. P.Y.75 is somewhat more lightfast than the above-mentioned mixture. Its lightfastness in full shade (5%) corresponds to step 7–8 in an air-dried alkyd resin paint, in reductions (1:5 TiO2), it matches step 7 on the Blue Scale. Its tinctorial strength is poor, and it is used mainly in traffic marking paints.
2.3.4.2.13 Pigment Yellow 97
Pigment Yellow 97 provides a medium yellow shade with properties that are somewhat of an exception compared to other monohydrazone yellow pigments. Its hue is similar to that of P.Y.1. P.Y.97 is considerably more resistant to most organic solvents than the other representatives of this group, its migration fastness properties are also superior. In baking enamels, for instance, the pigment resists blooming up to about 180 °C. However, its fastness to overpainting in such coatings is not always sufficient. In a white baking enamel on alkyd-melamine resin basis, P.Y.97 does not bleed after 30 min at 120 °C, but very slight bleeding is observed at 160 °C. Its high quality in terms of fastness to clear lacquer overcoatings and sterilization (Section 1.6.2.3), which are important aspects in the printing inks field, is very unusual for a monohydrazone yellow pigment.
In contrast to most other representatives of its class, P.Y.97 is also much more heat resistant. In a performance test, in which prints formulated at 5% pigment concentration are applied to sheet metal covered with white paint, most monohydrazone yellow pigments clearly change colour after 30 min exposure to a temperature up to 140 °C (Section 1.6.7). P.Y.97, however, being very heat stable, retains its colour for 30 min at 180 °C or for 10 min at 200 °C.
P.Y.97 is very lightfast and durable; in this respect it excels over P.Y.1, especially in white reductions. In various systems (air drying and baking paints), P.Y.97 exhibits a lightfastness that corresponds to step 6–7 on the Blue Scale, even if it is reduced by as much as 1:140 TiO2.
Its tinctorial strength is average and comparable to that of other monohydrazone yellow pigments, except P.Y.74. P.Y.97 disperses easily in all application media.
P.Y.97 is used in various fields. Even in pastel shades, it is used in industrial finishes; while its full shades lend colour to automobile refinishes. In emulsion paints, both its medium and full shades are suited to exterior application. The printing ink industry uses P.Y.97 in high grade printing products, especially where excellent fastness is required, such as in stable posters, and so on. It lends itself without difficulty to all printing techniques. However, lack of fastness to monostyrene and acetone and therefore a certain tendency to bleed in these media precludes its application in deco printing inks, that is, for decorative laminates.
P.Y.97 can also be applied in plastics. In rigid PVC, for instance, its lightfastness is excellent, both in transparent and in opaque shades with TiO2. Depending on its concentration, the lightfastness reaches values between steps 6–7 and 8 on the Blue Scale. In tests designed to determine the colour fastness in rigid PVC films containing 0.5% pigment, coconut fat, for instance, is not stained. In plasticized PVC, however, the pigment tends to migrate at low concentrations. P.Y.97 exhibits good thermal stability in polyolefins; in 1/3 and 1/25 SD HDPE foils, for instance, it is stable up to 240 °C for 5 min. In HDPE extrusion the pigment does not affect shrinkage. P.Y.97 performs similarly in polystyrene and PE as far as thermal stability is concerned. At common colouration concentrations, the pigment dissolves almost completely at temperatures above 200 °C. The lightfastness of transparent polystyrene colourations is excellent (step 7–8 on the Blue Scale); its opaque shades show good lightfastness (0.1% pigment/0.5% TiO2) (step 5). In ABS, the lightfastness is superior even in reduction with TiO2 (step 7 on the Blue Scale). If polymethacrylate is used as a medium, the pigment changes colour only after 5 min at 280 °C; in transparent and opaque colourations; the colour change is 1 CIELAB unit in the system of colour difference. The lightfastness of transparent colourations in such media corresponds to step 8 on the Blue Scale.
P.Y.97 is also used to lend colour to cast epoxy resins and to unsaturated polyester resins; it considerably accelerates the hardening process in the latter (Section 1.8.3.7).
Basically, P.Y.97 can also be applied in other areas, such as in pigmented water-based paints. However, various more economical organic pigments covering the same range of hues, such as P.Y.1, frequently compete with P.Y.97 in this area.
2.3.4.2.14 Pigment Yellow 98
This pigment has been removed from the market worldwide because one of the intermediates for its production is no longer available. P.Y.98 resembles P.Y.3 in shade, that is, it provides clean greenish shades of yellow. P.Y.98, like P.Y.3, is a particularly suitable pigment for clean green mixtures. No perfect replacement for P.Y.98 is commercially available. A mixture of P.Y.3 and P.Y. 111 has been introduced to the market which approaches the colouristic, application and fastness properties of P.Y.98 in printing inks. However, this mixture is not quite as lightfast as P.Y.98. In the paint field, P.Y.3 can replace P.Y.98 in many areas of application. Compared to P.Y.98, however, P.Y.3 is much less resistant to organic solvents. The interval in which blooming occurs in baking enamels is consequently much wider for P.Y.3, which is why this pigment is unsuitable for such applications.
The crystal data and powder pattern were reported by A. Whitaker [41].
2.3.4.2.15 Pigment Yellow 111
P.Y.111 has been commercially available since the 1970s. It is much redder and somewhat duller than P.Y.98, but considerably greener than P.Y.74. In printing inks it is tinctorially stronger than the more greenish P.Y.98 and less lightfast than the latter; the difference between printing inks containing the same amount of P.Y.74 and 98, respectively, equals one step on the Blue Scale. Prints that exhibit the same depth of shade are even more different in their lightfastness. Packaging gravure and flexographic inks, some of which are based on alcohol-soluble nitrocellulose, are much less subject to differences in tinctorial strength than are letterpress or offset inks. The commercially available version of P.Y.111 is considerably more opaque than P.Y.98 and 74 types with fine particle sizes.
2.3.4.2.16 Pigment Yellow 116
Pigment Yellow 116 is a product of somewhat less commercial importance. Its shade is a medium to reddish yellow. The commercial types with their low specific surface area (about 15–18 m2 g−1) exhibit good hiding power.
Areas of application include coatings, printing inks, and plastics. Compared to other representatives of its class, P.Y.116 is exceptionally resistant to common organic solvents. Its fastness to overpainting in baking systems, however, is not satisfactory at baking temperatures which are typically around 150 °C. The pigment is acid and alkali resistant and thermally stable up to 180 °C. It is very lightfast; in a medium-oil alkyd resin paint, its full shades (5% pigment) are equal to step 7–8 on the Blue Scale, while in 1/3 SD formulations it equals step 7 on the Blue Scale. P.Y.116 is also very weatherfast. It is applied especially in combination with inorganic yellow pigments and also in baking enamels that are baked at high temperature, such as 200 °C (30 min); and in automobile repair finishes. The pigment is also found in emulsion paints; exterior use is possible if the demands are not too high.
P.Y.116 generally exhibits good fastness properties in printing inks. In letterpress and offset application, for instance, it resists several organic solvents, such as the standard DIN 16 524/1 solvent mixture (Section 1.6.2.1), paraffins, butter, soap, alkali, and acid. Its thermostability is good up to 180 °C. The pigment is also recommended for metal deco printing. The lightfastness of 1/1 to 1/3 SD letterpress proof-prints is equal to step 6 on the Blue Scale. Its good solvent resistance qualifies P.Y.116 as a colourant for packaging gravure inks. The pigment can, for instance, be applied in mixed polymers based on vinyl chloride/vinyl acetate for PVC films.
P.Y.116 is also used in plastics. In plasticized PVC, it shows little tendency to bleed and is thermally stable up to 180 °C. The lightfastness of transparent PVC colourations (0.1% pigment) equals step 7–8; in 1/3 SD (with 5% TiO2), it corresponds to step 6 on the Blue Scale. Insufficient heat resistance limits the application of P.Y.116 in polyolefins, polystyrene, and other polymers which are processed at high temperature.
2.3.4.2.17 Pigment Yellow 130
This pigment is not marketed in Europe and enjoys only limited commercial importance in other parts of the world. As one of the less resistant representatives of its class, its lightfastness and resistance to organic solvents are particularly deficient. It is recommended to be used for air-drying paints.
2.3.4.2.18 Pigment Yellow 165
Its chemical constitution is identical with Pigment Yellow 10. The pigment enjoys limited commercial success, mainly in the Japanese market. Its hue is reddish yellow, its lightfastness is almost as good as the much greener and tinctorially stronger P.Y.97. P.Y.165 is recommended for use in paints.
2.3.4.2.19 Pigment Yellow 167
The constitution of this pigment differs from that of common monohydrazone yellow pigments, since it is synthesized from aminophthalic imide as a diazonium compound.
P.Y.167 enjoys only regional importance and is recommended for application in paints. The currently available type provides medium hiding power. In full shade and in similar deep shades, it shows a medium yellow; white reductions are greenish yellow and very clean. Its fastness properties correspond to those of other monohydrazone yellow pigments; P.Y.167 is not alkali resistant.
2.3.4.2.20 Pigment Yellow 203
This monohydrazone pigment exhibits a yellow shade. It is recommended for use in paints and printing inks as a substitute for lead containing pigments. P.Y.203 is scarcely found on the European market.
2.3.4.2.21 Pigment Orange 1
P.O.1 has lost most of its commercial significance. Its shade is a very reddish yellow; the Colour Index classifies it as a yellowish orange. Resistance to organic solvents is very low. P.O.1 dissolves more easily in common organic solvents than do other monohydrazone orange pigments, and it is also less lightfast.
P.O.1 is not entirely acid resistant; acids change its colour to a more reddish shade. The pigment has not been able to meet the increasingly stringent requirements of the printing ink and paint industry in recent years. It is now limited in scope to air drying paints and has largely been replaced by mixtures of P.O.5 or others with other monohydrazone yellow pigments.
2.3.4.2.22 Pigment Orange 6
P.O.6 has also suffered considerable loss of interest throughout the European market and is rarely sold today. It provides a very reddish yellow shade. Its lightfastness is very good and almost reaches the level of that of P.Y.1.
2.3.4.3 Monohydrazone Yellow Pigment Lakes
The structure of the monohydrazone yellow pigment lakes are shown in Table 2.4 (Section 2.3.4.1).
2.3.4.3.1 Pigment Yellow 61
This calcium pigment lake is of little commercial value. It is used to lend colour to plastics and to spin dyeing of polypropylene. Its shade is a greenish yellow, and 1/3 SD formulations in polyolefin are thermally stable up to 250 °C. Its tinctorial strength, however, is not satisfactory. 1/3 SD HDPE colourations (1% TiO2), for instance, require 0.7% pigment. The more costly but colouristically identical hydrazone condensation pigment P.Y.94 requires only 0.44% pigment in order to afford the same depth of shade, and only 0.13% of the somewhat redder diarylide yellow pigment P.Y.17 suffice for this purpose. In partially crystalline polymers, P.Y.61 has a considerable influence on the shrinkage during polymer extrusion. The pigment provides good lightfastness; in HDPE, transparent and opaque 1/3 SD colourations are equal to step 5–6 on the Blue Scale. Its lightfastness is even better in rigid and in plasticized PVC, but the pigment also exhibits very poor tinctorial strength in these media. 1/3 SD colourations require a 3.6% pigment concentration. Its migration resistance in plasticized PVC is good.
P.Y.61 is also used for industrial and trade sales paints. Its full shade and close to deep shades, frequently in combination with inorganic yellow pigments (especially Nickel Titanium Yellow), exhibit good-to-excellent lightfastness and durability. However, the pigment is technically unsuitable for exterior application. Its hiding power and its fastness to overpainting are good. However, P.Y.61 is of little overall importance to the paint industry.
2.3.4.3.2 Pigment Yellow 62
There is equally little commercial interest in the calcium pigment lake P.Y.62. Its use is in the plastics industry, where it produces colours in the medium to reddish yellow range. Although its solvent resistance is only average, P.Y.62 shows better fastness to plasticizers, such as dioctyl phthalate and dioctyl adipate, and consequently exhibits good bleeding fastness in plasticized PVC. It is also thermally stable. Transparent colourations and white reductions up to 1/3 SD equal step 7 on the Blue Scale for lightfastness. At 1/25 SD, the lightfastness drops to step 5–6.
P.Y.62 exhibits comparably low tinctorial strength. A 0.5% pigment concentration in HDPE is necessary to produce 1/3 SD colourations (containing 1% TiO2), compared to the 0.17% pigment concentration required to afford equal depth of shade with the somewhat more reddish diarylide yellow pigment P.Y.13.
P.Y.62 is thermally stable up to 250 °C. It has a considerable effect on the shrinkage of HDPE and other partially crystalline polymers. The pigment is an equally suitable colourant for polystyrene and polyurethane and lends colour to polypropylene spin dyeing products with minimal application requirements.
2.3.4.3.3 Pigment Yellow 100
This pigment is of little technical importance, except in the USA, where it is sometimes used to lend colour to NC-based flexographic inks and has also some usage in plastics. Owing to its heat stability of 280 °C it is used in food containers, for example HDPE milk bottles. P.Y.100 provides greenish yellow shades of little alkali and soap fastness. It also performs poorly in organic solvents and even water and exhibits little fastness to overcoating. Its tinctorial strength is inferior to that of other organic pigments, and it provides poor lightfastness.
As long as it complies with certain purity requirements, the pigment is approved as a colourant for food and cosmetics in the USA under FD&C Yellow 5 and in Europe under E102.
2.3.4.3.4 Pigment Yellow 133
This pigment is of regional commercial importance in East Asia. It is a strontium lake of a monohydrazone yellow pigment, which provides greenish to medium yellow shades and is used in plastics and for the spin dyeing of polyolefins. Its tinctorial strength is poor. 1/3 SD HDPE colourations (1% TiO2), for instance, require 0.85% pigment. In this medium, the pigment is thermally stable up to 260 °C.
2.3.4.3.5 Pigment Yellow 142
The pigment is of regional importance in East Asia. P.Y.142 provides a bright yellow shade and is used for the colouration of plastics.
2.3.4.3.6 Pigment Yellow 168
P.Y.168 is a calcium pigment lake and chemically closely related to the P.Y.61 and 62:1 lakes. It has been commercially available for some years, but has as yet gained only regional importance. It provides a clean, somewhat greenish yellow shade somewhere between the shades of P.Y.1 and 3. P.Y.168 is used in paints and plastics.
The pigment exhibits good fastness to aliphatic and aromatic hydrocarbons; but it shows only limited resistance to alcohols, esters and ketones. It is largely acid and alkali resistant. P.Y.168 is not fast to overpainting. It is used in inexpensive industrial finishes wherever the application requirements, especially as far as lightfastness and durability are concerned, are not high.
P.Y.168 shows good migration resistance in plasticized PVC, but its tinctorial strength is relatively poor. In transparent colourations, its lightfastness (0.1% pigment) is equal to step 6 on the Blue Scale; at 1/3 SD it corresponds to step 5. In HDPE, the pigment affects the shrinkage of extrusion products, which is typical of hydrazone pigment lakes of this class. P.Y.168 is recommended especially for use in LDPE.
2.3.4.3.7 Pigment Yellow 169
P.Y.169 is another calcium monohydrazone yellow pigment lake, which has been available for some years. Its hue is a reddish yellow. Its properties and application are similar to those of the much greener P.Y.168.
2.3.4.3.8 Pigment Yellow 183
P.Y.183 entered the market a few years ago and is considered a speciality product for the colouration of plastics.
This monohydrazone yellow calcium pigment lake affords reddish, somewhat dull yellow shades with poor tinctorial strength. 1/3 SD HDPE colourations (1% TiO2) require 0.32% pigment. In such media, the pigment is thermally stable up to 300 °C. Polymer shrinkage is practically unaffected. The pigment provides very good lightfastness; 1/3 SD colourations equal step 7–8 on the Blue Scale. P.Y.183 is also recommended for other high temperature processed plastics. In ABS, for example, it is thermally stable up to 300 °C.
P.Y.183 is bleed resistant in plasticized PVC, where its poor tinctorial strength is of some disadvantage. 1/3 SD samples (5% TiO2) require 1.64% pigment. Such systems provide a lightfastness which is equal to step 6 on the Blue Scale; 1/3 SD transparent colourations correspond to step 6–7 on the Blue Scale.
2.3.4.3.9 Pigment Yellow 190
The monohydrazone calcium pigment lake P.Y.190 is considered a speciality product for the colouration of plastics, but it has been removed from the market.
The commercially available type is tinctorially very weak. 0.66% pigment are required to produce 1/3 SD HDPE colourations (1% TiO2). P.Y.190 provides medium shades of yellow. In HDPE, the pigment resists heat up to 300 °C.
In polyamide, P.Y.190 is heat stable up to 270 °C. In this medium, the pigment has a somewhat redder shade and demonstrates noticeably higher tinctorial strength than in HDPE. 0.3% of the commercially available type are sufficient to produce 1/3 SD colourations.
This commercial grade is slightly soluble in water, which may cause problems during extrusion.
2.3.4.3.10 Pigment Yellow 191
The Monohydrazone calcium pigment lake P.Y.191 produces reddish shades of yellow, covering the same range of shades as the diarylide yellow pigment P.Y.83. P.Y.191, however, exhibits distinctly less tinctorial strength than P.Y.83. 0.32% P.Y.191 are needed to produce 1/3 SD colourations (1% TiO2) in HDPE. These colourations are heat stable up to 300 °C, while 1/3 SD colourations without TiO2 resist heat up to 290 °C. The pigment does not affect the shrinkage of the polymer. P.Y.191 shows good lightfastness: 1/3 SD colourations containing 1% TiO2 equal step 6–7 on the Blue Scale, while 1/3 SD colourations without TiO2 equal step 8. In media such as polystyrene and ABS, P.Y.191 shows similar values in terms of tinctorial strength, heat stability, and lightfastness. In polycarbonate, 1/3 SD colourations (containing 1% TiO2) are heat stable up to 330 °C; such colourations equal step 4–5 on the Blue Scale for lightfastness. P.Y.191 is migration resistant in plasticized PVC; up to a concentration limit of 0.005%, the pigment can be used at 180 °C and in rigid PVC up to a concentration limit of 0.005% at 200 °C.
P.Y.191 displays excellent solvent fastness in aliphatic and in aromatic hydrocarbons, as well as in the commonly used plasticizers. The pigment is almost completely fast to alcohols and esters but not to water, ketones and methyl-glycol.
P.Y.191 is also used for hot melting traffic paints in the USA.
The usual crystal phase of P.Y.191 is the α-phase. Four new crystal modifications of P.Y.191 (β, γ, δ, ɛ) have been described [42], which are obtained for example by recrystallization from different solvents. Phase conversions were achieved by solvation of the α-phase in high boiling organic solvents with subsequent cooling: the β-phase by recrystallizing from NMP (N-methylpyrrolidone) (180 °C), DMAA (dimethylacetamide) or DMSO (dimethyl sulfoxide); the γ-phase from diethylene-glycol dimethyl ether (150–170 °C), the δ-phase from ethylene-glycol (170–200 °C) and the ɛ-product from DMF (N,N-dimethylformamide. The β- and γ- phases can also be obtained by varying the synthetic procedure; correspondingly they are also found in commercial products.
2.3.4.3.11 Pigment Yellow 191:1
The Monohydrazone ammonium lake is a recent product and differs in its colouristic and fastness properties scarcely from the calcium lake types. Therefore, the recommended application media for the two lakes are the same. 0.34% P.Y.191:1 are required to produce 1/3 SD colourations (1% TiO2) in HDPE. Such colourations are heat stable up to 300 °C.
2.3.4.3.12 Pigment Yellow 205
This is a metallized hydrazone pigment with the exact chemical constitution remaining unpublished. The pigment exhibits very good heat stability in polyolefins where it can be used to 285 °C in masstone and in tint tone. The pigment is almost exclusively developed for plastic applications where lightfastness and weatherfastness are not important requirements. It is used in packaging and in disposable plastic items. Its relatively strong mid-shade tone allows concentrate manufacturers and moulders to replace diarylide yellows P.Y.14 and P.Y.17 where diarylides are restricted. P.Y.205 has been especially useful where P.Y.62 is tinctorially too weak to gain the intensity of colour and chroma required. P.Y.205 exhibits approximately 2–2.5 times the tinctorial strength of P.Y.62. By virtue of this increased strength, P.Y.205 is arguably the best valued alternative to diarylide yellows P.Y.14 and P.Y.17 for plastic applications where temperatures of 200 °C or higher will be reached.
2.3.4.3.13 Pigment Yellow 206
A pigment of the metallized hydrazone class in which the exact chemical constitution remains unpublished. P.Y.206 exhibits excellent heat stability in polyolefins and good compatibility and highly chromatic colour in polyester and polycarbonates as well as styrene. The heat stability reaches 285 °C in both masstone and tint tone. P.Y.206, although used mostly in plastics, exhibits good lightfastness in coatings as well, and can be used as shading pigment for many maintenance paints. In plastics it is a strong red shade yellow, almost yellow shade orange in masstone and a bright red shade yellow in tints. By virtue of this wide shift from almost orange masstone to red shade yellow tint-tone, the formulator gains a wide colour space of coverage and possibilities. The colour strength of P.Y.206 makes it a high value as a diarylide yellow P.Y.83 alternative where P.Y.83 is restricted.
2.3.4.3.14 Pigment Yellow 209 and 209:1
Both of these pigments are of the metallized hydrazone pyrazolone class. Pigment Yellow 209, the calcium salt, is the redder shade of the two pigments with Pigment Yellow 209:1, the strontium salt, being a more mid-red shade yellow. Both yellows exhibit good resin compatibility in plastics and excellent heat stability to 285 °C in polyolefins. These pigments were developed for the plastic packaging market segment as alternatives to diarylides and as products that comply with the FDA requirements for food contact under the conditions of use (A) through (H) as described in Table 2 of 21 CFR Part 176.170(c), where the pigments are allowed to be used at levels up to 1% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR part 178.3297. Both pigments exhibit excellent colour strength and good dimensional stability in moulding applications. Owing to marginal lightfastness and weatherfastness these products are limited to use in interior applications.
2.3.4.3.15 Pigment Yellow 212
P.Y.212 is a metallized hydrazone pyrazolone pigment exhibiting excellent heat stability in polyolefins, HDPE and polypropylenes exceeding 312 °C in masstone and tint. It is a bright medium shade yellow and provides an excellent alternative to diarylides and other yellows in this shade range. The pigment complies with the FDA requirements for food contact under the conditions of use (A) through (H) as described in Table 2 of 21 CFR Part 176.170(c), where this pigment is allowed to be used at levels up to 1% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR Part 178.3297.
P.Y.212 is not recommended for exterior use, but provides an excellent value for interior applications such as packaging and household durables of all types. It is tinctorially quite strong as metallized hydrazones go and exhibits very good dimensional stability, making it very useful in moulded items such as blow moulded containers.
2.4 Dihydrazone Pigments (Formerly Called Disazo Pigments)
In the industrial context, the term dihydrazone pigments is used exclusively to refer to pigments that possess two hydrazone groups and contain the diaminodiphenyl skeleton according to the general formula:

in which XCl, OCH3, or CH3, and Y = H, Cl, or the diaminobenzene moiety:

with U, V = H, CH3, OCH3, or Cl. The term is somewhat misleading, because it does not relate to all compounds that possess two hydrazone groups; dihydrazone condensation products, for instance, are not included (Section 2.9). This book follows the generally accepted definition.
Structurally, there are two basic types of dihydrazone pigments, depending on whether the bifunctional element is introduced through the diazonium compound or through the coupling component:
- Bifunctional diazo components:
A bifunctional diazo component results in products of the type:

Acetoacetarylides and 1-aryl-pyrazol-5-one are the two most common coupling components. The former affords diarylide yellow pigments (Section 2.4.1), while the latter produces dihydrazonepyrazolone pigments (Section 2.4.3).
- Bifunctional coupling components:
The general structure of a typical dihydrazone pigment based on a bifunctional coupling component is:

 ,
,  , and
, and  can be H, Cl, CH3, OCH3, OC2H5, or COOCH3. These products are known as bisacetoacetarylide pigments (Section 2.4.2).
can be H, Cl, CH3, OCH3, OC2H5, or COOCH3. These products are known as bisacetoacetarylide pigments (Section 2.4.2).
Diarylide yellow pigments reign supreme amongst dihydrazone pigments, followed by the commercially much less important dihydrazonepyrazolone pigments and the bisacetoacetarylide pigments. Dihydrazone pigments provide colours ranging from greenish yellow to reddish orange. Compared to monohydrazone yellow pigments, whose molecular weight is typically only about half of that of a dihydrazone pigment, the latter are considerably more solvent and migration resistant.
2.4.1 Diarylide Yellow Pigments
Diarylide yellow pigments were first patented as early as 1911 in the name of the company Griesheim-Elektron [43]. However, the new invention was not utilized for some time because monohydrazone yellow pigments, which had just entered the market under the name of Hansa Yellow, were faster to light than diarylide yellow pigments. It took as long as 25 years for diarylide yellow pigments to be appreciated. Their commercial value began to be rediscovered when they were used in Germany to lend colour to rubber products: monohydrazone yellow pigments, which show a tendency to bleed, proved unsatisfactory for this purpose.
The printing ink industry, currently the most important area of application for diarylide yellow pigments, first introduced these products in around 1938 in the USA.
The chemically most simple diarylide yellow pigment is made from bisdiazotized 3,3′-dichlorobenzidine and acetoacetanilide. This pigment exhibits high tinctorial strength, a property which is particularly useful for process printing inks, for which other diarylide yellow pigments were later developed.
It was not until after the Second World War that Europe followed this trend. During the early 1950s, the European printing ink industry experienced a change in pigments from monohydrazone yellow pigments to diarylide yellow pigments. In the course of this event, the German market added other diarylide yellow pigments with improved lightfastness and migration properties (Pigment Yellow 83, patented 1953 [44], Pigment Yellow 81, and 113). As a result, diarylide yellow pigments are by far the largest fraction of organic yellow pigments in the market today.
2.4.1.1 Chemistry, Manufacture and Crystal Structures
Commercially significant diarylide yellow pigments have the following chemical structure:

with Y = H or Cl and ![]() ,
, ![]() , and
, and ![]() = H, Cl, CH3, OCH3, or OC2H5.
= H, Cl, CH3, OCH3, or OC2H5.
3,3′-Dichlorobenzidine (Y = H) is the preferred bisdiazo component. It is synthesized by alkaline zinc reduction (Section 2.1.1) or by catalytic reduction of o-nitrochlorobenzene with subsequent rearrangement of the resulting 2,2′-dichlorohydrazobenzene with dilute hydrochloric acid.
To manufacture a diarylide yellow pigment, the diamine, dissolved in hydrochloric acid or sulfuric acid, is bisdiazotized with an aqueous sodium nitrite solution. The resulting diazonium compound is subsequently coupled onto two equivalents of acetoacetarylide. Since a material with a very fine particle size is needed for a fast and complete coupling reaction, the coupling component is prepared by dissolution in an alkaline medium and reprecipitation by an acid, such as acetic acid or hydrochloric acid. Dichlorobenzidine cannot be diazotized stepwise. A lack of sodium nitrite does not produce the monodiazonium compound, but an excess of 3,3′-dichlorodiaminodiphenyl remains.
Surfactants, dispersing and coupling agents are not the only additives used in pigment preparation. Depending on the application, the list includes resins, aliphatic amines and other compounds. Surfactants are primarily used as dispersing agents, while resins are responsible for the very fine particle size consistency of the pigments (Section 2.2.3).
Adsorption of for example rosin (abietic acid) at the pigment surface may – depending on the concentration of the rosin – reduce or accelerate the crystal growth. The presence of an excess amount of rosin during the production of diarylide yellow pigments of the Pigment Yellow 13 type affords an additional crystal modification, which can be identified by X-ray diffraction [45].
Additives may exert not only a physical but also a chemical influence on the coupling suspension: long-chain aliphatic or cycloaliphatic amines RNH2, which react partially with the pigment molecules, will enhance the effect of other additives. The carbonyl function of the acetyl group is converted into a ketimine (azomethine) and reacts to form an alkylammonium enolate [46]:
Pigment preparations containing such amines are widely used in publication gravure inks (see P.Y.12, Section 2.6.1.4.2.1).
The coupling reaction may be followed by more or less intense thermal processing of the crude aqueous pigment suspension. This finishing is sometimes carried out in the presence of organic solvents, especially to develop high hiding power. Solvent treatment is most economical if the solvent can easily be separated from the aqueous medium by distillation, to be recycled.
To extend the range of available products, mixed couplings are carried out with combinations of two or three different acetoacetarylide coupling components. The most common ones are acetoacetanilide, acetoacet-2-methylanilide, -2-methoxy anilide, -2-chloroanilide, -4-methoxyanilide, -2,4-dimethylanilide and acetoacet-2,5-dimethoxy-4-chloroanilide. It is also possible to carry out mixed couplings with two bisazo compounds. Combining 3,3′-dichlorobenzidine and 3,3′-dimethoxybenzidine, for instance, affords products which cover a wide range of colours and combine a variety of different properties [47].
The performance of a pigment produced by mixed coupling does not always equal the sum of the characteristics of the individually coupled products. A new crystal modification, for instance, may lead to an unpredictable deviation in the application properties. On a cost–performance basis, the best approach is to add to the reaction mixture traces of a costly coupling component that is known to confer excellent properties on the product, in an attempt to promote a more favourable crystal modification. The resulting mixed product is expected to assume the modification of the trace coupling component and thus to improve its commercial performance without representing a financial strain [48].
2.4.1.1.1 Crystal Structures
The crystal structures of P.Y1.2, P.Y.13, P.Y.14, P.Y.63 and P.Y.83 have been determined by single-crystal structure analysis and by X-ray powder diffraction [49–53]:

Although these five compounds differ only in the substitution on their terminal phenyl rings, their molecular conformation and the arrangement of molecules in the crystal vary considerably. In the commercial α-phase of P.Y.12, the central biphenyl fragment is not planar, but the phenyl rings form an interplanar angle of 22°. One of the terminal phenyl rings is nearly coplanar with the adjacent acetoacetyl fragment, the other one is rotated by 29° (Figure 2.17). The molecules are arranged in a complicated interdigitated herringbone pattern (Figure 2.18) [50].

Figure 2.17 Molecular geometry of P.Y.12 in the solid state: (a) and (b) α-phase (different view directions) [48]; (c) γ-phase [47]. The arrows denote the interplanar angles.

Figure 2.18 Crystal structure of the α-phase of P.Y.12, showing six molecules.
Lattice-energy calculations [53] show that this pattern is the optimal one for P.Y.12, but any additional substituent would cause steric hindrance with the neighbouring molecules, leading to an energetically unfavourable structure. Correspondingly, this structure is found for P.Y.12 only.
P.Y.13, P.Y.14 and P.Y.63 crystallize in a layer structure built from planar molecules (Figures 2.19 and 2.20) [51]. Such a layer structure is less sensitive to variations of the substitution pattern. P.Y.83 forms a herringbone arrangement of planar molecules (Figure 2.21) [52].

Figure 2.19 Crystal structure of P.Y.13; view perpendicular to the layers [49].
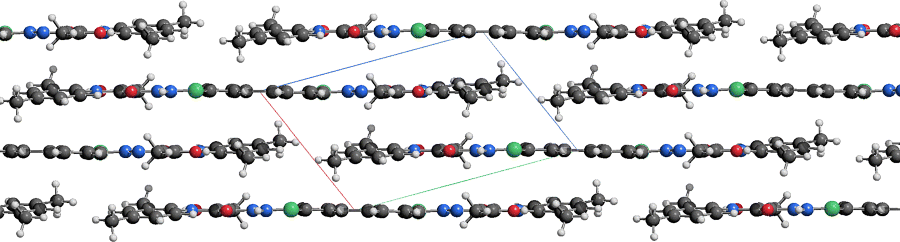
Figure 2.20 Crystal structure of P.Y.13; view along the layers. γ-P.Y.12, P.Y.14 and P.Y.63 are isostructural to P.Y.13.

Figure 2.21 Crystal structure of P.Y.83; herringbone pattern of nearly planar molecules [50].
According to quantum-mechanical calculations the preferred conformation of an individual molecule exhibits a mutual rotation of the two phenyl rings of the biphenyl fragment by about 40° [53]. The planar conformation of P.Y.13, P.Y.14, P.Y.63 and P.Y.83 is a packing effect. With a twisted conformation, an energetically favourable molecular arrangement is not possible, as is shown by lattice-energy calculations. Therefore, the molecules adopt a planar conformation, which easily allows a good and dense molecular packing. The planarization enhances the conjugation between the chromophoric systems of the two halves of the molecules. Apparently, this has no effect on the hue. The colour strength increases considerably, as shown by quantum-mechanical calculations [54]. Correspondingly, the intrinsic colour strength of P.Y.13 is about 50% higher than that of P.Y.12. The additional methyl groups in P.Y.13 have no electronic influence on the chromophoric system, but lead to a planar conformation in the solid state. Hence the increased colour strength of P.Y.13 (and P.Y.14, P.Y.63 and P.Y.83, too) is a packing effect.
Solid solutions of P.Y.13 and P.Y.14, as well as solid solutions of P.Y.12 with P.Y.13 or P.Y.14 also adopt the layer structure of P.Y.13, resulting in pigments with high colour strengths. Hence the crystal structure of P.Y.13 can be ‘diluted' with the cheaper P.Y.12 without changing the optical properties – an excellent example for crystal engineering [53].
2.4.1.2 Properties
Diarylide yellow pigments range in shade from a very greenish to an extremely reddish yellow. The more greenish types almost exclusively contain 2,2′,5,5′-tetrachlorobenzidine in place of the otherwise more common 3,3′-dichlorobenzidine.
Diarylide yellow pigments are colourants with high tinctorial strength. Their tinctorial strength is typically about twice as high as that of monohydrazone yellow pigments that cover the same range of colours (Section 2.3). In letterpress application, for instance, the strength of commercially available types of Pigment Yellow 12 is more than twice as high as that of transparent P.Y.1 varieties. In air drying alkyd systems, the intensely reddish P.Y.83 is three times as strong in white reductions as the similarly coloured monohydrazone yellow pigment P.Y.65. Structurally, it is the larger conjugated system that is the primary colour strength determinant in diarylide yellow molecules. Physical considerations, especially regarding the particle size distribution, also play a major role.
Most commercial varieties of diarylide yellow pigments are materials with comparatively fine pigment particles and specific surface areas between 50 and 90 m2 g−1. The specific demands of the printing industry often require surface-coated pigments with resins or other substances.
The specific surface areas mentioned in this context are frequently minimum values; pigment preparation tends to increase the surface area, so that the actual value may be much higher. A resinated P.Y.83 type, which is washed repeatedly with petrol ether, for instance, will slowly increase the measured specific surface area from 70 m2 g−1 to more than 100 m2 g−1 (Section 1.5.1). Notably, considerable amounts of resin remain on the pigment surface, even after repeated washing.
Monohydrazone yellow pigments, on the other hand, are products with larger particle sizes and with much smaller specific surface areas. P.Y.74 is the only species of which there are types with specific surface areas approaching those of diarylide yellow pigments (Section 2.3.4).
The pigment industry produces primarily the more transparent diarylide yellow pigment types. This has its advantages, particularly in the field of printing inks, since yellow is printed as the last colour in three- or four-colour printing (Section 1.8.1.1). Highly transparent varieties are almost exclusively resinated and are often easy to disperse.
Commercial diarylide yellow varieties are generally formulated with hard resins, primarily with rosin or its derivatives. These resins are easily used for the common types of printing ink systems, in which they dissolve more or less entirely during dispersion. Resins for pigment preparation may also be used advantageously in other application media, as long as the concentration is not too high. Polymers such as polyolefins are the only media in which a resin may decompose during processing if the degradation temperature is exceeded. Moreover, decomposition residue may adhere to parts of the production unit and present processing problems, owing to surface interaction.
Several diarylide yellow pigments are also available in the form of very opaque products with small specific surface areas. These are used to an appreciable extent in Chrome Yellow free paints, in packaging printing inks, and other media. Fastness to light and weather are much improved in opaque varieties, compared to the corresponding transparent types; their rheological characteristics are superior. Opaque pigments are not resinated.
Diarylide yellow pigments are typically surface treated to produce tailor-made products for specific applications, especially for printing purposes. Types that are treated with various amines are often very easily dispersible. However, they have their disadvantages in some applications. In offset inks, for instance, amine-treated types generally present printing problems, owing to a disturbed equilibrium between pigment and water. Use in nitrocellulose chips, that is, preparation on nitrocellulose basis, is not recommended because amines reduce the self-ignition temperature of nitrocellulose, which may lead to deflagration during chip production. Publication gravure printing inks, however, rely almost exclusively on diarylide yellow pigments prepared with amines to produce yellow shades.
In practice, diarylide yellow pigments are frequently selected for their excellent fastness to various organic solvents, in which they perform much better than monohydrazone yellow pigments. Properties such as migration or recrystallization resistance are thus distinctly superior. Several diarylide yellow pigments, such as P.Y.81 or 83, exhibit excellent fastness to overcoating (Section 1.6.3.2). Characteristics such as migration resistance in plastics, particularly in plasticized PVC, as well as fastness to acid and alkali, are equally superior.
Various diarylide yellow pigments are very fast to light and weather – although monohydrazone yellow and orange pigments are superior in this respect.
2.4.1.3 Application
The bulk of diarylide yellow pigments are targeted for use in printing inks, for which they are selected on the basis of their excellent tinctorial strength and versatile transparency, which can be optimized according to customer specification. The printing ink industry requires good or at least sufficient fastness to the commonly used solvents. Recrystallization can be minimized by maintaining an appropriate processing temperature and by keeping the dispersion temperature low, so that products with very fine particle sizes do actually provide highly transparent prints. This is a primary consideration in connection with modern processing technology. In agitated ball mills, for instance, which are used to prepare inks for Web offset printing presses, a medium based on mineral oil is used that is exposed to a temperature of at least 70–90 °C for several hours. Even the most effective cooling will not sufficiently reduce this temperature (Section 1.8.1.1).
In this type of application, there is no alternative to diarylide yellow pigments. It is not yet clear whether preparation agents such as hard resins, used in highly transparent types, enhance the recrystallization resistance of the product. The resin content of highly transparent varieties of diarylide yellow pigments, especially of the P.Y.12 and 13 grades, may vary considerably; this is also true for P.Y.127, derived from the above-mentioned pigments. The printing ink formulations are typically adjusted accordingly.
The effect of resin addition is a primary concern in offset printing. Good dispersibility is needed to guarantee easy incorporation of a diarylide yellow pigment into its offset vehicle by low shear equipment, such as agitated ball mills. During the dispersion process, the resin dissolves more or less in the ink, which exposes the surfaces of the previously resin-coated pigment particles. The units are distributed throughout the medium (i.e. dispersed). Test results may be distorted by partially undissolved or by swollen resin, which falsely indicates insufficient pigment dispersion. Various test methods, including microscopic examination of a printing ink, may fall prey to this error. The printed sample itself is usually exempt from such effects: tinctorial strength or gloss typically remain unaffected.
Good resistance to recrystallization also explains the frequent choice of diarylide yellow pigments as colourants in solvent containing gravure inks for packaging printing. The packaging industry does not generally require high transparency, as in offset printing inks; although transparency may be conferred on a product by applying nitrocellulose chips in order to preclude recrystallization during processing and to make for a glossy appearance. With P.Y.83, however, high transparency is usually expected, even for packaging purposes: it produces brilliant shades of gold on aluminium foil. Aluminium printing generally requires pigments with fine particle sizes and high transparency. This applies also to other diarylide yellow pigments, such as P.Y.17, and to various other organic pigments.
Diarylide yellow pigments exhibit good heat stability in printing inks; they withstand temperatures of 180–200 °C. The fastness of such prints thus far exceeds that of some of the monohydrazone yellow pigments. Diarylide yellow pigments have stimulated widespread interest as colourants for metal deco printing purposes. They are generally resistant to clear lacquer overcoating and to sterilization; fastness to alkali, acid and water are excellent, which is also true for several special application fastness properties (Section 1.6.2).
Several diarylide yellow pigments meet the lightfastness requirements for wallpaper printing. A certain tendency to migrate precludes, for instance, the more lightfast monohydrazone yellow pigments from use in PVC wallpaper.
Compared to the demand for printing inks, there is somewhat less of a demand for diarylide yellow pigments throughout the paint industry. In terms of lightfastness and weatherfastness, they frequently fail to meet the requirements for exterior application. Apart from P.Y.83 with its good properties, the paint market is dominated by monohydrazone yellow pigments, which are used in air drying paints; other suitable products are benzimidazolone, isoindolinone, flavanthrone and anthrapyrimidine yellow pigments, which lend themselves to use in baking enamels.
It is their moderate lightfastness compared to monohydrazone yellow pigments that restricts the use of most diarylide yellow pigments in emulsion paints, with the exception of P.Y.83.
In contrast, diarylide yellow pigments are used widely throughout the plastics field. This is particularly true for P.Y.13, 17, 81, 83 and 113. The first German edition of this book reported that the heat stability (Sections 1.6.7 and 1.8.3) of these pigments, for instance in polyolefins, was up to 200–270 °C for 5 min, depending on the depth of shade. Results that have been published in the meantime [55], however, require rectification of these numbers.
According to these studies thermal decomposition may occur during the processing or application of these pigments in polymers above 200 °C. The same is true for Pigment Orange 13, 34, and Pigment Red 38 types. The decomposition products are monohydrazone colourants and aromatic amines. The formation of such colourants had at earlier times escaped attention because they afford a range of shades that almost equals that of the respective basic pigment. Comparative colouristic studies, for instance, concerning such pigmented, thermally treated versus untreated polymeric materials do not show pigment decomposition or, respectively, formation of a colourant.
Analytical identification of monohydrazone colourants and the other decomposition products requires effective (analytical) methods of concentration, which is made possible by high-performance liquid chromatography (HPLC). Prior to HPLC analysis, the pigmented medium was extracted for 20 h with toluene in a Soxhlet extractor. These analytical methods also showed that above 240 °C, especially after prolonged exposure of the pigmented polymer material to heat, dichlorobenzidine (DCB) is also formed.
These results show that the described types of diarylide yellow pigments are not recommended for use in polymers that are processed at temperatures above 200 °C. This is true not only for their use in plastics, such as polypropylene and polystyrene, but also for applications such as metal deco prints, which are baked at temperatures above 200 °C, or powder coatings, which are processed above 200 °C.
The assumption is that thermal decomposition starts as soon as the diarylide yellow pigment begins to dissolve in the polymer. This means that the decomposition process proceeds via the dissolved state of the pigment. This assumption, if true, also explains why these pigment powders are heat resistant up to temperatures as high as 340 °C, as shown by differential thermoanalysis (DTA).
Blooming is not normally observed, but may occur under certain conditions. Depending on the processing temperature and the choice and amount of used plasticizer, a pigment concentration below 0.05% in plasticized PVC may have unacceptable results. Consideration must therefore be given to the respective threshold concentrations. Under certain conditions, several diarylide yellow pigments are very resistant to bleeding. These species are used to an appreciable extent in various PVC products, and their lightfastness also qualifies them for various applications throughout the plastics industry. These pigments exhibit high tinctorial strength in plastics, and favourable cost–performance considerations and generally good fastness properties broaden their scope within the plastics sector. Diarylide yellow pigments lend colour to rigid and plasticized PVC and polyolefins, polyurethane foam, rubber, other elastomers, and various cast resins. Diarylide yellow pigments are sometimes also used in spin dyeing.
High tinctorial strength and good fastness properties make diarylide yellow pigments important products in areas other than the printing inks, paints and plastics fields. Of the numerous applications, only a few options will be mentioned. Cleaning agents of various kinds, solvent stains, and office articles employ diarylide yellow pigments to lend colour to pencils, watercolours, chalks or artists' paints, provided the pigment is sufficiently lightfast. Diarylide yellow pigments are also used to an appreciable extent in textile printing inks.
2.4.1.4 Commercially Available Diarylide Yellow Pigments
2.4.1.4.1 General
Diarylide yellow pigments entered the market in 1935, when P.Y.13 was introduced by IG Farben in Germany. Sold as Vulcan-Echtgelb GR, this pigment was designed to lend colour to natural rubber articles. P.Y.12, which was produced and offered a few years later in the USA, replaced the P.Y.1 type monohydrazone yellow pigments, which up until then had dominated the yellow pigment market in most parts of the world. In subsequent years, methods for diarylide yellow pigment synthesis advanced steadily and resulted in a broad range of products, many of which have since then either vanished from the market or are now only regionally important. This is true for diarylide yellow pigments based on 3,3′-dimethoxy, 3,3′-dimethyl, or 2,2′-dimethoxy-5,5′-dichlorobenzidine as a bisdiazo component instead of the most commonly used 3,3′-dichlorobenzidine. P.O.15, 16 and 44 are the only species that have maintained a somewhat important position in the American and Japanese markets.
Table 2.5 lists the commercially significant diarylide yellow pigments. Notably, the acetoacetarylides in the aromatic moiety possess mostly methoxy or methyl substituents.
Table 2.5 Commercially available diarylide yellow pigments.

|
|||||||
| C.I. Name | C.I. Constitution Number | X | Y | Shade | |||
| P.Y.12 | 21090 | Cl | H | H | H | H | yellow |
| P.Y.13 | 21100 | Cl | H | CH3 | CH3 | H | yellow |
| P.Y.14 | 21095 | Cl | H | CH3 | H | H | yellow |
| P.Y.17 | 21105 | Cl | H | OCH3 | H | H | greenish yellow |
| P.Y.55 | 21096 | Cl | H | H | CH3 | H | reddish yellow |
| P.Y.63 | 21091 | Cl | H | Cl | H | H | yellow |
| P.Y.81 | 21127 | Cl | Cl | CH3 | CH3 | H | very greenish yellow |
| P.Y.83 | 21108 | Cl | H | OCH3 | Cl | OCH3 | reddish yellow |
| P.Y.87 | 21107:1a) | Cl | H | OCH3 | H | OCH3 | reddish yellow |
| P.Y.90 | — | — | — | H | H | H | reddish yellow |
| P.Y.106 | — | Cl | H | CH3 | CH3 | H | greenish yellow |
| OCH3 | H | H | |||||
| P.Y.113 | 21126 | Cl | Cl | CH3, | Cl | H | very greenish yellow |
| P.Y.114 | 21092 | Cl | H | H | H | H | reddish yellow |
| H | CH3 | H | |||||
| P.Y.121 | 21091 | Cl | H | Cl | H | H | yellow |
| P.Y.124 | 21107a) | Cl | H | OCH3 | OCH3 | H | yellow |
| P.Y.126 | 21101 | Cl | H | H | H | H | yellow |
| H | OCH3 | H | |||||
| P.Y.127 | 21102 | Cl | H | CH3 | CH3 | H | yellow |
| OCH3 | H | H | |||||
| P.Y.136 | — | Cl | H | — | — | — | yellow |
| P.Y.152 | 21111 | Cl | H | H | OC2H5 | H | reddish yellow |
| P.Y.170 | 21104 | Cl | H | H | OCH3 | H | yellowish orange |
| P.Y.171 | 21106 | Cl | H | CH3 | Cl | H | yellow |
| P.Y.172 | 21109 | Cl | H | OCH3 | H | Cl | yellow |
| P.Y.174 | 21098 | Cl | H | CH3 | CH3 | H | yellow |
| CH3 | H | H | |||||
| P.Y.176 | 21103 | Cl | H | CH3 | CH3 | H | yellow |
| OCH3 | Cl | OCH3 | |||||
| P.Y.188 | 21094 | Cl | H | CH3 | CH3 | H | yellow |
| H | H | H | |||||
| P.O.15 | 21130 | CH3 | H | H | H | H | yellowish orange |
| P.O.16 | 21160 | OCH3 | H | H | H | H | yellowish orange |
| P.O.44 | 21162 | OCH3 | H | H | Cl | H | reddish orange |
| P.O.47 | — | CH3 | H | CH3 | Cl | H | yellowish orange |
| a) CoIour Index constitution numbers 21107 (P.Y.124) and 21107:1 (P.Y.87) are misleading since both pigments exhibit different chemical structures. | |||||||
2.4.1.4.2 Pigment Yellow 12
P.Y.12 is a commercially important product; it makes up a large portion of the worldwide pigment production. The unsubstituted skeleton of the acetoacetanilide as coupling component makes P.Y.12 chemically the simplest representative of diarylide yellow pigments. The fastness properties of P.Y.12 are somewhat lower than those of the substituted derivatives. This is particularly true for the lightfastness. 1/1 to 1/3 SD letterpress proof prints exhibit a lightfastness around step 3 on the Blue Scale; in 1/5 SD, the lightfastness decreases to about step 2. At equal depth of shade, P.Y.12 is thus 1–2 steps less fast to light than other diarylide yellow pigments, such as P.Y.13, 83, 127 or 176, which cover the medium to reddish yellow range. The only exception is P.Y.14, whose lightfastness is about the same.
At equal pigment concentration, P.Y.12 is as much as 2–3 steps less lightfast than its counterparts. In other words, to achieve the same depth of shade, the tinctorially stronger diarylide yellow pigments in the medium to reddish yellow range require much lower pigment concentrations than a comparative P.Y.12 sample, which has to be more highly pigmented. Since the lightfastness of a pigment in an application medium increases with its concentration, differences between pigments become more obvious as the pigment level increases. Printed in equally thick layers (1 µm) and provided the particle sizes of the two samples are similar, a 1/1 SD P.Y.12 letterpress proof print requires a 10% pigment concentration, while a P.Y.13 sample will afford the same depth of shade if it is formulated at only about 7%.
P.Y.12 has a medium yellow hue, which is used to a large extent in letterpress and offset printing inks as the yellow component in three- and four-colour printing. To adjust P.Y.12 to the standard yellow of the European standard (Section 1.8.1.1), the hue may be shaded with traces of a redder component, such as one of the orange pigments P.O.13 or 34. No such shading is necessary for P.Y.12 to match the yellow on the Kodak scale.
The transparency of P.Y.12 can be optimized to meet various application requirements. The product line ranges from fairly opaque versions, which are targeted for packaging and newspaper printing inks, to semitransparent and highly transparent, typically resinated types. As the pigment particle size is reduced for the more transparent versions, the hue shifts increasingly towards more clean, greenish shades; fine pigment particles also render the resulting products more sensitive to light. In this respect, P.Y.12 types are satisfactory only in areas where fastness to light is of less concern. High gloss has its advantage for types that are to be used in the uppermost and very thin layer of a three- or four-colour print. Common printing techniques (Section 1.8.1.1) for letterpress and offset application produce roughly 1 µm ink films. This requirement explains why good dispersibility and optimized ink formulation are of major importance to the manufacturer.
While in Europe P.Y.13 and its derivatives are preferred to P.Y.12 for offset printing inks, the reverse is true in the USA, where P.Y.12 is almost exclusively used. In America, most oil-based inks are manufactured from pigment presscake with a fine particle size, which is transferred directly into the flush paste and then converted into the printing ink, avoiding agglomeration and, to a large extent, recrystallization (Section 1.6.5.8). This technique has become practically obsolete in Europe, considering the easy dispersibility of pigment powders for process colours, that is, colours for three- or four-colour printing in letterpress and offset application.
Compared to other diarylide yellow pigments, P.Y.12 is only moderately fast to organic solvents. Its tendency to recrystallize is particularly detrimental to modern agitated ball mills that are used to disperse pigments in Web offset printing vehicles. Under these processing conditions, highly transparent P.Y.12 types are inferior to the corresponding P.Y.13 types; improved reddish varieties of P.Y.12 with distinctly improved recrystallization stability have recently been introduced.
P.Y.12 is a typical diarylide yellow pigment in that its prints are fast to clear lacquer overcoating, which is used to protect tin prints against scratching and scrubbing. They are also fast to sterilization (Section 1.6.2.3), which is one of the major requirements of an ink to be printed on food cans.
Offset and letterpress inks are usually formulated at high pigment concentrations; most contain more than 15% pigment. This is a particular challenge to highly transparent products with very fine particle sizes. These depend on good rheology, which determines the flow behaviour of a material in a high-speed printing press.
Yellow shades for publication gravure printing inks are produced almost exclusively by speciality types of P.Y.12. These types have the additional advantage of providing extra depth of shade. A side reaction takes place between the aliphatic amines that are used to prepare the pigment, in which a portion of the pigment reacts to form ketimines (Section 2.4.1.1). The reaction products dissolve in the media commonly used to formulate publication gravure printing inks, shifting the colour of the ink to a dark red. Toluene is more effective than benzene in this respect. The result is a seemingly deeper colour in print. At equal pigment concentration, printing inks containing such speciality pigments also exhibit much better flow behaviour than inks made from traditional types.
Recently, amine-treated pigments have been developed that show less of a tendency to penetrate low-cost gravure paper (Section 1.8.1.2). In certain circumstances, the storage stability of a gravure printing ink is a major concern. This is particularly true for the transportation of such inks in summertime, when products are exposed to high temperature and agitation. The resins that are commonly used to formulate these printing inks are frequently unable to prevent a pigment from changing its colouristic properties in an ink. Ketimine formation is reversed and the previously dissolved compound precipitates as a crystalline pigment. The result is a shift towards a greener shade, reduced tinctorial strength and viscosity increase, owing to the formation of additional pigment surface [46]. The storage stability of a given publication gravure printing ink may be improved considerably by choosing appropriate resins for the product. Notably, in contrast to calcium resinates, the commonly used zinc resinates shorten the shelf life of an ink. However, the underlying mechanisms are not yet entirely known.
P.Y.12 types are used in packaging gravure and flexographic inks for their reasonable price, but they can also be utilized in aqueous printing inks, provided they are sufficiently fast to light. They cannot be applied as decorative printing inks to laminated paper.
The paint industry shows little interest in P.Y.12, since it is not sufficiently fast to overcoating for use in baking enamels. In air drying paints, lightfastness of P.Y.12 in white reduction (with 1:5 TiO2) only equals step 2 on the Blue Scale.
There is also little demand for P.Y.12 as a colourant for polymers. Although a certain tendency to migrate precludes its use in plasticized PVC, rigid PVC somewhat enhances its performance. Transparent varieties become sufficiently fast to light to equal step 6 on the Blue Scale; opaque types afford values between 2 and 5, depending on the pigment concentration and amount of TiO2 or depth of shade. Good heat resistance also qualifies P.Y.12 for use in polyurethane foam; aromatic polyurethanes themselves are not very fast to light.
Like modified P.Y.12 (P.Y.126), P.Y.12 is selected to lend colour to various speciality products, such as cleaning agents or office articles. Where better lightfastness is required, P.Y.12 is replaced by less intense and less solvent resistant monohydrazone yellow pigments, especially by P.Y.1 types, which provide a similar hue.
P.Y.12 exists in at least four crystal phases (α, β, γ, X). The α-phase is the usual phase. The metastable β-phase is claimed to be formed during special milling processes [56]. The γ-phase is obtained with additives such as dodecyldipropylenetriamine [57], and the X-phase is obtained with an additive formed by the coupling of diazotized dichlorobenzidine with acetoacetylated 5-amino-2-hydroxybenzoic acid (i.e. with the substituents ![]() in the coupling component) [58].
in the coupling component) [58].
2.4.1.4.3 Pigment Yellow 13
P.Y.13 is also a frequent choice throughout the printing ink industry, especially in offset application. Its hue corresponds to the standard yellow on the European Scale for three- and four-colour printing, and it also matches the yellow on the Kodak Scale (Section 1.8.1.1). High transparency is a major asset to a yellow pigment that is to be used as the uppermost layer in the multicolour printing sequence. P.Y.13 types, as well as their derivatives P.Y.127 and 176, are therefore surface coated with hard resins. At comparable specific surface area and particle size distribution and consequently similar transparency, prints containing P.Y.13 or its modifications are up to 25% stronger than those formulated with P.Y.12 or 126.
Although P.Y.13 types, especially the resinated, highly transparent versions, are generally easy to disperse, problems may arise with low shear equipment like agitated ball mills. Incompletely dispersed pigment may appear to recrystallize during dispersion, affecting both the transparency and the tinctorial strength of the product.
P.Y.13 is not only more resistant to solvents, but also shows less of a tendency to recrystallize than P.Y.12. This makes it much more advantageous to incorporate such pigments in offset vehicles containing large amounts of mineral oil by using agitated ball mills like the ones mentioned above (Section 1.8.1.1). P.Y.127 and 176 are even more recrystallization resistant than P.Y.13.
At equal depth of shade, the lightfastness of P.Y.13 exceeds that of similarly transparent types of P.Y.12 by one to two steps on the Blue Scale.
P.Y.13 is supplied as a wide selection of different types, ranging from highly transparent systems to semi-transparent and semi-opaque versions to highly opaque varieties.
P.Y.13 and its chemically modified derivatives, due to their higher solvent fastness compared to P.Y.12, are used in much greater volume in packaging gravure inks. P.Y.13 is also fast to protective clear lacquer overcoatings and may be sterilized and calendared.
P.Y.13 is not used in publication gravure printing inks based on toluene or toluene–benzene mixtures; amine-treated grades are not commercially available at present.
The paint industry shows only limited interest in either P.Y.13 or 12. Although the redder varieties of P.Y.13 are more lightfast than the P.Y.12 types by a few steps on the Blue Scale, they do not reach the lightfastness of Hansa Yellow type monohydrazone pigments. P.Y.13 is not fast to overpainting.
P.Y.13 is used to a more appreciable extent in plastics, where it meets average standards. Depending on the choice and the amount of plasticizer and processing temperature, a P.Y.13 content below 0.05% in plasticized PVC may cause some blooming (Section 1.6.3.1). Fastness to bleeding in plasticized PVC is much better than for P.Y.12; regarding bleed resistance, P.Y.13 is in fact more comparable to the more greenish P.Y.17. In 1/3 SD, which corresponds to a pigment concentration of about 0.3% with 5% TiO2, the lightfastness (daylight) of P.Y.13 in plasticized PVC is equal to step 6–7 on the Blue Scale; the pigment behaves similarly in rigid PVC, in which it does not migrate. In cable insulations made of plasticized PVC, P.Y.13 is used to match the standards according to DIN 47 002, RAL 1021 and RAL 2003 (Section 1.6.1.3); it is also used in PVC-based floor covering.
As a result of the discovered thermal decomposition of diarylide yellow pigments (Section 2.4.1.3), the use of P.Y.13 in HDPE must be limited to 200 °C, even though the pigment was previously said to survive a 5 min exposure to 200–260 °C (depending on the depth of shade and the commercial grade). Since P.Y.13 is a product with a high tinctorial strength, a pigment concentration of about 0.12% in HDPE will produce a 1/3 SD sample (1% TiO2 content). The shrinkage of plastics extrusion products is only affected if the processing temperature is low. In these materials, P.Y.13 is as lightfast as in PVC. P.Y.13 is used to an appreciable extent – usually in the form of pigment preparations – in rubber and other elastomers and in aromatic polyurethane foams. The spin dyeing market employs P.Y.13 to colour viscose rayon and synthetic wool.
2.4.1.4.4 Pigment Yellow 14
Although P.Y.14 is less important than P.Y.12 and 13, the packaging and textiles printing ink industries use it in large volume. P.Y.14 is somewhat greener than P.Y.12 and considerably more so in comparison with P.Y.13. A number of the P.Y.14 types are appreciably greener than the standard yellow on the European Scale. P.Y.14 is not only weaker than comparable P.Y.13 varieties with similar physical characteristics, such as specific surface area, but it is also less lightfast by 1 to 2 steps on the Blue Scale. Its resistance to solvents is also comparatively poor. This somewhat limits its use for process inks in offset and letterpress application to special cases, which is equally true for P.Y.14 blends with reddish pigments. Types with fine particle sizes, which match highly transparent versions of P.Y.12 and 13, are not available in Europe.
P.Y.14 is more resistant to solvents than P.Y.12. In contrast to P.Y.12, it is resistant to paraffin, which makes it a useful product for packaging inks. This is one of the reasons why P.Y.14 is used so much more widely throughout the USA, where it is produced in considerably larger volumes than P.Y.13. Where P.Y.12 is not fast enough to meet the requirements, P.Y.14 is usually the second choice in the USA; while most other countries replace P.Y.12 by P.Y.13.
Suitable P.Y.14 preparation with amines affords speciality types for publication gravure inks. Compared with corresponding varieties of P.Y.12, P.Y.14 speciality types provide a clean, particularly greenish hue; they are also colouristically somewhat weaker. However, the graphics industry currently prefers reddish shades for publication gravure printing inks, which explains why P.Y.14 speciality types currently have no value. However, untreated P.Y.14 is of regional importance as a colourant in publication gravure printing inks. The resulting inks exhibit poor rheology.
P.Y.14 is not utilized by the paint industry, and the interest of the plastics area varies according to the region. The pigment is more important in the USA, where it is used in polyolefins. It is thermally stable up to 200 °C, which makes it a useful colourant for elastomers, especially rubber. Whenever P.Y.14 is worked into plasticized PVC, there is a certain concentration threshold beyond which the pigment will bloom. P.Y.14 is also used for spin dyeing viscose rayon and viscose cellulose, and it is an especially important product for the mass colouration of viscose sponges. It is not particularly lightfast in these applications: in 1/1 to 1/9 SD, it equals only step 4 to step 5–6 on the Blue Scale. The important textile fastnesses are good.
2.4.1.4.5 Pigment Yellow 17
Used primarily in the printing ink field, this pigment is considerably greener than P.Y.14 and much more greenish than P.Y.12. At equal depth of shade, P.Y.17 is more lightfast than P.Y.14 by 1–2 steps on the Blue Scale. P.Y.17 is tinctorially weaker than P.Y.14. Printed in a standard layer (1 µm) on a letterpress proof printer, a P.Y.17 ink must be formulated at about 7.5% pigment to afford 1/3 SD prints; while an equally deep P.Y.14 ink only requires 3.7% pigment. In comparing the lightfastness of prints containing P.Y.17 and 13, respectively, it should be remembered that accelerated exposure to light with high pressure xenon lamps destroys P.Y.17 distinctly faster than P.Y.13. The reverse is true for daylight exposure, to which P.Y.17 is more resistant than P.Y.13. P.Y.17 is also more resistant to most organic solvents than P.Y.14.
Most commercially available P.Y.17 types are highly transparent, often resinated pigments, which have gained recognition in packaging inks. Combination with equally transparent, very reddish varieties of P.Y.83 affords a range of intermediate shades which exhibit good transparency and are very lightfast.
P.Y.17 is suitable for all printing techniques. It is somewhat more greenish than the standard yellow for offset and letterpress application on the European Scale. Shading with traces of P.Y.83, however, alleviates the problem. P.Y.17 is also applied in various packaging printing inks. The choice ranges from nitrocellulose and polyamide based inks to vinyl chloride/vinyl acetate mixed polymers on a ketone/ester basis for PVC or aluminium foil. In ester-based NC colours and in other media, the pigment, particularly in its fine particle size, highly transparent varieties, tends to exhibit poor flow properties and is highly viscous. It may also thicken, but this is rare. Publication gravure printing inks do not normally contain P.Y.17 but are mostly based on speciality types of P.Y.12 and 14. Reliable heat stability at temperatures up to 200 °C makes P.Y.17 suitable for metal deco printing.
P.Y.17 is only rarely employed in paints. It is used in interior linings of cans and other products for its high transparency. In air drying paints, its more opaque versions (organic pigment : TiO2 1:5) equal step 5 on the Blue Scale for lightfastness; full shades correspond to step 7. P.Y.17 is not completely fast to overpainting when employed in baking enamels.
The plastics industry, however, uses P.Y.17 extensively, although problems may occur in plasticized PVC, where blooming is observed if pigment concentrations are low. P.Y.17 is almost as lightfast as the somewhat redder P.Y.13 (step 6–7 at 1/3 SD). Likewise, both pigments are almost equally lightfast in rigid PVC. In polymers, as in other materials, P.Y.17 is also considerably weaker than P.Y.13.
P.Y.17 may be used for mass colouration and also to print PVC film. For these purposes, P.Y.17 is frequently prepared on a VC/VAc (vinyl chloride/vinyl acetate) mixed polymer basis. Good dispersibility in plastics makes these preparations suitable even for thin films. The dielectric properties of P.Y.17 allow its application in PVC cable insulations.
P.Y.17 is also frequently used in polyolefins, sometimes in the form of pigment preparations. Its heat stability in these media was said to be about 220–240 °C, but must now, as a result of the detected thermal decomposition of diarylide yellow pigments in plastics, be limited to 200 °C. This tendency to decompose excludes P.Y.17 from recommendation for use in polystyrene, in which the pigment largely dissolves under the processing conditions. The same is true for ABS.
Aromatic polyurethane foams are equally suitable media for P.Y.17, which is extremely lightfast in various cast resins. In 3 mm thick methyl methacrylate resins containing 0.025% pigment, P.Y.17 equals step 7–8 on the Blue Scale for lightfastness. Similar values are found for unsaturated polyester resins, whose hardening is not affected by the pigment (Section 1.8.3.5). In heat setting plastics such as melamine or urea/formaldehyde types, however, P.Y.17 is one to two steps on the Blue Scale less lightfast, which is true both for transparent and opaque versions. P.Y.17 is also used for the colouration of rubber.
The textiles printing industry has an appreciable interest in P.Y.17 and applies it in the form of pigment preparations. Where its fastness properties satisfy the specifications and where the use requirements are not too demanding, the pigment is also utilized for spin dyeing purposes. Manufacturer recommendations include media such as polyacrylonitrile and cellulose acetate fibres, on which 1/3 SD pigment prints exhibit a lightfastness which is equal to step 5 on the Blue Scale.
2.4.1.4.6 Pigment Yellow 55
P.Y.55 is less important than some other diarylide yellow pigments, especially the P.Y.12, 13, and 83 types. It affords a very reddish yellow, which is not quite as red as P.Y.83 or 114.
The main area of application for P.Y.55 is in the printing ink industry, where it is used particularly in speciality products for packaging inks. Various types are available with different specific surface areas to satisfy customer specifications regarding transparency or opacity. The flow properties are considerably different, especially in oil-based and aqueous systems, which are the most common media for P.Y.55. According to expectation, types with lower specific surface areas are redder, more opaque, show less tinctorial strength, and are less viscous than types with fine particle sizes. The pigment is occasionally highly resinated to enhance the transparency of the product. Most P.Y.55 types are distinctly weaker than comparable diarylide yellow pigments covering the reddish and medium yellow range. In 1/3 SD letterpress proof prints, P.Y.55 equals step 4 on the Blue Scale: it is as lightfast as the chemically and tinctorially similar diarylide yellow pigment P.Y.114.
The paints market shows only limited interest in P.Y.55, although the pigment is occasionally used to lend colour to low quality industrial paints. In 1/3 SD white reductions, the pigment matches the lightfastness of P.Y.13 samples; its full shades are more lightfast. Addition of TiO2 decreases its resistance to light.
P.Y.55 is suitable for application in polymers such as PVC and rubber. In plasticized PVC, it exhibits a certain tendency to bleed; its tinctorial strength is good-to-average. 0.7% pigment, incorporated in plasticized PVC, will produce a 1/3 SD sample (5% TiO2); while only 0.28% Pigment Yellow 83 is sufficient to afford the same depth of shade. The lightfastness equals step 7 on the Blue Scale. P.Y.55 is also used throughout the textiles printing ink industry.
2.4.1.4.7 Pigment Yellow 63
P.Y.63 continues to be manufactured in Japan, where it enjoys only limited regional importance. It provides a greenish to medium yellow hue with a lightfastness that is much inferior to that of other diarylide yellow pigments. Under standardized conditions, 1/1 SD letterpress proof prints equal only step 2 on the Blue Scale. Fastness to various organic solvents that are common in printing inks is good, and the pigment shows no tendency to recrystallize. Preparation with amines considerably shifts the hue of publication gravure printing inks towards greener shades, which is also observed with P.Y.14.
2.4.1.4.8 Pigment Yellow 81
This pigment affords a very greenish yellow. Its hue is similar to that of the monohydrazone yellow pigment P.Y.3, but white reductions are much stronger exhibiting considerably improved solvent and migration resistance. Although P.Y.81 shows satisfactory lightfastness, it is not quite as lightfast as P.Y.3. P.Y.81 resembles P.Y.113 as far as shade and fastness properties are concerned, although P.Y.113 is more resistant to solvents and also more migration resistant.
P.Y.81 is found in various media. It is equally suited to all printing purposes, including textile printing. Good resistance to many organic solvents facilitates its use in various solvent-containing printing inks, such as mixed polymer paints on ketone/ester basis for PVC. Its heat resistance qualifies the pigment for application in metal deco printing inks. The prints are resistant to clear lacquer coatings, sterilization, and calendering. In 1/3 SD, the lightfastness is equal to step 5–6 on the Blue Scale. Comparative values are: P.Y.3: step 6–7, P.Y.113: step 5–6.
P.Y.81 is recognized throughout the paint industry for its fastness to overcoating and very good solvent fastness in industrial application. Most types exhibit good hiding power. In an alkyd-melamine paint, the pigment equals step 7–8 on the Blue Scale in full shades and step 6–7 in white reduction (1:4 TiO2) for lightfastness. In paints, P.Y.81 provides a tinctorial strength which is similar to that of P.Y.3 of comparable shade, but it is not quite as lightfast and weatherfast.
At low pigment concentrations in plasticized PVC, the pigment may bloom, depending on the processing conditions and formulation of the polymer. In rigid PVC, however, P.Y.81 performs well, irrespective of its concentration. Its daylight fastness in 1/3 SD is equal to step 7 on the Blue Scale, in which it equals the distinctly stronger P.Y.113. At exposure to light from a xenon lamp, P.Y.113 is much more lightfast than P.Y.81, especially in transparent types. The difference is sometimes more than one step on the Blue Scale. P.Y.81 is very resistant to bleeding, provided the appropriate concentration limits are observed. Its heat resistance makes it a useful colourant for polyolefins. P.Y.81 was said to be heat stable up to 260 °C for 5 min, depending on the depth of shade. The pigment was thus much more heat resistant than other diarylide yellow pigments. As a consequence of new insight into the thermal decomposition of diarylide yellow pigments in the presence of plastics (Section 2.4.1.3), the heat stability of P.Y.81 must now be limited to 200 °C. The pigment hardly affects the shrinkage of preformed HDPE articles and those made from similar, partially crystalline plastics. It is also useful in other plastics and for spin dyeing of materials such as secondary acetate.
2.4.1.4.9 Pigment Yellow 83
P.Y.83 possesses excellent fastness properties, which make it almost universally applicable. It provides a reddish yellow hue, which is considerably more reddish than that of P.Y.13 and at the same time very strong.
P.Y.83 can be used for all printing techniques and purposes. The printing ink industry often prefers highly transparent, mostly resinated types. Printing such types on aluminium foil or on metal sheets produces brilliant shades of gold. Combining P.Y.83 with transparent versions of the greenish P.Y.17 affords transparent prints with good lightfastness, which cover the range of intermediate shades. Both pigments are similar in that their varieties with fine pigment particles afford transparent 1/3 SD prints whose lightfastness equals step 5 on the Blue Scale. For comparison, P.Y.12 equals step 2; P.Y.13 reaches step 3–4.
P.Y.83 is the standard pigment within the reddish yellow range. The similarly shaded monohydrazone yellow pigment P.Y.10 (see Table 2.4, Section 2.3.4.1.6) is only about half as strong as P.Y.83, but more lightfast (about 1.5 steps on the Blue Scale). At the same time, it is less transparent and considerably less solvent resistant, which may cause significant recrystallization problems during pigment incorporation into the medium of application.
P.Y.83 shows good-to-very good resistance to most solvents that are typically found in application media. Recrystallization is therefore rare under common processing conditions, even in highly transparent types. Resistance to clear lacquers, calendering and sterilization is consequently excellent.
P.Y.83 is also used to an appreciable extent in plastics. Because of its good solvent resistance, migration is no problem in plasticized PVC even at low pigment levels; it does not bleed or bloom. Its daylight fastness at 1/3 SD equals step 8 on the Blue Scale; at 1/25 SD, it still reaches step 7. The results in rigid PVC are similar. P.Y.83 is also very strong in polyolefins. The pigment concentration required for 1/3 SD HDPE colouration (1% TiO2) is only 0.08%. A considerable variety of pigment preparations is available for colouring several plastics. The choice ranges from pigment formulations on VC/VAc copolymer basis to pigment plasticizer pastes for PVC mass colouration, including application in thin films or cable insulations and printing on PVC films. Certain preparations are recommended for polyolefins or other plastics. The pigment has so far, on the basis of application tests in polyolefins, been said to be heat stable up to 250 °C. As a result of new insight into the thermal decomposition of diarylide yellow pigments in monohydrazone colourants and aromatic amines (Section 2.4.1.3), this heat stability must now be limited to 200 °C.
P.Y. 83 shows excellent lightfastness, even in methyl methacrylate or unsaturated polyester cast resins. The pigment does not effect the hardening of the latter.
The high quality of the fastness properties is the basis for frequent pigment use in textile printing. Dry cleaning with perchloroethylene or washing has almost no effect on the colour. P.Y.83, sometimes in the form of a preparation, is used for viscose spin dyeing, secondary acetate, and polyacrylonitrile.
In the paint industry, P.Y.83 types with fine particle sizes are intended for use in transparent and metallic finishes and are also generally employed in industrial coatings and emulsion paints, provided the demands on the light stability of the pigment are not too stringent. In full shades, the pigment reaches step 6–7 on the Blue Scale; some darkening is observed as a consequence of exposure to light. Opaque finishes with TiO2 (1:10) are equal to step 6 and mixtures of (1:125) correspond to step 4–5. P.Y.83 is fast to overcoating in baking enamels.
One of the P.Y.83 types is an extremely opaque form. It provides finishes with good flow properties and makes it easy to increase the pigment concentration, which in turn improves the hiding power. This special opaque type is considerably more weatherfast than its more transparent counterparts, which makes its full shades useful colourants for an original automobile finishes (O.E.M.). The product also lends itself to paints for automobile refinishes and tractor and implement finishes. It is found in various high grade industrial finishes.
Good overall fastness and considerable tinctorial strength broaden the scope of P.Y.83 application. The list includes office articles, artists' colours, and solvent based wood stains, in which the pigment is frequently combined with red pigments and carbon black to produce shades of brown.
2.4.1.4.10 Pigment Yellow 87
Although this pigment has recently been offered by several manufacturers throughout the USA and Japan, it is not produced in Europe. Its colour is a reddish yellow, similar to that of the chemically closely related P.Y.83. Although both provide almost equal strength, P.Y.87 shows very poor fastness to overcoating in baking enamels and plasticized PVC. It is also less lightfast. Under standard conditions, 1/1 SD letterpress proof prints are equal only to step 4 on the Blue Scale, while corresponding P.Y.83 prints reach step 5. The pigment is used to a certain extent in textile printing.
2.4.1.4.11 Pigment Yellow 90
P.Y.90 has little commercial impact, it is a regional product. The pigment provides very reddish yellow shades, similar to those of the much light-faster P.Y.114. 1/1 SD and 1/3 SD letterpress proof prints equal only step 3 on the Blue Scale, while corresponding P.Y.114 specimens reach step 4–5 and 4, respectively.
2.4.1.4.12 Pigment Yellow 106
The shade of P.Y.106 is greenish, somewhat redder than P.Y.17, and its tinctorial strength is greater than that of P.Y.17. Standard films of 1/1 SD letterpress proof prints are made with only 12% P.Y.106, while the necessary P.Y.17 content exceeds 25%. The required pigment concentrations for 1/3 SD proof prints are 6% and 7.5%, respectively. At equal SD, the lightfastness of P.Y.106 is inferior to that of P.Y.17; the difference is about 1/2 step on the Blue Scale. Both pigments lend themselves to similar purposes, mainly in the area of printing inks. P.Y.106 is thermally stable up to 200 °C (10 min); in prints, it is bleed resistant toward clear lacquer coatings and sterilization, which qualifies it for metal deco printing.
P.Y.106 has recently been removed from the market.
2.4.1.4.13 Pigment Yellow 113
A few years ago P.Y.113 has been removed from the market worldwide because one of the intermediates for its production is no longer internationally available. P.Y.113 is a yellow pigment with a very greenish shade. In its colouristic and application properties, the pigment largely resembles P.Y.81, by which it must widely be replaced. In contrast to P.Y.81, however, P.Y.113 is completely migration resistant in plasticized PVC. Even at very low pigment concentrations, P.Y.113 does not bloom in this medium and has no limitation in this respect. Both pigments display approximately equal lightfastness.
2.4.1.4.14 Pigment Yellow 114
P.Y.114 affords very reddish yellow shades which closely resemble those of P.Y.83, although the level of the fastness properties of P.Y.114 is lower; it is somewhere between those of P.Y.12 and 13. In print, P.Y.114 is less lightfast than P.Y.83; the difference is ½ to 2 steps on the Blue Scale, depending on the depth of shade; but P.Y.114 does not achieve the tinctorial strength of P.Y.83.
P.Y.114 is primarily supplied to the printing ink industry, where it is used especially for packaging inks. The pigment is utilized to produce prints at reasonable cost, especially where exceptional fastness, as provided by P.Y.83, is a minor consideration. Prints made from P.Y.114 are not entirely resistant to a number of organic solvents, including the standard DIN 16 524 solvent mixture, paraffin and butter; however, P.Y.114 prints are soap, alkali and acid resistant. The fact that the pigment does not withstand a temperature of 140 °C and is not stable to sterilization excludes P.Y.114 from use in metal deco printing.
2.4.1.4.15 Pigment Yellow 121
The pigment enjoys a certain regional importance and lends itself to various printing inks. It produces a medium yellow shade, which is somewhat less green than the standard yellow on the European Scale CIE 12-66 for process printing (Section 1.8.1.1). Commercial types show medium transparency. The pigment is weaker than similarly coloured diarylide yellow pigments, such as P.Y.126 or 176. P.Y.121 is fast to several organic solvents, which are commonly used in printing inks; the pigment does not recrystallize in these media. The list includes aromatic hydrocarbons, especially toluene, which qualifies the pigment for use in publication gravure printing inks. This is especially true for amine preparations, which shift the colour to a much greener shade. A similar colour trend is observed with P.Y.14.
2.4.1.4.16 Pigment Yellow 124
Its distribution is limited to the USA, where it enjoys little importance. The pigment affords a medium yellow shade.
2.4.1.4.17 Pigment Yellow 126
This pigment is roughly as fast as the chemically related P.Y.12. In types with a similar specific surface area, P.Y.126 is somewhat redder. In contrast to P.Y.12, printing inks containing P.Y.126 require little, if any, modification with reddish components to attain the European Standard Yellow (Section 1.8.1.1) for process printing. This feature makes P.Y.126 a suitable process colour pigment for offset printing. Highly transparent versions are designed for use as the uppermost layer in four colour printing.
P.Y.126 is tinctorially stronger than P.Y.12; to produce standard films of highly transparent 1/1 SD proof prints, 9% P.Y.126 is needed, as opposed to 10% P.Y.12. P.Y.126 prints are more resistant to light; the difference is one step on the Blue Scale. In 1/1 to 1/25 SD prints, the lightfastness of P.Y.126 and P.Y.12 equals step 4 and 3, respectively. Heat stability up to 200 °C (10 min) and complete resistance to clear lacquer coatings and sterilization facilitate its use in metal deco printing. In aqueous or water/alcohol-based printing inks P.Y.126 is also stronger and more lightfast than P.Y.12. Treatment with aliphatic amines leads to ketimine formation, which is also true for P.Y.12 (Section 2.4.1.1). The resulting products afford high tinctorial strength and prints with a comparatively reddish shade. Its storage stability and penetration characteristics parallel those of P.Y.12.
P.Y.126 is generally applied wherever P.Y.12 types suit the purpose, such as in office articles and cleaning agents.
2.4.1.4.18 Pigment Yellow 127
This pigment performs like the chemically related P.Y.13. Its commercial types are highly transparent, resinated materials. P.Y.127 is principally used in offset printing inks; in Europe it is considered a standard product for this purpose. The pigment provides a yellow shade, which matches the standard yellow for process printing according to CIE 12-66 (Section 1.8.1.1). P.Y.127 has a very high tinctorial strength. 1/1 SD letterpress proof prints require 6.5% pigment, 1/3 SD proof prints contain 3.3% pigment. Comparative values for several other pigments covering the same range of colours are listed under the respective name.
The fact that P.Y.127 recrystallizes only very slightly facilitates its dispersion in modern agitated ball mills. The pigment is easy to disperse and its heat stability parallels that of P.Y.13. Its fastness to clear lacquer coatings and to sterilization are excellent, which is why the pigment is suitable for metal deco printing.
P.Y.127 is also used to an appreciable extent in packaging gravure printing inks. Highly transparent inks lend gloss especially to nitrocellulose inks. P.Y.127 prints are very fast, in which they largely parallel P.Y.13 prints.
2.4.1.4.19 Pigment Yellow 136
This pigment has been commercially available for several years. Chemically, it is a modified version of P.Y.13 and 14. Its colouristic and application properties are comparable to those of P.Y.13 and 14 mixtures. P.Y.136 is supplied to the printing ink industry, primarily for the production of oil-based inks, that is, offset inks. Both its hue and its tinctorial strength are somewhere between those of P.Y.13 and 14.
2.4.1.4.20 Pigment Yellow 152
Although P.Y.152 enjoys little appreciation in Europe, it is used to a considerable extent in the USA. It produces a reddish, somewhat dull yellow shade. Most of the commercial varieties are very opaque versions with good hiding power, which frequently displace Chrome Yellows in paints. P.Y.152 types are more viscous than opaque varieties of the much greener P.Y.83. The pigment is only moderately fast to overcoating: the tendency to bleed is very strong in an alkyd-melamine resin paint at a baking temperature of 120 °C. Its lightfastness is average. White reductions (1:5 TiO2) equal step 5 on the Blue Scale, while the corresponding P.Y.83 paint reaches step 7–8. In full and deep shades, there is some darkening, as with P.Y.83; the lightfastness of such shades equals step 7. White reductions of P.Y.152 in air drying alkyd resin systems are almost twice as strong as the somewhat more greenish yellow monohydrazone pigment P.Y.65; full shades are considerably more opaque.
2.4.1.4.21 Pigment Yellow 170
P.Y.170 is produced in Japan and in Europe. Its hue is yellowish orange or very reddish yellow. The commercial products with a coarse particle size exhibit good hiding power and correspondingly little tinctorial strength in white reductions. It is considerably weaker than the more yellowish benzimidazolone pigment P.O.62, which is also far superior in light and weather resistance. P.Y.170 withstands overcoating in baking enamels at commonly used temperatures. Its prints are thermally stable up to 200 °C, are fast to clear lacquer coatings, and may be sterilized.
2.4.1.4.22 Pigment Yellow 171
The only manufacturer of this pigment has recently cancelled its production.
P.Y.171 was found in the Japanese market. Its prints are considerably redder than those made from P.Y.13; besides, its colour is off the fastness limits for yellows on the European colour scales for offset and letterpress application. P.Y.171 has a dull hue and is weaker than P.Y.13. The pigment is targeted for the colouration of plastics, such as PVC and PE.
2.4.1.4.23 Pigment Yellow 172
P.Y.172, like P.Y.171, was produced in Japan. The two pigments were not only colouristically alike but also possessed similar application properties. P.Y.172 is no longer found on the market.
2.4.1.4.24 Pigment Yellow 174
P.Y.174 joined the market a few years ago and bears some resemblance to P.Y.13. The commercially available varieties are very strong, highly resinated products which are correspondingly transparent. They are of great interest to the offset printing industry.
P.Y.174 provides a yellow shade that matches the CIE 12-66 standard yellow for process colour printing. The excellent tinctorial strength of the commercial products is accompanied by the high viscosity of the ink.
2.4.1.4.25 Pigment Yellow 176
P.Y.176 exhibits application properties which are similar to those of the chemically related P.Y.13. Only highly transparent varieties are available. The hue of P.Y.176 is somewhat redder than that of P.Y.13.
P.Y.176 finds extensive use in offset printing, where it matches the CIE 12-66 standard yellow for process colour printing (Section 1.8.1.1). It is a particularly strong pigment. Standardized 1/1 SD proof prints are made from inks containing only 6% pigment, while 1/3 SD samples require 3% pigment. P.Y.176 is stronger than P.Y.13 and 127. In certain vehicle systems, however, this advantage can be compromised by increased viscosity and poor flow behaviour, respectively. Fastness in prints, including lightfastness, heat stability, and resistance to clear lacquer coatings and sterilization, common organic solvents, and the DIN 16 524 solvent mixture are good-to-excellent. In this respect, P.Y.176 largely parallels P.Y.13.
2.4.1.4.26 Pigment Yellow 188
P.Y.188, which was released onto the market only a few years ago, is closely related to Pigment Yellow 13 in both its chemical and application properties.
The commercially available, highly transparent type is primarily applied in offset printing inks. In this field, P.Y.188 matches the CIE 12-66 standard yellow shade for process printing (Section 1.8.1.1). The commercially available type of P.Y.188 is highly resinated and shows very good tinctorial strength. It displays good flow behaviour.
2.4.1.4.27 Pigment Orange 15
P.O.15 produces yellowish orange shades, which resemble those of P.O.13. Compared to P.O.13, however, P.O.15 has largely lost its importance. Currently, it is only produced in the USA. Although the pigment is less lightfast and less stable to organic solvents than P.O.13, P.O.15 is used to lend colour to printing inks and rubber.
2.4.1.4.28 Pigment Orange 16
Structurally based on 3,3′-dimethoxybenzidine (o-dianisidine) as a diazo component, P.O.16 is also known as Dianisidine Orange. At present, the pigment only enjoys some importance in Europe, the USA and in Japan.
Its colour is a yellowish orange, which is considerably redder than P.O.13 and 34. Increasingly stringent application requirements regarding lightfastness and durability, solvent and migration resistance present a considerable challenge to P.Y.16 and have largely eliminated the pigment from the market.
The largest area of application for P.Y.16 is in the printing ink industry. The pigment is used particularly to adjust the shades of P.Y.12 type diarylide yellow pigments, to which it is most closely related as far as application performance is concerned. Such blends are employed in low-cost packaging and speciality inks. Resinated types are produced to provide higher transparency. There is a certain disadvantage to the poor rheology of several P.Y.16 types, which makes it difficult to formulate highly pigmented systems.
P.O.16 is not lightfast and durable enough to be used in paints. In this respect, it performs even more poorly than P.Y.12. Low-cost production, however, makes it a suitable colourant for rubber and for textile printing.
2.4.1.4.29 Pigment Orange 44
P.O.44 has lost most of its commercial importance and is at present only applied to a limited extent. This is also true for other pigments whose synthetic route involves 3,3′-dimethoxybenzidine as a diazo component. P.O.44 provides a very reddish orange shade, which is much redder than the colour of the β-naphthol pigment P.O.5. Although P.O.44 is more resistant to solvents than P.O.5, the reverse is true for lightfastness. Standardized letterpress proof prints containing P.O.44, for instance, equal step 3 on the Blue Scale, while equally deeply shaded P.O.5 prints equal step 6 on the Blue Scale.
The main area of application for P.O.44 is in the field of textile printing, although it is less lightfast than P.O.5. 1/1 SD prints containing P.O.44 equal step 5–6, as opposed to step 7 for P.O.5 prints; 1/3 SD P.O.44 samples equal step 4–5, again as opposed to step 7; and 1/6 SD P.O.44 prints only reach step 3–4 on the Blue Scale, while P.O.5 still scores as high as step 6–7.
The application fastness of the pigment, however, is more satisfactory. P.O.44 may be dry cleaned and resists dry heat up to 210 °C for 30 s; while P.O.5 and also the somewhat redder Naphthol AS pigment P.R.10 are less fast. P.O.44 is also used in PVC coatings.
2.4.1.4.30 Pigment Orange 47
Pigment orange 47 is one of the few diarylide yellow pigments which is based on 3,3′-dimethylbenzidine instead of 3,3′-dichlorobenzidine as disazo component. The coupling compound is the same as in P.Y.171. The replacement of 3,3′ dichlorobenzidine by 3,3′dimethylbenzidine results in a colour shift from yellow (P.Y.171) to a bright yellowish orange (P.O.47).
P.O.47 is mainly produced in the USA. Specific applications are not reported.
2.4.2 Bisacetoacetarylide Pigments
2.4.2.1 Chemistry, Manufacture and Crystal Strctures
Bisacetoacetarylide pigments are dihydrazone pigments that are obtained from bifunctional coupling components, which are synthesized by bisacetoacetylation of aromatic diamines, especially of 4,4′-diaminodiphenyl or 1,4-diaminobenzene derivatives with 2 equivalents of diketene or acetoacetic ester:

There is a large number of patents describing bisacetoacetarylide pigments based on the bifunctional coupling components 1,4-bisacetoacetylaminobenzene and its derivatives [59], but only a few of these pigments enjoy commercial importance.
Pigments obtained from bisacetoacetylamino-p-phenylene are also called ‘DAEP pigments', derived from the German name DAEP (Di-acetessig-p-phenylendiamin) for the coupling compound.
Only common aromatic amines are used as diazonium components. Coupling reactions between aminobenzamides or aminobenzanilides and bisacetoacetylaminophenylenes are described under dihydrazone condensation pigments (see Section 2.9). Bisacetoacetarylide pigments thus have the general chemical structure:

A commercially available product is Pigment Yellow 155, a yellow pigment that is prepared by coupling the diazonium salt of aminoterephthalic acid dimethyl ester onto the bifunctional coupling component 1,4-bisacetoacetylaminobenzene. With an additional chloro substituent at the central ring Pigment Yellow 219 is obtained:

Scheme 2.1 Molecular structures of P.Y.155 and P.Y.219.
Using 2-methoxy-4-nitroaniline as diazo component instead and coupling onto the same nonchlorinated bifunctional coupling component yields Pigment Yellow 198:

Scheme 2.2 Molecular structure of P.Y.198.
Similarly, bisacetoacetarylide pigments obtained from 4,4′-bisacetoacetylaminodiphenyl derivatives as coupling components are of little commercial value. One exception is Pigment Yellow 16, 20040:

Scheme 2.3 Molecular structure of P.Y.16.
It is obtained by coupling two equivalents of diazotized 2,4-dichloroaniline onto bisacetoacetylated 3,3′-dimethylbenzidine, also known as Naphtol AS-G.
Molecules of P.Y.155 adopt the usual tautomeric hydrazone form (Figure 2.22). The crystal structure was determined from X-ray powder data (Figure 2.23). In the crystal the molecules are planar and form a layer structure [60].

Figure 2.22 Molecular structure of P.Y.155 in the solid state.

Figure 2.23 Crystal structure of P.Y.155 [58].
2.4.2.2 Properties and Application
Bisacetoacetarylide pigments are characterized by good solvent resistance and tinctorial strength, although they are not quite as strong as diarylide yellow pigments.
Bisacetoacetarylide pigments are used for paints, plastics and printing applications. P.Y.155 is especially important for laser printers.
2.4.2.3 Commercially Available Bisacetoacetarylide Pigments
2.4.2.3.1 Pigment Yellow 16
P.Y.16 covers the medium yellow range. Increasingly stringent industrial requirements in some areas are slowly restricting the use of this originally versatile product.
P.Y.16 shows good-to-very good resistance to various organic solvents, including alcohols and esters; however, it exhibits no fastness to some other solvents, such as xylene and other aromatic hydrocarbons. Recrystallization may be a problem if the pigment is to be processed in systems containing aromatic solvents, such as some baking enamels and packaging gravure printing inks, resulting in a considerable shift toward red shades. By thermic treatment P.Y.16 undergoes a similar colour shift in the presence of certain solvents in the medium of application, owing to a change of crystal modification. Different solvent treatment has led to three more crystal modifications. Compared with the β-form, the γ-modification is greener, the δ-form considerably redder and the ɛ-form somewhat redder and cleaner in shade. The results have been confirmed by X-ray diffraction studies [61]. Both the δ- and ɛ-modifications exhibit improved lightfastness compared with the α- or β-form.
In the paint field P.Y.16 is primarily used in industrial finishes. The similarity to P.Y.1 is limited to a likeness in shade; but P.Y.16 is fast to overcoating and shows no tendency to bleed in baking enamels. Although its full shade lightfastness equals step 7–8 on the Blue Scale, types that are only slightly reduced with TiO2 (1:5) reach only step 5. Only full shades provide good weatherfastness.
To produce chromium-free full shade finishes with good hiding power, special opaque P.Y.16 types are available. Their good rheology makes it possible to further increase the hiding power by increasing the pigment concentration without adversely affecting flow, gloss or other inherent features of the paint. Although the weatherfastness of such opaque versions with coarse particle sizes is somewhat better than that of traditional types, it is frequently insufficient for long-term exterior application. The opaque varieties are faster to aromatic solvents and solvents other than the standard types, and they recrystallize less readily.
P.Y.16 is supplied as a pigment preparation to the emulsion paint industry.
P.Y.16 is suitable for all printing techniques used in the printing ink industry. In some systems, however, it tends to recrystallize. P.Y.16 shows high tinctorial strength and relatively good resistance to light. The pigment is as lightfast as the good lightfast diarylide yellow pigments. 1/1 SD printed samples equal step 5 on the Blue Scale, 1/3 SD prints reach step 4–5. Very opaque types are even faster to light; depending on the depth of shade, these score ½ to 1 step higher on the Blue Scale. The prints are resistant to clear lacquer (Section 1.6.2.1) and stable to calendering and sterilization. Heat stability at 30 min exposure up to 200 °C makes P.Y.16 a suitable pigment for metal deco printing. Similarly, it is utilized for textile printing purposes. Application in the plastics field has diminished considerably. P.Y.16 migrates in PVC, which leads to blooming and bleeding. Moreover, its heat resistance in plasticized PVC is unsatisfactory, due to recrystallization. The pigment shows good tinctorial strength in polyolefins. Its heat stability in this medium has been said to equal approximately 230–240 °C for 5 min, depending on the depth of shade. But as a result of the thermal decomposition (Section 2.4.1.3) the heat stability is to be limited to 200 °C.
Besides its use in cast resin, such as methyl methacrylate mixtures, P.Y.16 has gained recognition as a colourant for felt-tip pens, watercolours and several related applications. It lends colour to leather, mass and surface paper colouration, and paper pulp.
2.4.2.3.2 Pigment Yellow 155
P.Y.155 provides clean, somewhat greenish yellow shades and is characterized by high tinctorial strength, good solvent resistance, and fastness to alkali and acid.
The pigment is recommended for use in paints, plastics and printing inks. P.Y.155 is one of the most important yellow pigments for laser printers. It shows excellent fastness to overcoating in baking enamels: in alkyd-melamine systems, it withstands 30 min of exposure to up to 140 °C without bleeding. P.Y.155 is characterized by good lightfastness; its full shades are as fast as P.Y.16. In full shades and in white reductions, P.Y.155 is more weatherfast than P.Y.16, but it is not as weather resistant as the somewhat greener monohydrazone yellow pigment P.Y.97. A new type consisting of coarser particles, which provides better weatherfastness, has recently been introduced to the market. This type is recommended as a replacement for Chrome Yellow pigments for its higher hiding power.
P.Y.155 is used to an appreciable extent in industrial finishes that are targeted for commercial vehicles, tractors and farm implements. In paints, it resists temperatures up to 160 °C.
Incorporated in plasticized PVC, P.Y.155 withstands temperatures up to 180 °C, but it shows a certain tendency to bleed under common processing conditions. Its tinctorial strength is good-to-average. 1/3 SD colourations (5% TiO2) require 0.7% pigment. In PVC, the ability of both transparent (0.1%) and opaque (0.1% pigment +0.5% TiO2) versions to withstand light is equal to step 7–8 on the Blue Scale, while such types are less weather resistant. In HDPE, 1/3 SD P.Y.155 colourations (1% TiO2) may be exposed to temperatures up to 260 °C for 5 min without changing colour. As in PVC, the tinctorial strength in HDPE is average. Recommendations for application include polypropylene and styrene, but exclude polyesters.
In printing inks, P.Y.155 lends itself to all printing techniques. The prints provide good fastness properties; they are even soap and butter resistant. P.Y.155 is one of the most important pigments for inkjet printing and laser printing.
2.4.2.3.3 Pigment Yellow 198
The pigment was recently introduced to the market.
P.Y.198 is primarily recommended for packaging gravure printing, in particular for printing inks based on nitrocellulose with alcohols and esters as solvents and for water-based printing inks as well. The lightfastness of the corresponding prints in 1/1 SD with 4.7% of pigment equals step 5, in 1/3 SD with 2.5% of pigment it matches step 4 of the Blue Scale.
Although fast to bleeding in petroleum ether and dibutyl phthalate, very little bleeding is found in ethanol and methoxypropanol and a slight bleeding occurs in toluene, ethyl acetate, paraffin and the solvent mixture according to DIN 16 524.
2.4.2.3.4 Pigment Yellow 212
A metallized bisacetoacetarylide pigment with a chemical constitution is as yet unpublished. This pigment exhibits excellent heat stability in polyolefins, HDPE and polypropylenes exceeding 310°C in masstone and tint. It is a bright medium shade yellow and provides an excellent alternative to diarylides and other yellows in this shade range. The pigment complies with the FDA requirements for food contact under the conditions of use (A) through (H) as described in Table 2 of 21 CFR Part 176.170(c) where this pigment is allowed to be used at levels up to 1% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR Part 178.3297.
P.Y.212 is not recommended for exterior use, but provides an excellent value for interior applications such as packaging and household durables of all types. Pigment Yellow 212 is tinctorially quite strong as metallized azos go and exhibits very good dimensional stability, making it very useful in moulded items such as blow moulded containers.
2.4.2.3.5 Pigment Yellow 219
A rather new development is P.Y.219, exhibiting a particularly bright greenish yellow shade, high colour strength and good opacity. The pigment is suitable for automotive paints and all kinds of industrial paints, both for water-based as well as solvent-based coatings. It is ideal for combinations with bismuth vanadate, as the colour does not change over a wide array of mixture ratios.
It can also be used in plastics, toners and inks. The lightfastness (DIN EN 105-B01) in deep shade is 7–8, in 1/25 SD 6–7; weatherfastness in deep shade is 4–5 and shows 4 in 1/25 SD.
Six different crystal modifications of the pigment could be obtained by heat treatment with organic solvents/water. The amorphous crude pigment suspended in dimethylformamide/water at 140 °C yields the β-polymorph. If this modification is heat-treated with N-methylpyrrolidone, dimethyl sulfoxide, long-chain alcohols, 1,2-dichlorobenzene, pyridine and/or nitrobenzene, the α, γ, δ, ɛ, ξ, and η modifications are obtained [62].
2.4.3 Dihydrazonepyrazolone Pigments (Formerly Called Disazopyrazolone Pigments)
Like monohydrazone yellow and diarylide yellow pigments, the first dihydrazonepyrazolone pigments were developed as early as 1910 [63]; commercial application, however, was delayed by some 20 years. This prolonged lack of interest was caused by a simultaneously ongoing search for high strength, bleed resistant organic pigments to produce orange and yellowish red shades for the colouration of rubber. It was not until the early 1950s that P.O.34 gained recognition as a commercial product.
The preparation of dihydrazonepyrazolone pigments is based on a synthetic pathway discovered by Ludwig Knorr in 1883, in a successful attempt to make phenylmethylpyrazolone. It was not until the 1930s that the first pyrazolone pigment, an orange (Pigment Orange 13), became commercially available. Likewise, pyrazolone red pigments were patented at the time; they were released to the market without much delay. Only a few dihydrazonepyrazolone pigments are still of interest to manufacturers; those that are, however, comprise a relatively large portion of organic pigment production.
2.4.3.1 Chemistry, Manufacture and Crystal Structures
Industrially produced disazopyrazolone pigments are based on the following general chemical structure:

The originally large number of commercially significant pyrazolone pigments has dwindled considerably, leaving only a few of them that meet modern application standards. Their synthesis parallels that of diarylide yellow pigments: bisdiazotization of a 4,4′-diaminodiphenyl dihydrochloride derivative, primarily 3,3′-dichlorobenzidine dihydrochloride or 3,3′-dimethoxybenzidine dihydrochloride (o-dianisidine), is followed by coupling onto 2 equivalents of the corresponding pyrazolone derivative, which finally affords the crude pigment. Using 3,3′-dichlorobenzidine as the diazo component yields orange pigments, while the increased bathochromic effect of 3,3′-dimethoxybenzidine produces pigments with red shades. Likewise, using 1-aryl-3-carbalkoxypyrazol-5-one as a coupling component affords pigments with red shades. It is possible to modify the physical parameters of a pigment by adding certain agents during or after the coupling reaction, by adjusting the diazotization or coupling technique, or by adapting the aftertreatment of the pigment to the intended use of the product.
Dihydrazonepyrazolone pigments, for instance, can be tailor-made for various applications to provide features such as high transparency or good hiding power, easy dispersibility and high tinctorial strength.
The crystal structures of dihydrazonepyrazolone pigments have not yet been published.
2.4.3.2 Properties
Dihydrazonepyrazolone pigments range in shade from reddish yellow to orange, red and maroon. The currently available commercial types, however, come exclusively in orange or red hues. The application properties and fastnesses of these pigments are very versatile. P.O.34, for instance, is as fast as a good diarylide yellow pigment, while P.O.13 is somewhat less fast. This applies to its lightfastness as well as to fastness to solvents and the tendency to migrate. Pigments obtained from 3,3′-dimethoxybenzidine instead of 3,3′-dichlorobenzidine as the bisdiazo component perform much less well as far as fastness to solvents, migration and light are concerned. They enjoy only restricted commercial significance.
2.4.3.3 Application
Dihydrazonepyrazolone pigments are broad in scope. Depending on their physical characteristics, different types are targeted for the printing ink, paint or plastics industry.
2.4.3.4 Commercially Available Dihydrazonepyrazolone Pigments
2.4.3.4.1 General
P.O.34 and 13 are the two most frequently used dihydrazonepyrazolone pigments obtained by bisdiazotization of 3,3′-dichlorobenzidine. P.O.13 comprises the largest portion of dihydrazonepyrazolone pigment production and also reigns supreme amongst organic orange pigments in general. P.R.38 and 37 have less of an impact on the pigment industry. Both were introduced around the same time as P.O.13. P.R.37 and 41 are obtained with 3,3′-dimethoxybenzidine as bisdiazonium component. Table 2.6 lists the commercially available dihydrazonepyrazolone pigments.
Table 2.6 Commercially available dihydrazonepyrazolone pigments.

|
|||||
| C.I. Name | C.I. Constitution Number | X | R1 | R2 | Shade |
| P.O.13 | 21110 | Cl | CH3 | H | yellowish orange |
| P.O.34 | 21115 | Cl | CH3 | CH3 | yellowish orange |
| P.R.37 | 21205 | OCH3 | CH3 | CH3 | yellowish red |
| P.R.38 | 21120 | Cl | COOC2H5 | H | Red |
| P.R.41 | 21200 | OCH3 | H | H | Red |
| P.R.111 | — | — | — | — | Red |
2.4.3.4.2 Pigment Orange 13
P.O.13, sold in the USA as pyrazolone orange, comes in semi-transparent types with specific surface areas between about 35 and 50 m2 g−1. It is colouristically very similar to P.O.34, but generally somewhat yellower. Occasionally weaker than P.O.34, it is also slightly less fast in many media. Considering its tendency to migrate, incorporation into plasticized PVC is not recommended. The pigment blooms over a large concentration range and bleeds considerably. At concentrations below about 0.1%, neither P.O.34 nor 13 are suited for use in rigid PVC.
Application of P.O.13 in polyolefins is limited. It is recommended for use at temperatures up to 200 °C (Section 2.4.1.3). This is equally true for polystyrene and other plastic materials that are processed above 200 °C, such as polymethacrylate, in which P.O.13 is used. P.O.13 is one of the pigments that do not affect the extrusion shrinkage of HDPE, but it is nevertheless rarely employed to colour such materials. In LDPE, there is some danger of blooming.
P.Y.13 is extensively used in the rubber industry. It is fast to vulcanization and bleeding in natural rubber. Owing to its excellent fastness to water the pigment can be applied for bathing articles, sponges and sealing rubbers for preservation glasses.
The pigment is very fast to detergents. It is also employed in viscose spin dyeing and in mass dyeing, for example for cellulose sponges and foils. In full shades (1/1 SD) the lightfastness equals step 6–7 of the Blue Scale and decreases with reduction (1/12 SD) to step 4. The most important fastness properties for textile printing are excellent or very good.
P.O.13 shows less stability in paints than P.O.34 types of similar particle size. This includes both fastness to overpainting in baking enamels and lightfastness in air drying paints. The volume of trade sales for this purpose is accordingly limited.
The graphics industry, on the other hand, uses P.O.13 to an appreciable extent for packaging printing inks. Its fastness to light is average and corresponds to that of the diarylide yellow pigment P.Y.12, with which it is frequently combined as a shading component. The stability of pigmented prints to several organic solvents is excellent or almost perfect. Similarly, the prints are fast to paraffin, butter and soap. They withstand heat very well and are stable up to 200 °C. P.O.13 thus lends itself to metal deco printing, provided its lightfastness suits the purpose. Likewise, its resistance to clear lacquer coatings and to sterilization are excellent.
2.4.3.4.3 Pigment Orange 34
P.O.34 is supplied in various types, which differ considerably in their particle size distributions. Specific surface areas range from 15 m2 g−1 in highly opaque versions to about 75 m2 g−1 in transparent types. It is these physical characteristics that determine the colouristic and fastness properties of each type. Even varieties of P.O.34 with fine particle sizes are generally not resinated.
Transparent P.O.34 versions represent the most frequent choice for printing inks. They provide a clean, yellowish orange hue and high tinctorial strength. Standardized 1/1 SD letterpress proof prints require inks formulated at 7.6% pigment. The same is true for corresponding yellow shades produced by the tinctorially strong diarylide yellow pigment P.Y.13. P.O.34 is somewhat redder than the similarly strong P.O.13. At equal depth of shade, prints obtained from P.O.34 tolerate light better than do prints containing P.O.13; the difference is about 1 step on the Blue Scale. The lightfastness of 1/1 and 1/3 SD prints equals step 4 on the Blue Scale, which makes the pigment almost as fast as P.Y.13.
P.O.34 shows good solvent resistance to several organic solvents. Its prints are more stable in this respect than those made from P.O.13, which is also true for the standard DIN 16 524 solvent mixture (Section 1.6.2.1). Despite these comparatively good fastness properties, P.O.34 may recrystallize in various printing inks, depending on the processing conditions. P.O.34 prints are fast to paraffin and dioctyl phthalate; likewise, they tolerate clear lacquer coatings and may be sterilized.
Transparent P.O.34 is somewhat sensitive to heat and generally only withstands temperatures up to 100–140 °C. Higher sterilization or metal deco printing temperatures may produce a colour shift towards a redder orange.
P.O.34 is used for all printing techniques. Packaging printing inks, especially nitrocellulose inks, often use the orange version of the cheaper and more lightfast P.O.5 in areas where fastness to organic solvents is unimportant. The shade of the product may have to be modified accordingly. P.O.34, as do diarylide yellow pigments, exhibits insufficient solvent fastness to be used in decorative printing inks; it is particularly not fast enough to monostyrene and acetone (Section 1.8.1.2). The pigment also performs poorly as far as lightfastness is concerned, and it bleeds into solutions of melamine resins, which precludes its use in such fields.
The textiles printing industry, however, uses P.O.34 to an appreciable extent. The pigment provides average lightfastness (at 1/3 SD, it equals step 5–6 on the Blue Scale); its dry cleaning solvent resistance is excellent, and it withstands exposure to dry heat up to 200 °C. P.O.34 performs similarly in connection with spin dyeing of polyacrylonitrile.
Throughout the plastics industry, P.O.34 is used to colour plasticized PVC, although a certain tendency to bloom precludes its application at levels below about 0.1%. At higher concentrations, the pigment tends to bleed in plasticized PVC, but it is considerably more stable to light than the weaker P.O.13. At equal depth of shade, 1/3 SD samples equal step 6 on the Blue Scale, while the corresponding P.O.13 sample reaches only step 4. Transparent P.O.34 colourations in rigid PVC are even more resistant to light. Pigment concentrations of less than about 0.1% are likewise unsuitable, due to the necessity to avoid blooming. The pigment is also used in vinyl floor coverings and in cable insulations.
P.O.34 is rarely used in polyolefins. In such media, it only withstands exposure to 200 °C, and its opaque colourations show insufficient lightfastness. P.O.34 tends to bloom, especially in extrusion products made of low molecular weight LDPE types. The pigment is, however, recommended for various other media. These range from aromatic polyurethane foams to cast resins of unsaturated polyester, in which the pigment slightly delays the hardening process.
In the paint industry, transparent types are used only to a limited extent. In air drying systems, P.O.34 equals step 6–7 on the Blue Scale in full shades, while opaque colourations with TiO2 (1:5) only reach step 3 for lightfastness. In baking enamels, the pigment is not fast to overpainting.
Highly opaque versions of P.O.34 with coarse particle sizes and specific surface areas between about 15 and 25 m2 g−1, however, are gaining recognition within the paint field. Excellent flow properties make it possible to further increase the pigment level and the opacity, in which P.O.34 affords exceptionally good results for an organic pigment. Even at equal pigment concentration, P.O.34 is more opaque than commercially available Molybdate Red pigments which cover the same range of hues. Full shades of this very opaque type are very fast to light and weather, and they have a much better ability to tolerate solvents and migration. This makes P.O.34 attractive as a partial or complete replacement for Molybdate Red in industrial finishes, tractor and agricultural implement finishes, house paints, and so on. Its temperature stability is similarly superior. This is also true for application in print, where P.O.34 exhibits an increase in thermal stability from less than 120 °C in transparent prints to 200 °C in specialized opaque varieties. Opaque types are also much more reddish, which changes the DIN colour (Section 1.6.1.4) of 1/3 SD prints from 4.06 to 5.93. Combinations with other coloured pigments, such as iron oxides or titanium mixed oxides, such as nickel titanium yellow and colourations that are reduced with TiO2 are much less fast.
2.4.3.4.4 Pigment Red 37
P.R.37 is a yellowish red pigment that performs poorly in many applications and is largely restricted to the colouration of rubber and plastics.
Lightfastness in rubber, which meets almost all requirements, is accompanied by good curing properties and migration resistance. The resulting pigmented articles are very resistant to water and detergent solutions.
P.R.37 is a very strong pigment in PVC, but deficient in its fastness to light. 1/3 SD coloured samples equal step 2–3 on the Blue Scale, while 1/25 SD specimens only reach step 1. In its fastness to light, the pigment thus performs much poorer by several steps on the Blue Scale than diarylide yellow pigments, such as P.Y.13 or P.Y.17. Its fastness to bleeding in plasticized PVC (at 1/3 SD), however, parallels that of these yellows. Pigment preparations of P.R.37 are also available. Owing to its good dielectric characteristics, P.R.37 is frequently selected for use in cable insulations. However, application recommendations exclude polyolefins.
2.4.3.4.5 Pigment Red 38
P.R.38 affords a medium red shade. It is used primarily in rubber and plastics. Much faster to several organic solvents than P.R.37, rubber colourations are very fast to light and are used under almost any application conditions. P.R.38 is completely resistant to curing and bleeding into natural rubber and fabric backing (Section 1.8.3.6). The coloured articles are resistant to water, soap and detergent solutions, and equally fast to various organic solvents, including gasoline. P.R.38 shows high tinctorial strength in PVC, but tends to bloom at low pigment levels. In contrast to P.R.37, low P.R.38 levels in rigid PVC are equally prone to bloom. However, P.R.38 is much more lightfast than P.R.37: in 1/3 SD, it reaches step 6 on the Blue Scale, in which it parallels the properties of P.Y.13. Combinations of the two are frequently used to produce intermediate shades. In PVC, the pigment withstands temperatures up to 180 °C; its good dielectric properties make it a useful colourant for PVC cable insulations.
In polyethylene, the pigment tolerates temperatures up to 200 °C; such systems are used to make films. Higher processing temperatures carry the risk of inducing blooming and decomposition by heat in LDPE types. Depending on the depth of shade, its lightfastness in HDPE equals between step 3 and step 6 on the Blue Scale. In this medium, it does not affect the shrinkage of the plastic. Especially throughout the USA, P.R.38 is primarily used for paper coatings, mass colouration of paper, artists' colours, crayons, and similar specialized media, and also in specialized printing inks.
2.4.3.4.6 Pigment Red 41
P.R.41 is also known as pyrazolone red. It has lost most of its commercial importance in recent years. P.R.41 production is now limited to the USA, where it is mostly employed to lend colour to rubber. To a lesser extent, P.R.41 is found in PVC; excellent dielectric properties make it a suitable candidate for PVC cable insulations. The pigment provides a medium to bluish red; of limited brilliance, it is much bluer than P.R.38. P.R.41 is somewhat less fast than P.R.38, which is also true for its stability to various organic solvents. However, it parallels P.R.38 in its alkali and acid resistance. P.R.41 is very lightfast in rubber; 1% pigment concentrations equal step 6–7 on the Blue Scale, which meets practically any requirement. The pigment also withstands migration, that is, it does not bleed into natural rubber or fabric backing (Section 1.8.3.6). P.R.41 is also completely resistant to curing conditions. Coloured rubber articles tolerate boiling water, acid and soap.
2.4.3.4.7 Pigment Red 111
P.R.111 is chemically related to the pyrazolone pigments P.O.34 and P.R.37. Its hue is somewhat bluer than that of its pyrazolone counterparts; it equals that of Signal Red (RAL 3000). The lightfastness of P.R.111 is somewhere between that of P.O.34 and of P.R.37. P.R.111 performs similarly as far as other fastness properties are concerned. The pigment lends itself particularly to the colouration of rubber and PVC; excellent dielectric properties also render it suitable for cable insulations. Thermally, P.R.111 is not sufficiently stable to be used in polyolefins, styrene, ABS and similar plastics.
2.5 β-Naphthol Pigments
The same chemistry is involved as with monohydrazone yellow pigments, except that β-naphthol pigments are obtained by coupling with 2-hydroxynaphthalene (β-naphthol) instead of acetoacetarylides. They have the general chemical structure:

β-Naphthol colourants are amongst the oldest synthetic dyes known. Likewise, β-naphthol pigments, first manufactured in 1889, were the earliest products of their kind in the pigment industry. Th. and R. Holliday (at Read Holliday & Sons in the UK) applied for the patent in 1880. The compounds became known as developing dyes in connection with so-called ‘ice dyeing' or ‘azoic dyeing'. They are applied by padding cotton goods through an alkaline β-naphthol solution and drying them evenly. The goods are then processed through tubs containing the cold diazonium compound and acetate buffer, where the colour ‘develops' through coupling. Insolubility of the colourant in water makes for very washfast products. This is the pathway found by Gallois and Ullrich in 1885, who obtained Para Red (Pigment Red 1) by coupling β-naphthol with diazotized 4-nitroaniline. The resulting ‘pigment' was one of the first successful compounds used in textile dyeing and printing – it is even considered the oldest of all known synthetic organic pigments.
β-Naphthol had been discovered by Schaeffer as early as 1869. It was used initially as a starting material for colourants (‘Orange II', Echtrot AV).
o-Nitroaniline Orange followed in 1895 (o-nitroaniline → β-naphthol), and the beginning of the twentieth century saw a steady increase in the number of β-naphthol pigments, including improved products such as Toluidine Red (P.R.3), launched in 1905, chlorinated Para Red (P.R.4), which appeared in 1906, and Dinitroaniline Orange (P.O.5), introduced in 1907. Most of these are still used in a host of applications.
The history of β-naphthol pigments reflects the development of organic pigments in general. First used as developing dyes, the colourant was changed into a pigment by adding an inorganic carrier. As knowledge and expertise progressed and it became clear that such carriers have no effect on the fastness properties of the pigments, these compounds developed into independent products (‘para toners'). Likewise, β-naphthol pigment lakes followed the same course of events. To date, two β-naphthol pigments (Toluidine Red and Dinitroaniline Orange) and one β-naphthol pigment lake (Lake Red C) still reign supreme amongst organic pigments worldwide.
2.5.1 Chemistry, Manufacture and Crystal Structures
The general formula for β-naphthol pigments is:

In commercial products, RD primarily stands for CH3, Cl, NO2; and m = 1–2.
The pigments are obtained by treating the appropriate amine with water/hydrochloric acid to form the amine hydrochloride. In most cases, subsequent diazotization is carried out in the cold (0–5 °C) with an aqueous sodium nitrite solution (a). The resulting diazonium salt solution is then transferred onto sodium naphtholate that is dissolved in a sodium hydroxide solution. An acetic acid/sodium acetate buffer maintains a slightly acidic pH throughout the coupling reaction (b). When the coupling process is completed, no diazonium salt may remain in the reaction solution.
Nitrosylsulfuric acid, prepared by dissolving sodium nitrite in concentrated sulfuric acid, is employed for amines of low basicity, whose diazonium salts will hydrolyse in dilute acid. To synthesize Pigment Orange 5, for instance, 2,4-dinitroaniline is dissolved in concentrated sulfuric acid and diazotized preferably with nitrosylsulfuric acid. Coupling is carried out with a β-naphthol suspension, produced by acidifying a sodium naphtholate solution:


Comparatively low-cost starting materials and easy synthesis make it possible to manufacture these pigments economically.
2.5.1.1 Crystal Structures
Most crystal structures of β-naphthol pigments have been determined by single-crystal structure analyses. They show that all commercial β-naphthol pigments exist in the solid state in the tautomeric o-quinonehydrazone form exclusively; hence they contain a 1,2-naphthalenedione-1-(2-phenylhydrazone) fragment (20), and not a hydroxyazo structure (21). The NH group of the hydrazone fragment forms an intramolecular hydrogen bond with the keto group of the naphthalenedione system. This hydrogen bond is bifurcated, if R2 is an acceptor group (Figure 2.24).

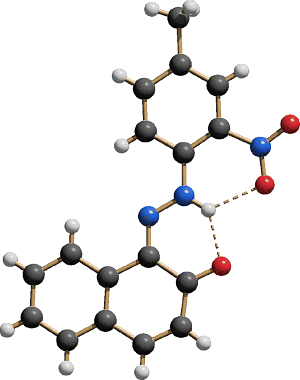
Figure 2.24 Molecular structure of P.R.3 in the solid state.
The naphthalenedione system is planar. The phenyl group may be slightly inclined against the naphthalene moiety (11° in the γ-phase of P.R.1). In the crystal, the commercial β-naphthol pigments do not form intermolecular hydrogen bonds; the molecules are held together only through van der Waals and Coulomb interactions.
The β-naphthol pigment Pigment Red 1 was the first red hydrazone pigment, whose crystal structure was determined by X-ray structure analysis [64]. Today, crystal structures of three polymorphic forms are known, which exhibit different hues and crystal shapes [65]:
- α – brownish red
- β – deep red needles
- γ – deep red blade-shaped crystals.
The polymorphic forms differ in their molecular packing (Figure 2.25), which is the reason for the different properties of the polymorphs.

Figure 2.25 Crystal structures of P.R.1 in the solid state: (a) α-phase, (b) β-phase and (c) γ-phase. The molecular layers in the α- and γ-phases are similar, but in the γ-phase the molecules in subsequent layers are rotated by 180°.
The crystal structures of P.R.3 [66], P.R.6 [67], P.O.2 [68] and P.O.5 [69] have also been determined. As an example, Figure 2.26 shows the structure of P.O.5.

Figure 2.26 Crystal structure of P.O.5, determined by X-ray powder diffraction, lattice-energy minimization and Rietveld refinement [67].
2.5.2 Properties
β-Naphthol pigments come in shades from yellowish orange to bluish red. They are tinctorially weak and in some applications considerably less strong even than monohydrazone yellow pigments.
Their solvent stability is poor, it approaches that of the above-mentioned yellows (Section 2.3.3). Migration characteristics (i.e. fastness to bleeding and blooming) are consequently inferior, although the pigments are generally resistant to alkali and acid.
Most β-naphthol pigments are supplied as opaque products with low specific surface areas, designed to provide good hiding. Large particles, on the other hand, are frequently responsible for loss of gloss in paints and prints. Systems that dry under volume contraction, particularly air drying alkyd resin systems, are sensitive to hazing, a special kind of surface disturbance that also occurs in monohydrazone yellow pigments with coarse particle sizes (Section 1.7.6).
Speciality products with fine particle sizes, high specific surface areas and enhanced transparency are characterized not only by increased tinctorial strength, but also by inferior stability to solvents and light.
Although standard types resist light well, especially in full shades, they are not as fast as most monohydrazone yellow pigments. Lightfastness is lost rapidly with decreasing depth of shade and decreasing pigment area concentration, respectively. The commercially available types disperse easily in most media.
2.5.3 Application
The technical applicability of β-naphthol pigments is severely limited by poor fastness to organic solvents and migration.
The more significant members of this family have not lost their impact in the field of paints, which is the main market for β-naphthol pigments.
Pigment Orange 5 and Pigment Red 3 are standard types covering the orange and red range in air drying systems. Pigments used for this purpose are generally characterized by very small specific surface areas between about 7 and 20 m2 g−1, which makes for products with extremely coarse particle sizes. There is therefore little danger of recrystallization during processing and application. The pigments are used mainly in masstone and similar deep shades. Several β-naphthol pigments are also incorporated into emulsion paints. In baking enamels, the pigments may bloom considerably over a large concentration range, which practically precludes their use in such systems. In special cases and in concentrations in which there is no danger of blooming, the pigments may be applied in low-temperature curing systems. Application in plastics, however, is severely limited by poor fastness properties, including a tendency to migrate and little fastness to heat. A few special types, which may be incorporated into rigid PVC, are the only exception.
Although β-naphthol pigments, such as P.R.3, have suffered considerable loss of impact in the field of printing inks, this remains an important market. The pigments are preferably used for packaging purposes. They also lend themselves very well to flexo and offset printing. Such prints, like the pigment powders themselves, are fast to water, acids and bases, but they perform poorly regarding several special fastness properties: they are not resistant to butter and paraffin (Sections 1.6.2.2 and 1.6.2.3). Other deficiencies are poor fastness to clear lacquer, overpainting and sterilization. β-Naphthol pigment prints do not tolerate heat: they do not withstand exposure to 140 °C. This is another one of the aspects in which this family approximates monohydrazone yellow pigments.
The grades with coarse particle sizes provide easy dispersion and good rheology. Types with finer particle sizes are tinctorially stronger and somewhat more transparent, and they show less of a tendency to bronze than the products with coarse particle sizes.
β-Naphthol pigments are broad in scope in many fields. Special applications include cleaners and detergents, office articles and artists' colours, as well as match-head compositions and fertilizers.
2.5.4 Commercially Available β-Naphthol Pigments
2.5.4.1 General
Very few β-naphthol pigments continue to play an important role in today's pigment industry. The list of important products includes Toluidine Red (P.R.3) and Dinitroaniline Orange (P.O.5). Other compounds, such as P.R.6, Parachlor Red, which is the positional isomer of P.R.4; P.O.2, Orthonitroaniline Orange, which is the positional isomer of the para toner P.R.1, are only of regional importance. Table 2.7 lists the commercially available β-naphthol pigments. The Colour Index numbers are listed along with the common names, since older products are frequently referred to by these names.
Table 2.7 Commercially available β-naphthol pigments.

|
||||
| C.I. Name | C.I. Constitution Number | R2 | R4 | Common Name |
| P.O.2 | 12060 | NO2 | H | Orthonitraniline Orange |
| P.O.5 | 12075 | NO2 | NO2 | Dinitraniline Orange |
| P.R.l | 12070 | H | NO2 | Para Red/Para Toner |
| P.R.3 | 12120 | NO2 | CH3 | Toluidine Red |
| P.R.4 | 12085 | Cl | NO2 | Chlorinated Para Red |
| P.R.6 | 12090 | NO2 | Cl | Parachlor Red |
It is impossible to clearly assign a distinct shade to each of these pigments because each compound produces a very different range of red, depending on the manufacture, the additives and the particle size distribution. Variation of choice and rate of reactant addition during diazotization and coupling, pH, and concentration, choice and amount of additives for the coupling reaction are the factors determining the exact shade of each pigment. Commercially available β-naphthol pigments possess a notable feature: they all carry at least one nitro group, either in an ortho or a para position relative to the hydrazone bridge. The two most important species are obtained by coupling with o-nitroaniline derivatives as diazo components.
2.5.4.2 Pigment Orange 2
This pigment has only limited impact outside Europe; it is used especially in the USA. Compared to its positional isomer P.R.1, it performs slightly better as far as fastness to various solvents, such as aliphatic hydrocarbons, is concerned. Commercial P.O.2 types afford clean orange shades with good hiding power. Resinated and transparent types are not known. The pigment is primarily used to colour aqueous flexo inks, paper, air drying coatings and artists' colours.
2.5.4.3 Pigment Orange 5
P.O.5 is one of the most significant organic pigments. Two product lines with different particle sizes are available that differ considerably in their colouristic properties. The varieties with coarser particle sizes and specific surface areas between about 10 and 12 m2 g−1 are much more reddish and duller than the types with somewhat finer particle sizes and specific surface areas between 15 and 25 m2 g−1.
P.O.5 is principally applied in air drying systems. The type with a coarser particle size provides good hiding power. In full shades, there is some darkening upon exposure to light, although its lightfastness equals step 6 on the Blue Scale. Similarly, the pigment exhibits very good weatherfastness. Its lightfastness, however, deteriorates rapidly in white reductions, although this trend is much less pronounced than with other pigments within the same family. The 1:5 reductions with TiO2 equal step 5 on the Blue Scale for lightfastness; at 1:40 TiO2 reduction, the pigment only reaches step 4. Consequently, P.O.5 is used only in full or similarly deep shades, partially in combination with inorganic pigments such as Molybdate Orange.
The yellower, cleaner types with finer particle sizes are almost as lightfast as opaque varieties. Good lightfastness and weatherfastness make for good colouration of emulsion paints; only the deeper shades are used for exterior paints. P.O.5 is not entirely fast to alkali and lime. It shows a strong tendency to bloom in baking paints. The concentration limits (Section 1.6.3.1) are 0.5% at 120 °C, and 1% at 140 °C. At higher baking temperatures, P.O.5 always blooms, irrespective of the concentration. It is not suited to use in epoxy resin coatings, the colour of which changes to a brownish shade.
P.O.5 is used to an appreciable extent in printing inks, especially for offset, flexo and packaging gravure printing inks. Transparent types with fine particle sizes are particularly important for these purposes. They are considerably stronger, some by more than 30%, and afford much yellower, cleaner prints that are glossier. These prints are tinctorially only about half as strong as similarly coloured P.O.34, but much more lightfast.
Lightfastness at common pigment area concentrations, that is, at 1/1 to 1/25 SD, equals approximately step 6 on the Blue Scale. P.O.5 is a typical member of its class in that the prints are more or less sensitive to organic solvents and to the standard DIN 16 524 solvent blend (Section 1.6.2.1). While the pigment lacks butter and paraffin fastness, it is completely resistant to soap. P.O.5 is not used for metal printing or for decorative printing.
P.O.5 is in high demand in the field of the textile printing. As far as most of the relevant fastness properties are concerned, P.O.5 performs less well than the somewhat yellower and much more expensive perinone pigment P.O.43. Compared to the somewhat yellower P.O.34, however, its fastness to light is superior. 1/3 SD P.O.5 samples equal step 7 on the Blue Scale, as opposed to step 5–6 reached by P.O.34. In other respects, such as fastness to dry-cleaning with perchloroethylene or petroleum ether, to laundering with peroxide bleach or alkali, P.O.5 performs less well than P.O.34.
In the plastics industry, P.O.5 is limited to application in rigid PVC. Transparent colourations (0.1% pigment) afford a lightfastness that equals step 8 on the Blue Scale, although the shade darkens somewhat upon exposure. 1/1 to 1/25 SD opaque samples equal step 6 on this scale. The 0.5% pigmented films (Section 1.6.2.3) pass the coconut test. P.O.5 is also suitable for various other applications, including office articles and artists' colours. In the latter, it is used particularly where good lightfastness is required: in pigmented drawing inks, in coloured pencils, wax and marking crayons, and watercolours. Likewise, P.O.5 is used in the mass and surface colouration of paper.
2.5.4.4 Pigment Red 1
Known also as para red or para toner, P.R.1 has proven to be incompatible with ever increasing industrial demands and has lost most of its significance. It provides a very dull, somewhat brownish red hue. P.R.1 is less stable to organic solvents than other members within the same family. Moreover, it is also less fast to light. In air drying paints, full shades of P.R.1 equal step 5 on the Blue Scale; however, there is some darkening. Even trace amounts of TiO2 reduce the lightfastness considerably. Poor solvent resistance is accompanied by a tendency to bloom and bleed in baking enamels.
P.R.1 is used in printing inks for low-cost articles. It used to be important for newsprint purposes, although the prints are affected by numerous media. In contrast to other pigments of its class, P.R.1 is sensitive to acids, bases, soaps and even water.
2.5.4.5 Pigment Red 3
Toluidine Red, like P.O.5, is by volume one of the 20 largest organic pigments in the world. It shows insufficient fastness towards solvents; in fact, it is partially inferior even to monohydrazone yellow pigments, which is also true for other members of this class. Its stability to alcohols, aliphatic and aromatic hydrocarbons, and dibutyl phthalate equals step 3 on the 5-step scale; P.R.3 is even less fast to esters and ketones.
P.R.3 is used primarily in air drying paints. Its hue varies considerably with the particle size, therefore pigment manufacturers usually offer a range of brands. The products with coarser particle sizes are bluer. Very bluish varieties, however, partially contain chemically modified P.R.3.
As paint films with coarse particle sizes are dried, the volume of the air drying system contracts with increasing drying time and the glossy surface turns hazy. A phenomenon known as ‘Toluidine Red haze' appears (Section 1.7.6). Coarse pigment particles, at the surface with adsorbed binder components, protrude more and more from the surface of the coating, scatter light, and reduce the gloss. Varieties with finer particle sizes, which are also more yellowish, show less of a tendency to turn hazy. Intensive dispersion, however, improves the gloss, even of bluish types, resulting in more yellowish materials. This implies that the gloss can only be improved by reducing the size of the coarser particles, which has been confirmed by electron microscopic studies of ultrathin layers.
Full shades of Toluidine Red are extremely lightfast and weatherfast, but deteriorate rapidly as the pigment is reduced with white pigment. In full shades, the lightfastness equals step 7 on the Blue Scale, while 1:4 TiO2 reductions only reach step 4. The pigment is therefore used preferably in full or similarly deep shades. Recommendations include emulsion paints for interior application or short-term advertisement and marking purposes.
P.R.3 is very likely to bloom in baking enamels. At 120 °C, the concentration limit for blooming is 2.5%; beyond about 140 °C, blooming occurs with certainty, irrespective of the concentration. Therefore, only full shades of P.R.3 are used to colour baking enamels, and only where application temperatures are low. The pigment is used extensively in combination with Molybdate Red.
P.R.3 application in printing inks is restricted; the pigments are being displaced by their stronger Naphthol AS pigment counterparts. P.R.3 is used primarily in flexo printing. The problem with offset application is that it frequently provides poor gloss. The prints are very sensitive to organic solvents, including the standard DIN 16 524 blend; but they are soap, alkali and acid fast.
In plastics, Toluidine Red is practically limited to rigid PVC. Its lightfastness in full shades and slight white reduction is fair. In addition, the pigment is also used to colour a number of specific media, such as normal wax crayons and pastel chalks or low-cost watercolours.
2.5.4.6 Pigment Red 4
P.R.4 is also known as chlorinated Para Red. It has lost much of its commercial impact in recent years. The pigment affords a yellowish red shade, somewhere between the more yellowish P.O.5 and the more bluish P.R.3. Tinctorially, P.R.4 is the weakest of the three. To formulate equal depth of shade in an air drying alkyd system, three equivalents of TiO2 are necessary per equivalent of P.R.4, while P.O.5 and P.R.3 require 5 and 6 parts of TiO2, respectively, per equivalent of organic pigment; this is provided all other parameters are equal. Full shades exhibit good lightfastness (step 6), but darken somewhat upon exposure. Addition of even small amounts of TiO2 reduces the lightfastness considerably. Likewise, only full and similarly deep shades tolerate weather.
In the print field the pigment is almost exclusively used in air drying systems. P.R.4 is very likely to bloom in stoving enamels. At only 120 °C, the concentration limit for blooming is as low as 2.5%, and at 140 °C the limit is at 5%, which makes it necessary to carry out test experiments. Application is consequently restricted to baking enamels targeted for low temperature purposes. In epoxy resins, the pigment turns brown, as does P.O.5, and is therefore unsuitable for use in these media.
In the printing inks field, P.R.4 is employed as a clean, yellowish red colourant for purposes where no solvent fastness is required. The prints do not tolerate most organic solvents, including the standard DIN 16 524 solvent blend. They are even more sensitive to some solvents than prints made with P.O.5 or P.R.3. A series of other fastness properties, such as fastness to paraffin, butter and grease, are poor. Nevertheless, P.R.4 is of regional importance for both offset printing and packaging gravure and flexo printing. Its prints are strong compared to other members of this class that cover the same range of shades. Under standard conditions, 1/1 SD P.R.4 letterpress proof prints are prepared with printing inks containing 13% pigment, while P.R.3 samples require between 16 and 20%, or, alternatively, 16% P.O.5.
At typical pigment area concentrations, that is, standard depths of shade in the range between 1/1 and 1/25 SD, the lightfastness equals step 4 or 3 on the Blue Scale, respectively. Application in metal deco printing is not feasible, which is also true for other pigments within the same class.
Application in rubber blends, which used to be an important market for P.R.4, has suffered from increasingly stringent requirements. P.R.4 bleeds considerably during curing into overlaid white rubber sheets, while it hardly bleeds into the fabric backing (Section 1.8.3.6).
P.R.4 is also used in specialized applications. The pigment lends colour to cleaning agents and detergents, including shoe polish and floor polish, office articles and artists' colours, including coloured pencils, wax crayons for schools and artists, and pastel chalks, and to low price watercolours. It is also used in decorative cosmetics, for which types are available that meet the legal requirements. In the USA it is registered by FDA as D & C Red 36, in Japan as Red No. 228.
2.5.4.7 Pigment Red 6
This pigment, also known as Parachlor Red, has lost most of its commercial impact. It provides a yellowish red hue, somewhat on the yellow side of Pigment Red 3 and bluer than Pigment Red 4. P.R.6 parallels these two pigments in its fastness properties; the only exception is its stability to light. In air drying alkyd systems, full and similarly deep shades of P.R.6 are as lightfast as Toluidine Red, while in white reductions the pigment tolerates light much better. Compared to P.R.3, 1/3 SD P.R.6 samples equal step 6–7 as opposed to step 4 on the Blue Scale, while 1/25 SD samples reach step 5–6 as opposed to step 1.
2.6 Naphthol AS Pigments
For Naphthol AS pigments (Naphthol Red pigments), the same chemistry is involved as with monohydrazone yellow pigments, except that coupling is carried out with arylides of 2-hydroxy-3-naphthoic acid. The reference compound, Naphtol AS1) is the anilide of 2-hydroxy-3-naphthoic acid:

Substitution in the anilide ring affords a series of Naphthol AS derivatives, although only a limited number are commercially recognized.
In 1892, the chemist Schopf, in an attempt to prepare 2-phenylamino-3-naphthoic acid, developed a synthetic route leading to the anilide of 2-hydroxy-3-naphthoic acid. His method continues to be used today, if only in a slightly modified form. He added phosphorus trichloride to a molten reaction mixture containing aniline and 2-hydroxy-3-naphthoic acid (β-oxynaphthoic acid, also known as BONA or BONS) and obtained Naphthol AS in good yield. Modern processes differ from this principle only in terms of reaction control; the synthesis is now carried out in the presence of organic solvents, such as aromatic hydrocarbons.
Naphthol AS was not further used until 1909, when BASF in Germany claimed a patent for a diazotization dye that could be developed by diazotizing primuline2) on the fibre and then coupling with Naphthol AS in an alkaline solution.
In 1911, A. Winter, H. Laska and A. Zitscher at Griesheim-Elektron, later the Offenbach site of Hoechst AG in Germany, made a discovery that was to prove an important breakthrough in the Naphthol AS field. They synthesized hydrazone colourants from diazotized anilines or toluidines (with Cl or NO2 substituents) and Naphthol AS as the coupling component. The resulting pigments (‘Grela Reds'), although superior to β-naphthol pigments in terms of lightfastness and solvent stability, were initially disregarded, since the pigment industry at the time focussed on the more economical and immensely commercially successful β-naphthol pigments (Toluidine Reds).
In 1912, Griesheim-Elektron first replaced β-naphthol pigments by Naphthol AS, to be used for ice dyeing. Naphthol AS has a much higher substantivity and can therefore be fixed much more evenly than β-naphthol. Intermediate drying on the fibre prior to coupling can thus be eliminated. Moreover, alkaline naphthol solutions are much more stable in air than the corresponding β-naphthol solutions. This discovery initiated the rapid development of Naphthol AS dyeing into a well established technology within a few years, followed by continuous introduction of new Naphthol AS pigments. These events stimulated the discovery of a vast number of substituted anilides of 2-hydroxy-3-naphthoic acid.
Naphthol AS derivatives that have retained commercial importance are listed in Table 2.1, together with their name, constitution and Colour Index number (Section 2.1.2).
During the 1920s and 1930s, the development of Naphthol AS technology in Germany was initiated by IG Farben. New Naphthol AS pigments were synthesized, based on combinations previously used in Naphthol AS dyeing. In the USA, the development of Naphthol Red pigments commenced in the 1940s. The pigments were developed further by varying the substitution pattern of the dihydrazone component, attempting to improve solvent and migration fastness. Sulfonamide and, to an even higher extent, carbonamide groups as substituents were most successful.
Technically significant Naphthol AS pigments are therefore divided into two groups, according to whether their diazo component carries:
- simple substituents, such as Cl, NO2, CH3 or OCH3,
- sulfonamide groups and/or carbonamide groups.
In the latter case, a second carbonamide group may be introduced through the coupling component (Section 2.6.2).
Today, more than 100 years after their discovery, Naphthol AS pigments continue to play a major role amongst organic pigments. Although comparatively many derivatives are known, only a few are produced in large volume.
2.6.1 Chemistry, Manufacture and Crystal Structures
Naphthol AS pigments have the following general chemical structure:

Commercial derivatives are primarily substituted as follows:
- RD = RK, COOCH3, CONH2, CONHC6H5, SO2N(C2H5)2
- RK = CH3, OCH3, OC2H5, Cl, NO2, NHCOCH3
- m and n are numbers between 0 and 3.
The reaction sequence is the usual one. The hydrochloride of the aromatic amine is diazotized with sodium nitrite/hydrochloric acid and subsequently coupled onto a Naphthol AS derivative.
In the earlier stages of development of these pigments, the coupling components often presented solubility problems. Although sodium naphtholates dissolve most easily in alcohol/water mixtures, organic solvents increase the price of pigment manufacture and also present ecological problems. The coupling component is therefore heated to 60–90 °C in the presence of a 7–10% aqueous sodium or potassium hydroxide solution and thus converted into the soluble dialkali salt of the amine enolate:

Acetic acid or hydrochloric acid, possibly together with a tenside, reprecipitates the compound. This method affords the Naphthol AS derivative as a material with a very fine particle size, ready for coupling. A slightly acidic pH can be optimized and maintained by adding a sodium acetate buffer.
Coupling is usually carried out at 10–25 °C, although it sometimes requires as much as 40–70 °C – provided the diazonium salt tolerates such temperatures.
For group I Naphthol AS pigments, further treatment is not necessary. The pigment suspension may be heated to 60–80 °C for a short time before it is filtered.
Manufacture of group II pigments, on the other hand, is typically followed by intensive thermal aftertreatment in water or water/organic solvents to produce a pigment that is easy to disperse.
2.6.1.1 Crystal Structures
The first crystal structure determinations of Naphthol AS Pigments were carried out by E. Paulus in cooperation with J. Ribka at Hoechst in the early 1970s on chloro derivatives of Pigment Brown 1 and Pigment Red 9 [70]. Studies by Whitaker on Pigment Red 2 followed [71]. Further X-ray structure analyses confirmed the structural features, which are similar to those found for β-Naphthol pigments (Section 2.5.1):
- The compounds exist exclusively in the hydrazone tautomeric form in the solid state.
- Both NH groups (from the hydrazone fragment and the CONH fragment) form hydrogen bonds with the keto group of the naphthalenedione system. These hydrogen bridges are bifurcated, if the compound contains substituents X and Y with lone electron pairs, for example, OCH3, OC2H5, COOR, NO2 or Cl, in the ortho-positions of the phenyl rings:

- The naphthalene moiety is almost coplanar with the phenyl ring of the diazo compound; but the phenyl ring of the coupling component may be rotated.
- Intermolecular hydrogen bonds are only formed if the substituents contain hydrogen-donating groups like CONH2 or CONH-Ph.
X-Ray powder diffractograms show that Pigment Red 170 has three polymorphs: The α-phase is formed during synthesis as a nanocrystalline powder. Heating in water to about 100 °C with additives gives the β-phase; and heating in water under pressure at 130 °C results in the γ-phase [72], which is more yellowish and more opaque than the β-phase. The β- and γ-phases are sold commercially.

Scheme 2.4 Molecular structure of P.R.170.
In the α-phase of P.Y.170 the molecules are arranged in a herringbone structure. The CONH2 group forms two hydrogen bonds with neighbouring molecules (Figure 2.27).

Figure 2.27 Section of the crystal structure of the α-phase of Pigment Red 170; view along the a axis.
Generally, an ethoxy group is not a favourable substituent for organic pigments, since it tends to increase the solubility. However, for P.R.170 it was observed that the ethoxy group is necessary to achieve good pigmentary properties. Substituting the ethoxy group by a methoxy group or by a propoxy group increases the solubility and deteriorates the application properties. The reason as to why the ethoxy group provides the best application properties is based on the crystal structure. The crystal structure of the commercial γ-phase of P.R.170 was determined from X-ray powder diffraction data (Figures 2.28 and 2.29) [73]. In the crystal the molecules are arranged in layers. The ethoxy groups are necessary to fill the space between the molecules. A methoxy group (P.R.266) would leave a hole, whereas a propoxy group (in its usual trans conformation) would overlap with the neighbouring molecule.

Figure 2.28 Crystal structure of Pigment Red 170, γ-phase; view perpendicular to the layer.
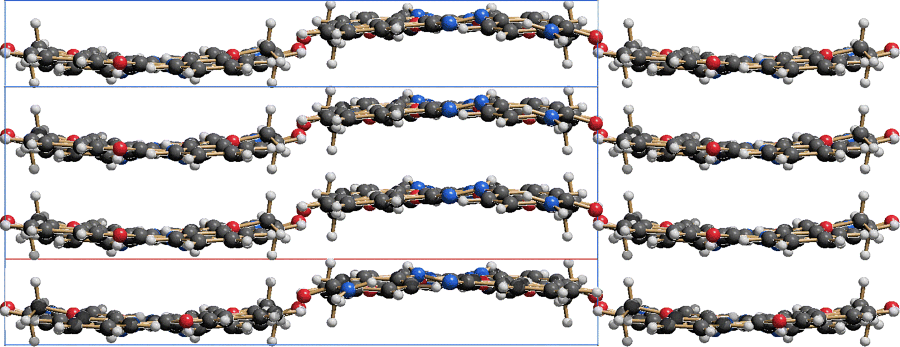
Figure 2.29 Crystal structure of Pigment Red 170, γ-phase; view parallel to the layers.
The crystal structure of the β-phase of P.R.170 was determined by a combination of single-crystal X-ray analysis, X-ray powder diffraction, electron diffraction and lattice-energy minimisations [74]. The crystal structure of the β-phase is similar to the γ-phase, with two main differences: (1) the layers are almost planar in the β-phase; (2) subsequent layers of the β-phase are laterally shifted either in the +y direction (‘right’) or in the −y direction (‘left’). The sequence is not periodic, but irregular, leading to a so-called stacking disorder. Extensive lattice-energy minimisations revealed that the probability for a shift in right or left direction depends not only on the neighbouring layers, but also on the next-neighbouring layers. Typical sequences are right-left-right-left or right-left-left-right, see Figure 2.30 [74b].
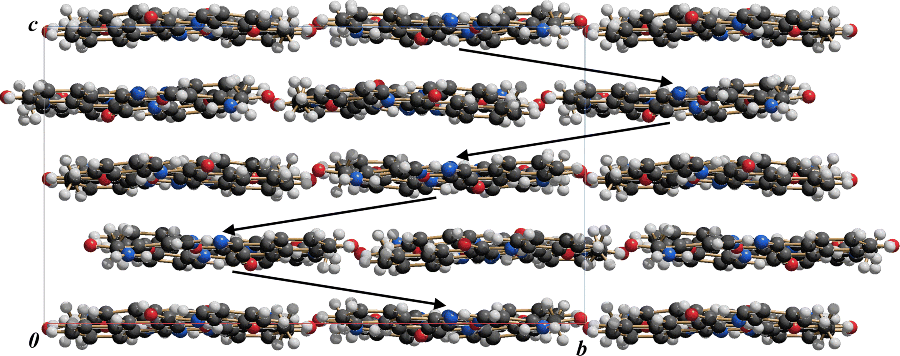
Figure 2.30 Crystal structure of P.R.170, β-Phase. View parallel to the layers. The layers are affected by a stacking disorder. The Figure shows a typical sequence of five layers with lateral shifts in the order right-left-left-right, as indicated by the arrows.
P.R.266 differs from P.R.170 only by an OCH3 group instead of an OC2H5 group. The molecules form the same hydrogen-bond pattern as in the γ-phase of P.R.170, but the layers are less wavy, and subsequent layers are shifted against each other by a different vector as in γ-P.R.170 [75].
2.6.2 Properties
Commercially available Naphthol AS pigments afford various shades ranging from yellowish to very bluish red; including all possible intermediate shades, such as bordeaux, maroon, violet and brown. Most are tinctorially very strong compared to other pigments that cover the same range of shades.
Amongst the components used to obtain Naphthol AS pigments with a simple substitution pattern (Cl, CH3, NO2, OCH3), that is, group I Naphthol AS pigments, chloroanilines produce orange to scarlet hues, chlorotoluidines primarily make bluish reds, and nitrotoluidine and nitroanisidine frequently afford bordeaux shades. Most of the Naphthol AS pigments that have been developed in the USA are obtained from dihydrazone components that possess nitro groups.
Various Naphthol AS pigments are polymorphous: they display at least two crystal modifications. The list includes P.R.9, 12, 170 and 187. A wide variety of diazonium compounds and coupling components with different substitution patterns have been developed to alter the properties of the resulting Naphthol AS pigments. This is particularly true for parameters such as fastness to solvents, in which most Naphthol AS pigments perform poorly (group I). Sulfonamide and carbonamide moieties in particular confer higher solvent resistance on their parent structure (group II). As pigment powders, various members of this class show a resistance to ethyl acetate that equals step 1 or 2 on a 5-step scale; while other pigments, such as P.R.146, reach step 4–5 on the same scale (Section 1.6.2.1). Each type is affected differently by plasticizers such as dioctyl phthalate or dibutyl phthalate that are frequently used in polymers.
The fastness of a pigment to solvents controls a variety of its resulting properties, such as the tendency to migrate, which differs considerably between types. There are media in which pigment behaviour may range from strongly migrating, blooming and bleeding pigments to nonblooming and more or less bleed resistant types.
Table 2.8 lists several commercially available pigments, along with their chemical structures, to illustrate the different structural types of Naphthol AS pigments. Fastness to solvents and migration resistance improve from top to bottom, that is, with increasing number of CONH groups in the molecule. The first example, a simple β-naphthol pigment, is the skeleton from which all other species are derived.
Table 2.8 Structural principles of β-Naphthol and Naphthol AS pigments. The fastness properties improve with the number of CONH2 groups.
| C.I. Name | C.I. Constitution Number | Structure | Number of CONH Groups |
| P.R.3 | 12120 |  |
0 |
| P.R.2 | 12310 |  |
1 |
| P.R.170 | 12475 |  |
2 |
| P.O.38 | 12367 |  |
3 |
| P.R.187 | 12486 |  |
3 |
The tendency of a pigment to migrate may be reduced further by introducing heterocyclic substituents into the coupling component (Section 2.8). Naphthol AS pigments are fairly lightfast. Even though a few members do not quite reach the fastness of β-naphthol pigments, such as full shades of P.O.5 or P.R.3, they are distinctly superior in white reduction. Full shades of P.R.9, for instance, equal step 7 for lightfastness, while 1:6 reduction with TiO2 equals step 6, and 1:500 formulations are still lightfast enough to reach step 4–5 on the Blue Scale.
The heat sensitivity of a Naphthol AS pigment is largely a function of its chemical constitution and may, for instance in print, range from under 120 to 200 °C.
2.6.3 Application
Increasingly stringent application requirements have in the course of the last two decades forced several Naphthol AS pigments out of the market. Only a few of them continue to be produced locally or regionally. Others, such as P.R.22 or 23, have long been produced in large volume in certain areas, while they are almost non-existent in other countries. Requirements vary considerably, and it is frequently impossible to refer to a standard. Several particularly high grade Naphthol AS pigments, however, have enjoyed increasing commercial recognition in recent years.
Naphthol AS pigments are used primarily in paints and printing inks. The applicability of a pigment in paints is determined or restricted largely by its fastness to organic solvents. Very fast pigments are used not only in air drying paints, nitrocellulose combination lacquers, and other paints that are processed at room temperature, but they find additional application in baking enamels. A few Naphthol AS pigments are even used in high grade systems, such as those used for various automotive finishes, including original equipment manufacture (O.E.M.) finishes. Chemically simple species bloom in baking enamels, while high grade pigments are nonblooming in all concentrations. Others may be used within a certain concentration range in baking enamels (Section 1.6.3.1), provided the pigment concentration is sufficiently high and the baking temperature is as low as possible. Depending on their chemical constitution, however, these pigments show a more or less pronounced tendency to bleed; none of them is entirely fast to overcoating.
Many Naphthol AS pigments derive most of their commercial importance from the application in printing inks. Some pigments that show high tinctorial strength produce exceptionally brilliant shades. Corresponding to the solvent fastness, other fastness properties limit the application of Naphthol AS pigments in certain fields, such as packaging printing inks. Fastness to soap and paraffins is almost always excellent or at least good, which is also true for water, acid and alkali resistance. However, the pigments exhibit a certain lack of stability to clear lacquer coatings and sterilization, which is very poor in some types. Application in print is therefore possible only where no overlacquering fastness is required. Naphthol AS pigments are primarily used in offset, flexo and packaging gravure printing inks.
Almost all Naphthol AS pigments, with a few exceptions, are excluded from metal deco printing. However, a number are used in large volume by the textile printing industry. Poor migration and heat resistance make most members of this class inapplicable to plastics.
Naphthol AS pigments are used to an appreciable extent in special areas, such as in office articles, artists' colours, cleaning agents and detergents, including soaps. They are used to colour paper, both mass coloured paper and surface coated paper.
2.6.4 Commercially Available Naphthol AS Pigments
2.6.4.1 General
The number of Naphthol AS pigments that have gained commercial recognition is comparatively large (Table 2.9). There is a dual classification system according to the substitution pattern (Section 2.6).
Table 2.9 Commercially available Naphthol AS pigments.

|
||||||||
| C.I. Name | C.I. Constitution Number | Shade | ||||||
| Group I: Pigments with simple substituents | ||||||||
| P.R.2 | 12310 | Cl | H | Cl | H | H | H | red |
| P.R.7 | 12420 | CH3 | Cl | H | CH3 | Cl | H | bluish red |
| P.R.8 | 12335 | CH3 | H | NO2 | H | Cl | H | bluish red |
| P.R.9 | 12460 | Cl | H | Cl | OCH3 | H | H | yellowish red |
| P.R.10 | 12440 | Cl | H | Cl | H | CH3 | H | yellowish red |
| P.R.11 | 12430 | CH3 | H | Cl | CH3 | H | Cl | ruby |
| P.R.12 | 12385 | CH3 | NO2 | H | CH3 | H | H | bordeaux |
| P.R.13 | 12395 | NO2 | CH3 | H | CH3 | H | H | bluish red |
| P.R.14 | 12380 | NO2 | Cl | H | CH3 | H | H | bordeaux |
| P.R.15 | 12465 | NO2 | Cl | H | OCH3 | H | H | maroon |
| P.R.16 | 12500 | OCH3 | NO2 | H | a) | bordeaux | ||
| P.R.17 | 12390 | CH3 | H | NO2 | CH3 | H | H | red |
| P.R.18 | 12350 | NO2 | CH3 | H | H | H | NO2 | maroon |
| P.R.21 | 12300 | Cl | H | H | H | H | H | yellowish red |
| P.R.22 | 12315 | CH3 | H | NO2 | H | H | H | yellowish red |
| P.R.23 | 12355 | OCH3 | H | NO2 | H | H | NO2 | bluish red |
| P.R.95 | 15897 | OCH3 | H | SO2OC6H4NO2(p) | CH3 | H | H | carmine |
| P.R.112 | 12370 | Cl | Cl | Cl | CH3 | H | H | red |
| P.R.114 | 12351 | CH3 | H | NO2 | H | H | NO2 | carmine |
| P.R.119 | 12469 | CH3 | H | SO2OC6H4CO2CH3 | OCH3 | H | H | yellowish red |
| P.R.136 | — | — | — | — | — | — | — | bordeaux |
| P.R.148 | 12369 | Cl | Cl | H | CH, | H | H | orange |
| P.O.22 | 12470 | Cl | H | Cl | OC2H5 | H | H | orange |
| P.O.24 | 12305 | H | H | Cl | H | H | H | orange |
| P.Br.1 | 12480 | Cl | H | Cl | OCH3 | H | OCH3 | brown |
| Group II: Pigments with sulfonamide or carbonamide groups | ||||||||
| P.R.5 | 12490 | OCH3 | H | SO2N(C2H5)2 | OCH3 | OCH3 | Cl | carmine |
| P.R.31 | 12360 | OCH3 | H | CONHC6H5 | H | H | NO2 | bluish red |
| P.R.32 | 12320 | OCH3 | H | CONHC6H5 | H | H | H | red |
| P.R.146 | 12485 | OCH3 | H | CONHC6H5 | OCH3 | Cl | OCH3 | carmine |
| P.R.147 | 12433 | OCH3 | H | CONHC6H5 | CH3 | H | Cl | pink |
| P.R.150 | 12290 | OCH3 | H | CONHC6H5 | b) | carmine | ||
| P.R.164 | 212855 | c) | H | OC2H5 | H | yellowish red | ||
| P.R.170 | 12475 | H | CONH2 | H | OC2H5 | H | H | red |
| P.R.184 | 12487 | H H |
CONHC6H5 CONHC6H5 |
CH3 OCH3 |
H Cl |
ruby | ||
| P.R.187 | 12486 | OCH3 | H | CONHC6H4-(p)CONH2 | OCH3 | Cl | OCH3 | bluish red |
| P.R.188 | 12467 | COOCH3 | H | CONHC6H3-Cl2(2,5) | OCH3 | H | H | yellowish red |
| P.R.210 | 12474d) 12475 |
H H |
CONH2 CONH2 |
H H |
OCH5 OC2H5 |
H H |
red | |
| P.R.212 | 12360 | OCH3 | — | CONHC6H5 | H | H | NO2 | very bluish red |
| P.R.213 | 12290 | OCH3 | — | COC6H5 | b) | bluish red | ||
| P.R.222 | 123665 | OCH3 | H | CONHC6H4(m)CF3 | H | NHCOC6H5 | H | bluish red |
| P.R.223 | 125075 | Cl | H | CONHC6H2-Cl3(2,4,5) | 1-naphthyl | bluish red | ||
| P.R.238 | e) | OCH3 | H | CONHC6H5 | OCH3 | H | Cl | bluish red |
| P.R.245 | 12317 | OCH3 | H | CONH2 | H | H | H | bluish red |
| P.R.253 | 12375 | Cl | SO2NHCH3 | Cl | CH3 | H | H | red |
| P.R.256 | 124635 | Cl | SO2N(CH3)2 | Cl | OCH3 | H | H | yellowish red |
| P.R.258 | 12318 | OCH3 | H | SO2CH2C6H5 | H | H | H | |
| P.R.261 | 12468 | OCH3 | H | CONHC6H5 | OCH3 | H | H | |
| P.R.266 | 12474 | H | CONH2 | H | OCH3 | H | H | bluish red |
| P.R.267 | 12396 | CH3 | H | CONH2 | CH3 | H | H | |
| P.R.268 | 12316 | CH3 | H | CONH2 | H | H | H | red |
| P.R.269 | 12466 | OCH3 | H | CONHC6H5 | OCH3 | H | Cl | bluish red |
| P.O.38 | 12367 | Cl | H | CONH2 | H | NHCOCH3 | H | reddish orange |
| P.O.74 | — | Cl | SO2N(CH3)2 | Cl | H | Cl | H | reddish orange |
| P.V.13 | 125085 | OCH3 | NHCOC6H5 | CH3 | 1-naphthyl | violet | ||
| P.V.25 | 12321 | OCH3 | NHCOC6H5 | OCH3 | H | H | H | violet |
| P.V.44 | — | OCH3 | NHCOC6H5 | OCH3 | H | H | H | violet |
| P.V.50 | 12322 | OCH3 | NHCOC6H5 | CH3 | H | H | H | violet |
| P.B.25 | 21180 | f) | H | H | H | reddish blue | ||
| a) P.R.16 contains 2-hydroxy-3-naphthoic acid-1-naphthylamide as coupling component. b) P.R.150 and P.R.213 contain 2-hydroxy-3-naphthoic acid amide as coupling component. c) P.R.164 is synthesized with the following bisdiazo component:  d) P.R.210 as mixed coupling product has constitution number 12477. e) The chemical structure is identical with P.R.269. f) 3,3′-Dimethyl-4,4′-diaminobiphenyl as bisdiazo component. |
||||||||
Somewhat of an intermediate position is assumed by a compound that may be considered both a dishydrazone and a Naphthol AS pigment. Pigment Blue 25 is an interesting example of a ‘cross' compound, derived from coupling bisdiazotized 3,3′-dimethoxy-4,4′-diaminodiphenyl with Naphthol AS:

Scheme 2.5 Molecular structure of P.B.25.
As far as intermediates for the manufacture of group I Naphthol AS pigments are concerned, 2-methyl-5-nitroaniline and 2,5-dichloroaniline reign supreme amongst diazo components. The list of important coupling components, apart from the anilide of 2-hydroxy-3-naphthoic acid itself, includes primarily derivatives that are substituted in the ortho position by methyl or methoxy groups. All of the group II pigments with known structures carry either an SO2N group or a CONH group in the 4 or 5 position on the aromatic ring of the diazo component.
2.6.4.2 Naphthol AS Pigments with Simple Substituents
2.6.4.2.1 Pigment Red 2
P.R.2 provides a medium red shade, which is somewhat yellower than the Naphthol AS pigment P.R.112. Its main area of application is the printing inks field. In some systems, P.R.2 is even slightly stronger than P.R.112, although it does not quite achieve the same lightfastness. 1/1 SD letterpress proof prints equal step 5 on the Blue Scale, 1/25 SD prints reach step 4. The corresponding values for P.R.112 are ½ to 1 step higher. Most of the commercially available products have specific surface areas between about 20 and 30 m2 g−1. They afford prints with correspondingly poor transparency. The types with comparatively coarse grains, on the other hand, which are frequently used to formulate highly pigmented printing inks, show good flow properties and satisfy the requirements for corresponding application. The prints exhibit good gloss.
P.R.2 does not show perfect performance in special applications (Section 1.6.2.3) in prints, which is also true for several other members of this class. In this respect, P.R.2 is inferior to P.R.112. This may have a particular impact on marginal areas of pigment applicability. P.R.112 letterpress proof prints, for instance, tolerate mineral spirits and soap, while P.R.2 prints only reach step 4 on the 5 step fastness scale. P.R.2 specimens are also sensitive to clear lacquer coatings and to sterilization.
P.R.2 is primarily used in offset and packaging gravure and flexo printing inks. The pigment also lends itself to textile printing. Besides, P.R.2 also functions as a pigment in connection with the spin dyeing of viscose rayon and viscose cellulose and in the mass colouration of viscose sponges and films. Leather coverings are also frequently coloured with P.R.2.
The paint industry has little demand for P.R.2. The pigment finds some use in household paints, especially in air drying systems.
2.6.4.2.2 Pigment Red 7
P.R.7 provides bluish red shades and is used in various media in the printing inks, paints and plastics field. Only one or two decades ago, P.R.7 was considered the leading Naphthol AS pigment. The pigment has been removed from the market altogether, because the starting materials for its synthesis were no longer available. There are other Naphthol AS pigments covering the same range of shades, such as P.R.170, which may replace P.R.7.
2.6.4.2.3 Pigment Red 8
Pigment Red 8 affords clean, bluish shades of red. It is primarily used in the printing ink industry. P.R.8 exhibits high tinctorial strength and produces brilliant prints. Commercially available types with specific surface areas between about 50 and 60 m2 g−1 afford transparent prints. P.R.8 is used in prints that require no particular solvent resistance. However, the pigment tolerates solvents much better than the yellower P.R.7; in this respect, P.R.8 matches the yellower, but more lightfast P.R.5. The prints are fast to soap but not entirely stable to butter and paraffin. P.R.8 is sensitive to clear lacquers coatings and to sterilization. It tolerates exposure to 140 °C for 30 min.
The lightfastness (such as in letterpress proof prints) deteriorates drastically with decreasing pigment area concentration. 1/1 SD prints equal step 5, while 1/25 SD specimens only reach step 3 on the Blue Scale. P.R.8 is used in letterpress and offset inks, in packaging gravure printing inks and in various flexo inks. Types with finer particle sizes, dispersed in vehicles for various printing techniques, may recrystallize and drastically deteriorate in tinctorial strength and transparency. P.R.8 is also used in textile printing, although its lightfastness does not satisfy high demands.
P.R.8 is used in various special media outside the paints, printing inks and plastics field, which is also true for other members of this class of pigments. One such application is in the paper industry, where the pigment is used for mass colouration and surface coating formulations. It also lends itself to application in artists' colours and office articles.
2.6.4.2.4 Pigment Red 9
P.R.9 affords clean, yellowish shades of red, which show very good lightfastness. The fact that the pigment is supplied in the form of an unstable crystal modification, which is very sensitive to aromatic hydrocarbons and some other organic solvents, restricts its use to media that do not contain aromatic solvents. P.R.9 is therefore used in letterpress and offset printing and aqueous flexo inks. Although the prints are comparatively sensitive to several solvents, they are soap and butter fast and almost fast to paraffin, dibutyl phthalate and mineral spirits. Compared to the slightly yellower P.R.10, P.R.9 is more lightfast if the pigment area concentration is low. In comparison with the slightly bluer 1/3 SD P.R.53:1 prints, P.R.9 is more lightfast by a few steps on the Blue Scale; it is also alkali and acid resistant. Commercial types with low specific surface areas around 20 m2 g−1 afford little transparency in print.
The paints field is another area in which P.R.9 is restricted to media containing aliphatic hydrocarbons only. Application in systems containing aromatic hydrocarbons, ketones, esters or glycol ethers changes the crystal modification and causes recrystallization, shifts the colour considerably, and drastically reduces the tinctorial strength. In suitable finishes, however, P.R.9 demonstrates very good stability to light, even in white reduction. This qualifies it for use in emulsion paints, where it shows equal perfection in lightfastness and weatherfastness. Even its full shades, however, are frequently not fast enough for exterior application.
Applications outside the above-mentioned areas include coloured pencils.
2.6.4.2.5 Pigment Red 10
This pigment exhibits a clean, yellowish red shade, similar to that of P.R.9. It is supplied as a stable crystal modification and is thus not as sensitive to aggressive solvents as P.R.9. This broadens its scope to include packaging gravure and flexo printing inks containing solvents. The pigment provides good lightfastness. 1/3 or 1/25 SD letterpress proof prints, however, score ½ to 1 step less on the Blue Scale than corresponding P.R.9 prints. P.R.10 prints are not suitable for clear lacquer coatings and may not be sterilized; their heat stability is low.
Textile prints score poorly in dry cleaning tests involving agents such as perchloroethylene, a feature that is typical of Naphthol AS pigments. P.R.10 is affected by dry heat and is equally unsuitable for PVC coatings. The prints equal step 6–7 on the Blue Scale for lightfastness, depending on the depth of shade.
P.R.10 is used to a certain extent in office articles, artists' colours and cleaners.
2.6.4.2.6 Pigment Red 11
P.R.11 has largely been replaced by other products. Its hue is a bluish red that resembles the colour of ruby; P.R.11 is more bluish than its positional isomer P.R.7. Both pigments behave similarly as far as fastness properties are concerned, which includes fastness to most of the solvents that are used in paints and printing inks, fastness to overlacquering, heat stability and so on. However, P.R.11 is much less lightfast than P.R.7; incorporated in medium-oil alkyd systems at 1/3 SD, P.R.11 scores 2 steps less on the Blue Scale (step 4).
2.6.4.2.7 Pigment Red 12
P.R.12 is one of the more significant bordeaux coloured pigments. Two crystal modifications are known, of which only the thermodynamically unstable bordeaux one is commercially used. Little activation energy is required to convert the bordeaux modification into the stable red modification. Conversion is, for instance, initiated as the pigment is dispersed in an oily binder containing zinc oxide using low shear equipment, such as a Hoover muller. Change of crystal modification may also occur if P.R.12 is milled in dry state with dry baryte. The transition occurs also very rapidly in the presence of chlorohydrocarbons or ketones in the dispersion medium. Exceptionally large pigment crystals are formed as the crystal modification changes, considerably reducing the viscosity of the dispersion medium. Poor lightfastness renders the stable red modification unsuitable for commercial application.
The graphic industry uses P.R.12 in offset inks, for packaging gravure and flexo printing inks. The pigment is comparatively lightfast; 1/1 to 1/25 SD letterpress proof prints equal step 5–6 to step 4 on the Blue Scale. P.R.12 demonstrates very high tinctorial strength; the fastness of the prints to solvents and other media is, however, moderate. The prints are affected by paraffin, butter and other fats. P.R.12 is similarly sensitive to alkali.
P.R.12 textile prints are excellently lightfast; 1/1 to 1/6 SD prints equal step 7 and 6–7, respectively, on the Blue Scale. However, the pigment fails dry cleaning tests and migrates considerably in PVC coatings. P.R.12 tolerates dry heat up to 150 °C; at 180 °C, the pigment still largely retains its initial colour value.
The paint industry uses P.R.12 primarily in air drying paints. Baking may be a problem, since a pigment concentration of less than 1% will cause blooming between 140 and 180 °C; at 200 °C, the concentration limit is 2.5%. P.R.12 is therefore only used in highly concentrated formulations, especially in full and related shades. Ketones and other aggressive solvents induce a change of the crystal modification, accompanied by a drastic colour change.
However, P.R.12 demonstrates a satisfactory degree of lightfastness. Air drying paints equal step 6 on the Blue Scale in full shade, while baking enamels reach step 6–7, but show some darkening. In white reductions (1:10 TiO2), the pigmented systems equal step 4–5 and 5–6, respectively. In baking enamels, P.R.12 bleeds considerably, irrespective of the temperature. The pigment is, however, occasionally used in emulsion paints wherever its fastness properties satisfy the specifications.
P.R.12 is also employed in a series of special applications, such as automotive cleaners, floor polish, shoe polish and so on, and it is frequently used to colour office articles and leather.
2.6.4.2.8 Pigment Red 13
Produced in China, this pigment is of limited commercial interest. Its colour is a bluish red.
2.6.4.2.9 Pigment Red 14
P.R.14 provides an intense, lightfast bordeaux shade, which is much yellower than that of P.R.12. Like P.R.12, it also possesses two crystal modifications. Again, the commercially available form is thermodynamically unstable, but, in contrast to P.R.12, conversion into the stable modification, which is technically less interesting because of its poor lightfastness, requires something of an effort. It is facilitated, for instance, by aromatic hydrocarbon and ketone solvents.
The main market for P.R.14 is in the paint industry, but the pigment is also used in printing inks and in some other applications.
Apart from air drying systems, P.R.14 is also used in baking enamels; however, it blooms beyond certain concentration limits. In an alkyd-melamine resin system, for instance, the pigment shows resistance to blooming up to 160 °C, independent of the concentration; the concentration limit at 180 °C is 0.2%, and at 200 °C it is 1%. Blooming is accompanied by considerable bleeding.
P.R.14 is very lightfast; its full shade lightfastness in air drying systems equals step 6–7 on the Blue Scale; in baking enamels, it reaches step 7–8. In white reductions (1:7 TiO2), the lightfastness of the same systems equals step 5–6 and 6–7, respectively.
Very lightfast red shades can be produced by combining P.R.14 with Molybdate Red. P.R.112 is a member of the same class of pigments, behaves similarly and is particularly suitable as a partner for P.R.14 in blends that approach the colours of Toluidine Red (P.R.3) but provide much improved lightfastness and weatherfastness.
In applications where the pigment is fast enough to solvents to satisfy the requirements, it may also be used for offset, packaging gravure and flexo printing inks. The resulting prints are soap, alkali and acid resistant. They are not completely fast to paraffin and quite sensitive to butter and several other fats. P.R.14 prints are not fast to clear lacquer coatings and may not be sterilized. Heat stability is less than 140 °C. 1/1 SD letterpress proof prints equal step 6 on the Blue Scale for lightfastness, 1/3 SD prints equal step 5–6 and 1/25 SD prints reach step 4.
2.6.4.2.10 Pigment Red 15
P.R.15 continues to be offered in the Japanese market, but is of limited commercial value. Its hue is a brilliant medium maroon.
2.6.4.2.11 Pigment Red 16
The bluish bordeaux crystal modification of this polymorphous pigment is produced in Japan and China. It is used in printing inks wherever tolerance to solvents is unimportant. Although the pigment exhibits average lightfastness, it fails to meet current industrial standards. The shade can be adjusted by tinting P.R.12, which is a member of the same pigment family.
2.6.4.2.12 Pigment Red 17
P.R.17 provides medium reddish shades. As a result of poor fastness properties, its commercial significance is somewhat limited and it is sold only in small volume. P.R.17 has the advantage of being fast to acid, alkali and soap. It is therefore used in offset, gravure and flexo printing inks wherever tolerance to alkali and soap is a major concern. Moreover, P.R.17 is also employed in connection with mass colouration and surface colouration of paper.
2.6.4.2.13 Pigment Red 18
P.R.18, a positional isomer of P.R.114, is currently unavailable in Europe. Production abroad, especially in Japan and Central America, is limited. P.R.18 produces shades of maroon, with properties that parallel those of other members of its class with somewhat poorer fastness levels. The pigment is registered in the USA as D&C Red No. 38.
2.6.4.2.14 Pigment Red 21
P.R.21 is produced and sold in the USA and in Japan. It affords yellowish shades of red. Since it fails to meet the increasingly stringent industrial requirements, P.R.21 is no longer extensively used.
2.6.4.2.15 Pigment Red 22
P.R.22 affords yellowish shades of red. Its commercial significance varies considerably with the region. Less common in Europe, the pigment maintains an important position in the USA and especially in Japan. Its main market is in textile printing, but it is also used throughout the graphics field, such as in offset and gravure printing, especially in NC-based inks. P.R.22 is advantageous where soap and alkali resistance are required. A pigment with high tinctorial strength, it is somewhat lighter than P.R.9 in high pigment area concentrations, while at lower pigment area concentrations it is yellower. Compared to P.R.2, P.R.22 is distinctly yellower and less lightfast; this tendency is even more pronounced in comparison with P.R.112. As opposed to Lake Red C pigments (P.R.53:1), P.R.22 is bluer to yellower, depending on the choice of printing ink, but its lightfastness is better by about 1 step on the Blue Scale.
The paint industry employs P.R.22 in air drying systems, in emulsion paints and occasionally in industrial finishes, although there is some danger of blooming, and the appropriate limit has to be observed. Again, P.R.22 is much less lightfast in these media than P.R.112. Areas of application include paper mass and surface colouration, coloured pencils, artists' colours and other purposes.
2.6.4.2.16 Pigment Red 23
P.R.23 provides bluish, dull red shades, yellower and much duller than P.R.146. Its commercial significance, like that of P.R.22, varies considerably with the region. Fastness to solvents and application performance are mostly inferior, which is also true for lightfastness: 1/1 SD letterpress proof prints equal step 3 on the Blue Scale, while P.R.146, at equal depth, reaches step 5. Commercial P.R.23 types usually have smaller particles than P.R.146; they are stronger and more transparent, but at the same time provide higher viscosity in ink. Inadequate solvent resistance and, as a result, a tendency to recrystallize is a disadvantage, especially in packaging gravure printing inks. Compared to P.R.146, P.R.23 will produce more opaque, weaker prints. The pigment lends itself to application in aqueous printing inks and NC-based inks. It is also used for textile printing.
P.R.23 is recommended for use in industrial paints, although this is one of the markets where it competes primarily with P.R.146. Despite being tinctorially stronger and somewhat yellower, P.R.23 is also less fast to overpainting than P.R.146 and is considerably less lightfast.
2.6.4.2.17 Rigment Red 95
P.R.95 produces a bluish red, a carmine.
The pigment is used for printing inks and paints, where it satisfies average requirements. 1/1 SD letterpress proof prints equal step 4–5 on the Blue Scale for lightfastness; the prints are not fast to butter and various fats. They are acid and alkali fast but not entirely so to soap. They are also not completely resistant to the DIN 16 524/1 standard solvent mixture. The pigment tolerates heat up to 180 °C for 10 min, but it is not entirely stable to calendering and sterilization, a feature that is typical of its class.
P.R.95 is suitable for various printing techniques. Poor migration resistance and insufficient fastness to plasticizers render the pigment unsuitable as a colourant in special gravure inks for plasticized PVC.
The paint industry employs P.R.95 primarily in various industrial finishes and in paints. Relatively lightfast in full shade, its fastness to light rapidly deteriorates upon reduction of the shade with TiO2. The 5% full shade finishes (medium-oil alkyd resin) equal step 6–7 on the Blue Scale; 1/3 SD coatings reach step 4–5, and 1/25 SD samples afford step 3. P.R.95 is also frequently used in blends with Molybdate Red. The commercial variety shows good transparency and is therefore suited to purposes such as metallic coatings and ‘hammertone finish'. These systems, however, do not satisfy the stringent requirements with regard to lightfastness; besides, they are not fast to overcoating. At high temperature and low pigment concentration, blooming is observed. Incorporated in an alkyd-melamine resin system, for instance, at a pigment concentration below 0.1%, blooming may be observed above 140 °C. P.R.95 is heat stable up to 140 °C for 30 min.
2.6.4.2.18 Pigment Red 112
P.R.112 produces highly brilliant medium red shades. Patented as early as 1939, it has only been marketed for a few decades. However, production has increased rapidly due to its superior application properties. Its main market is in printing inks, finishes and paints, but it is also used in various other areas.
The printing inks field employs P.R.112 primarily in letterpress and offset inks, in packaging gravure inks and in flexo inks. It is tinctorially very strong. At standard thickness of layer, 1/1 SD letterpress proof prints are formulated at 17% pigment concentration, but, in practice, the amount is often much higher. The prints show excellent lightfastness. 1/1 to 1/25 SD letterpress proof prints, which, after all, cover a wide range of pigment concentrations per surface area unit, uniformly equal step 5–6 or step 6 on the Blue Scale. The fastness to light increases with increasing pigment concentration, until it reaches step 6–7 on the Blue Scale (25% printing inks). The prints are fast to soap, although they do not completely tolerate paraffin and are only moderately fast to butter and other fats (step 3). They are also affected by clear lacquer coatings and sterilization. The pigment demonstrates poor heat stability; the prints do not withstand exposure to 140 °C.
P.R.112 is very lightfast on textiles; 1/1 to 1/3 SD (deep shade) prints equal step 7 on the Blue Scale. Exposure to dry heat at 150 and 180 °C has no effect. However, like other members of its class, P.R.112 fails the dry cleaning test with perchloroethylene. The pigment may not be brought into contact with PVC coatings, into which it bleeds.
In paints, P.R.112 produces a shade that is referred to as signal red. It may be used not only in air drying paints but also in baking enamels, provided appropriate conditions are maintained to avoid blooming. At baking temperatures between 140 and 160 °C, the concentration threshold is 0.1%; between 180 and 200 °C, the limit is 2.5%. However, the fastness to overcoating is unsatisfactory. Lightfastness and weatherfastness are excellent, even in white reductions. Air drying finishes in full shade equal step 7–8 on the Blue Scale, while baking enamels reach step 8. There is not much difference in white reduction: 1:10 reductions with TiO2 afford step 6–7 and step 7, respectively.
The shade of P.R.112 approaches that of Toluidine Red (P.R.3), which it may consequently replace in applications with demanding requirements. P.R.112 may also be combined with the bordeaux P.R.12, which behaves similarly, to provide a broad range of colours. It is somewhat bluer than P.R.9 and considerably more lightfast and weatherfast. Types that provide optimized flow are used particularly in opaque full shade finishes. Excellent fastness properties make P.R.112 suitable for high grade paints, such as automotive finishes and general industrial coatings. Electrophoretic painting is one of its special applications; the pigment is also found in emulsion paints, despite the disadvantage of comparatively poor fastness in exterior application.
Rigid PVC is one of the polymers that are sometimes coloured by P.R.112. Transparent systems (0.1% pigment) equal step 8 on the Blue Scale for lightfastness, while the lightfastness of white reductions is only step 5–6. The spin dyeing industry employs P.R.142 for viscose rayon and viscose cellulose, in which the pigment exhibits excellent lightfastness and performs, if not perfectly, then almost satisfactorily.
In addition, P.R.112 is also used in various special media and applications. It is encountered in aqueous wood stains, in which it may also be combined with yellow pigments, such as P.Y.83, or with violet colours, such as P.V.23, or with carbon black to produce shades of brown. In these media, its lightfastness equals approximately step 5 on the Blue Scale. However, the pigment bleeds when oversprayed by a white nitrocellulose combination paint; it may, though, be coated with a nitro or acid hardening varnish or a polyester varnish. The list of special applications includes office articles and artists' colours, such as pigmented felt-tip pen inks, watercolours and coloured pencils, poster paints, as well as cleaning agents and detergents. In paper mass colouration, optimum lightfastness is only realized in full shade.
2.6.4.2.19 Pigment Red 114
P.R.114, an isomer of P.R.18, is sold only in Japan, where it is of minor importance. It produces a bluish red shade, carmine. The pigment largely parallels P.R.22 in terms of application properties, especially in its fastness properties.
2.6.4.2.20 Pigment Red 119
Although it is considered a member of the Naphthol AS pigment series, the exact chemical constitution of P.R.119 has not yet been published. The pigment used to be marketed, but its production has recently been discontinued. It affords a brilliant yellowish red shade and is used throughout the paint and printing ink industry. A typical representative of group I Naphthol AS pigments as far as application performance goes, the pigment exhibits very good lightfastness in finishes. Incorporated in medium-oil alkyd varnishes, for instance, or in alkyd-melamine resin systems, the full shade lightfastness of a 5% formulation equals step 7–8 on the Blue Scale, although some darkening is observed. In white reduction, at 1/25 SD, the pigment still scores step 5 on the Blue Scale for lightfastness, in which it resembles the somewhat bluer P.R.112. Comparisons of the weatherfastness, however, are in favour of P.R.112.
P.R.119 is not resistant to overcoating; however, no blooming has been observed at low pigment concentrations and typical baking conditions. The pigment is also used in emulsion paints, although exterior application is not recommended.
Throughout the printing ink industry, P.R.119 is in direct competition with other members of the Naphthol AS pigment series. Its shade, for instance, closely resembles that of P.R.10, although it is somewhat weaker. Regarding fastness to light, P.R.119 scores ½ to 1 step less on the Blue Scale than P.R.112. P.R.119 is mainly used in flexo and wallpaper inks. It migrates in gravure inks printed on plasticized PVC, a typical feature of members of the Naphthol AS series. P.R.119 is resistant to clear lacquer coatings, may be sterilized and is highly suitable for metal deco printing. The pigment is heat stable up to 180 °C for 10 min. It is also found in crayons and artists' colours.
2.6.4.2.21 Pigment Red 136
Although P.R.136 is a Naphthol AS pigment, its exact chemical constitution remains to be published. The pigment is currently not being marketed. Two versions have been commercially available. The transparent type has a specific surface area of almost 70 m2 g−1 and shows a considerable degree of structural viscosity. Its shade is a cherry red. The bordeaux grade, on the other hand, has a specific surface area of about 25 m2 g−1, is much more opaque and exhibits a more favourable flow behaviour. It is used primarily in close to full shade colourations, for applications where hiding power is an issue. The shade of RAL 3004 is matched by adding appropriate amounts of TiO2.
Both types, however, enjoyed limited commercial success. They were found in paints, in which they are heat stable up to 160 °C. The fastness of the pigment to solvents is typical of a member of the Naphthol AS pigment series, which includes inadequate fastness to overcoating. At low pigment concentrations, high baking temperatures may induce blooming. The pigment will bloom, for instance, at 160 °C in an alkyd-melamine resin baking enamel formulated at less than 0.05% pigment concentration. Both P.R.136 types demonstrate good lightfastness and weatherfastness, but darken in full shade. The more opaque version is more lightfast and weatherfast and is recommended for automotive refinishes.
2.6.4.2.22 Pigment Red 148
A few years ago P.R.148 was withdrawn from the market. It used to be employed in printing inks, paints, coloured pencils and also for the spin dyeing of viscose rayon and viscose cellulose. Although P.R.148 is lightfast, its behaviour towards solvents and its corresponding fastness properties, such as migration resistance, fail to meet current industrial standards. The pigment is very heat stable, but shows a strong tendency to bloom, which precludes its use even in rigid PVC. It affords a very yellowish red or reddish orange shade, which may also be produced by appropriate blends of other Naphthol AS pigments.
2.6.4.2.23 Pigment Orange 22
A reddish orange pigment, P.O.22 is used for spin dyeing viscose rayon and viscose cellulose, for which it is sold in the form of a pigment preparation. Properties that have a bearing on textile printing and colouration, such as fastness to light, as well as fastness to wet and dry rubbing, dry cleaning, and bleaching with peroxide, are excellent.
2.6.4.2.24 Pigment Orange 24
P.O.24 is manufactured exclusively in Japan and enjoys only minor recognition. Its resistance to organic solvents is inferior to that of other Naphthol AS pigments. The pigment fails to meet most lightfastness requirements.
2.6.4.2.25 Pigment Brown 1
P.Br.1 is a neutral brown pigment of very good lightfastness. However, it has lost most of its importance in recent years; therefore, production has currently been discontinued. The pigment is not stable to organic solvents. Its main market is in printing inks wherever solvent resistance is not required. The prints are not fast to soap and butter, do not tolerate clear lacquer coatings, and cannot be calendered or sterilized without colour change. The 1/1 to 1/25 letterpress proof prints equal step 6–7 on the Blue Scale for lightfastness. In high grade prints, P.Br.1 is being displaced successively by newer pigments, such as P.Br.23 and 25, which have much better application properties and are calender and sterilization resistant. P.Br.1 is also falling prey to blends of orange, red and yellow pigments with carbon black, which show more acceptable performance.
The pigment is not used in paints. Rigid PVC is one of the few polymers in which it is employed. P.Br.1 is found in bottles, in which its transparent formulations (0.1%) equal step 7 on the Blue Scale for lightfastness, while opaque versions reach step 6–7.
P.Br.1 is also used in polystyrene, in which it produces an orange shade as it dissolves. P.Br.1 used to play a role in the spin dyeing of viscose rayon and viscose cellulose. The pigment continues to be employed in connection with textile printing, where it exhibits excellent lightfastness and durability. Its lightfastness in full shade (1/1 SD) equals step 8 on the Blue Scale, while in white reduction (1/6 SD) it reaches step 7. Although the pigment does not tolerate dry cleaning with tetrachloroethylene, other facets of its performance are excellent or almost excellent. The pigment also lends colour to coloured pencils.
2.6.4.3 Naphthol AS Pigments with Sulfonamide or Carbonamide Groups
2.6.4.3.1 Pigment Red 5
P.R.5 affords a bluish red shade, somewhat similar to that of P.R.8, although the former is much more lightfast. Its commercial significance and its primary use depend on the region: In Europe it is primarily employed in printing inks, while the American and Japanese markets mainly utilize the pigment in the paint field.
Compared to other members of its class, P.R.5 exhibits good fastness to solvents.
In the printing inks field, P.R.5 is in direct competition with the much more bluish P.R.146, which provides more brilliant prints and is considerably more solvent resistant. P.R.146, on the other hand, is less lightfast. P.R.5 letterpress proof prints score between step 6–7 and step 5 on the Blue Scale for lightfastness, depending on the depth of shade (1/1 to 1/25 SD). The corresponding values for P.R.146 are 5 and 4, respectively. Prints made from P.R.5 are fast to soap and resistant to clear lacquer coatings, but are not entirely stable to butter and paraffin wax.
Since the prints do not tolerate calendering, the pigment is primarily used in offset inks, as well as in packaging gravure inks and in flexo inks.
In finishes, P.R.5 does not bloom under normal processing conditions, but it bleeds in baking enamels. The pigment is lightfast in full shade and in white reductions. Full shade lightfastness equals step 7 on the Blue Scale, while some darkening is observed. At 1:6 reduction with TiO2, the lightfastness equals step 6 in air drying systems and step 7 in baking enamels. At 1:60 TiO2 reduction, the values are step 4–5 and step 5 on the Blue Scale, respectively. P.R.5 is generally used in industrial and in trade sales paints. It may be blended with Molybdate Orange to produce opaque shades of red.
Types containing comparatively large particles and therefore featuring very low specific surface areas produce duller shades; these varieties are used mainly in Japan. They are employed to formulate dark red shades for automotive finishes, in which they compete with more opaque types of P.R.170, which produce a cleaner shade, tolerate solvents better and are fast to overlacquering.
Owing to the disadvantage of comparatively poor migration resistance, P.R.5 is not used in plasticized PVC, but it can be applied in rigid PVC. Its lightfastness is excellent in this medium, transparent and opaque colourations (up to 0.01% pigment + 0.5% TiO2) equal step 7 and, respectively, step 6–7 on the Blue Scale. Dispersed pigment preparations are available for the mass colouration of viscose films as well as for spin dyeing of viscose rayon and viscose cellulose.
The pigment performs satisfactorily on textiles, it shows good lightfastness. Depending on the SD, its lightfastness equals step 5 to step 7 on the Blue Scale (1/12 and 1/1 SD).
P.R.5 also lends itself to various special applications. The list includes (decorative) cosmetics such as lipstick, eye shadow, powder, nail polish and others. Application in this area depends on special purity requirements, and the commercially available types are tested accordingly. The pigment is listed in the European Cosmetics List.
2.6.4.3.2 Pigment Red 31
The pigment enjoys regional significance on the North American Continent and in Japan, but has vanished from the European market. It provides bluish shades of red, bordeaux. Its principal application is in rubber, in which it shows good lightfastness. No bleeding into natural rubber or into the fabric backing is observed (Section 1.8.3.6). The pigmented products resist cold and boiling water, soap, detergent solutions, 5% aqueous acetic acid, and 50% aqueous ethanol.
In polystyrene (PS), polymethacrylate (PMA), unsaturated polyester resins or similar media, P.R.31 affords highly transparent colourations of medium red shades, which are used as automobile tail lights and for other signalling purposes. Its heat stability in PS and PMA extrusion products (5 min) is good up to about 280 °C. P.R.31 is stable to the usual peroxide catalysts. The pigment is lightfast (0.025% formulations in PMA, 1.5 mm thickness of layer: step 7 on the Blue Scale). P.R.31 is also used in textile printing.
2.6.4.3.3 Pigment Red 32
P.R.32 is made in Japan and South America and enjoys only limited local significance. Its fastness properties and application performance largely parallel those of the chemically related Naphthol AS pigment P.R.31.
2.6.4.3.4 Pigment Red 146
P.R.146 is a bluish red pigment with very good solvent resistance, even compared to other group II Naphthol AS pigments. It is primarily used in printing inks, coatings and paints.
The printing ink industry uses P.R.146 for letterpress and offset inks and also in packaging gravure and flexo printing inks. The pigment lends itself to various special applications: it is used, for instance, to print bank notes and securities. A certain tendency to migrate, however, precludes its use in print on plasticized PVC films.
P.R.146 is somewhat yellower than P.R.57:1; it therefore fails to match the standard magenta for three- and four-colour printing. However, P.R.146 prints have the advantage of good application performance (Section 1.6.2.3), corresponding to the above-mentioned solvent fastnesses.
The prints tolerate white spirit, dibutyl phthalate, butter, soap, alkali and acid. Moreover, they are also stable to clear lacquer coating; their fastness to sterilization, on the other hand, exceeds that of P.R.57:1, but is not perfect. Where sterilization fastness is required, P.R.146 is superseded by P.R.185, a benzimidazolone pigment, which is its colouristically closest neighbour.
As far as heat stability goes, P.R.146 retains its colour value for 10 min at 200 °C or for 30 min at 180 °C; it thus scores 20 °C higher than P.R.57:1. The 1/1 and 1/3 SD letterpress proof prints equal step 5 on the Blue Scale for lightfastness, while 1/25 SD specimens match step 4. P.R.146 is thus ½ to 1 step on the Blue Scale more lightfast than P.R.57:1. Prints made from commercially available types are semi-transparent.
P.R.146 also shows good lightfastness in textile printing. 1/1 SD prints equal step 7 on the Blue Scale, while 1/3 SD samples score step 6–7 and are therefore somewhat less lightfast than those of the yellower P.R.7, but superior to the likewise yellower P.R.170. Weatherfastness in print, however, is much inferior to that of P.R.7 and 170. P.R.146 prints are not entirely stable to dry cleaning and dry heat; they match step 4 on the 5 step evaluation scale.
P.R.146, which is also used in paints, is primarily applied in emulsion and architectural paints; it also lends colour to general industrial paints in applications where durability is unimportant.
In air drying systems, its full shade lightfastness equals step 5–6 on the Blue Scale; in baking enamels, the lightfastness equals step 6 and some darkening is observed. In white reduction, at 1:7 reduction with TiO2, however, fastness to light drops to step 3–4. The pigment thus satisfies most requirements for emulsion paints in interior application. As far as durability is concerned, full shade paints only score step 2 to 3 (Section 1.6.6) on the 5 step scale. P.R.146 exhibits high tinctorial strength and does not bloom in baking enamels. Its fastness to overcoating is much superior to that of group I Naphthol AS pigments, but it is not perfect. P.R.146 may be used in combination with Molybdate Orange pigments to produce bright opaque shades of red.
In polymers, P.R.146 is only used to colour rigid PVC. Transparent colourations (0.1%) afford a lightfastness that matches step 8 on the Blue Scale, while opaque specimens equal between step 6–7 and step 6, depending on the standard depth of shade and on the TiO2 content. Insufficient heat resistance in polyolefins (less than 200 °C) precludes its use in such media.
P.R.146 is a suitable candidate for various special applications. The list includes wood stains, in which it is frequently blended with yellow pigments, especially with P.Y.83, and also with black to afford shades of brown. The products are fast to overcoating and stable to nitro and acid catalysed and polyester varnishes. Intense shades match step 5 on the Blue Scale for lightfastness. Other areas of application include office articles and artists' colours, cleaning agents, paper mass colouration, laundry markers and so on. In connection with cosmetics, the pigment frequently lends colour to soaps.
2.6.4.3.5 Pigment Red 147
P.R.147 produces a very bluish, clean red, a pink. Its main field of application is in printing inks. Inks formulated at ca 15% pigment concentration afford the standard magenta for multicolour printing on the European Scale CIE 12-66. As far as fastness to solvents is concerned, the pigment may be compared to the yellower P.R.184 or to the even yellower P.R.146. The prints are correspondingly fast to soap, paraffin, dibutyl phthalate, white spirit and toluene, but are not entirely fast to butter and other fats. They are suitable for clear lacquer coatings, but may not be sterilized. The heat stability of the pigment equals 200 °C for 10 min or 190 °C for 30 min, which is considered excellent.
Compared to P.R.146, lower pigment area concentrations of P.R.147 are less lightfast. 1/1 SD letterpress proof prints equal step 5 on the Blue Scale for lightfastness, and 1/3 SD specimens match step 4, while 1/25 SD prints are equal to step 3. P.R.147 is used in offset inks, in packaging gravure printing inks and in various flexo inks. Like other Naphthol AS pigments, P.R.147 is also employed in a series of special applications.
2.6.4.3.6 Pigment Red 150
P.R.150 is of limited regional significance. Depending on the area of application and on the reduction ratio, the pigment affords shades from bluish purple to carmine. P.R.150 is used in textile printing. In PVC colouration, which used to be its main market, it has been superseded by other products. P.R.213 is identical to P.R.150.
2.6.4.3.7 Pigment Red 164
This dihydrazone pigment on a Naphthol AS basis provides a somewhat dull, yellowish red shade of little strength. P.Y.164 was withdrawn from the market a few years ago. Used in printing inks, paints and plastics, the pigment was produced only in small volume. Lack of lightfastness generally precluded its use in exterior application. Incorporated in air drying alkyd paint, its full shade lightfastness (5%) equals step 6–7 on the Blue Scale, while addition of TiO2 (1:5 TiO2) reduces this value to 5–6.
P.R.164 is heat stable up to 200 °C, which made it a suitable candidate for applications where good temperature resistance is required. At baking temperatures of 140–160 °C and higher, the fastness to overcoating begins to decrease.
Letterpress proof prints with 30% P.R.164 in the inks reach step 5–6 on the Blue Scale for lightfastness. The prints are inert to paraffin and butter, as well as to various other fats, but they do not tolerate acid and alkali. The same is true for soap. P.R.164, however, is stable to sterilization.
Good heat stability made P.R.164 an interesting colourant for a series of polymers. It was being worked into polystyrene, in which full shades retain their colour value up to 270 °C before turning yellow; white reductions are stable up to 250 °C. The pigment shows average lightfastness. P.R.164 also lends itself to the colouration of rigid PVC. Its heat stability in PE is only 200–220 °C. A tendency to bloom made it necessary to observe certain concentration limits.
P.R.164 was also found in cast resin composed of methacrylate and unsaturated polyester. The pigment does not affect the hardening process of such media, which may be carried out, for instance, by using peroxides. An important field of application was in the colouration of various polyurethanes, for which the pigment was also sold in the form of a pigment preparation.
2.6.4.3.8 Pigment Red 170
P.R.170 provides medium shades of red, which in tints are somewhat bluish. Developed in the 1960s, excellence in application performance soon made this one of the most recognized pigments.
Like some other Naphthol AS pigments, P.R.170 is polymorphous (α-, β- and γ-phases); the shades of the commercial β- and γ-modifications are within the same range of colours. The α-phase is obtained from synthesis; finishing in water results in the β- or γ- phases.
Commercially available types, which are made from the β- or γ- phases, differ primarily in terms of opacity. The more transparent version (β-phase) is also somewhat more bluish. The very opaque modification (γ-phase) is much more stable to a variety of agents than the more transparent type. The opaque type is, for instance, slightly more resistant to organic solvents than the transparent one. Notably, however, even transparent varieties are very resistant to solvents, compared to other members of this class of pigments. They are not affected by white spirit and are largely inert to alcohol, esters, xylene and other solvents. The stability of the more transparent version to ethyl-glycol and methyl ethyl ketone equals step 3 on the 5 step scale, while the opaque variety matches step 4.
As a result of its excellent fastness properties, P.R.170 is used in high grade industrial paints. The pigment lends colour to finishes for tools, to implements, agricultural machinery, and commercial vehicles; the opaque varieties are also used for automotive finishes, such as automotive refinishes. Thorough testing is necessary before a product can be used in original automotive finishes, for which full shades of P.R.170 are sometimes employed.
P.R.170 does not bloom in stoving enamels, but it does bleed. Opaque varieties show better overcoating fastness than the more transparent ones. Very pure shades of red, which exhibit good hiding power, are produced by blending P.R.170 with Molybdate Orange. Combinations with quinacridone pigments afford bluer red shades. P.R.170 may, as a result of its excellent lightfastness and durability, be incorporated into high grade systems. The standard transparent type, for instance, equals step 6 on the Blue Scale in full shade, while the opaque version matches step 7–8 for lightfastness. Full shades show some darkening upon exposure to light and weathering. Opaque types have been introduced to the market which are characterized by noticeably improved weatherfastness compared to previously available grades. P.R.170 is less costly than similarly coloured perylene tetracarbonic acid pigments or diketopyrrolopyrrole pigments such as P.R.254, which are completely fast to overpainting and also more weatherfast. The opaque versions exhibit good flow behaviour, which makes it possible to enhance the hiding power of a product by increasing the pigment concentration without affecting the gloss.
The transparent type is preferred for printing inks. It is tinctorially very strong and lends itself to use in various high grade formulations. Inks formulated at only 15% pigment concentration will under standardized conditions produce 1/1 SD letterpress proof prints; the resulting prints are very lightfast. 1/1 to 1/3 SD letterpress samples equal step 6 on the Blue Scale. The opaque, yellower and somewhat weaker version is more lightfast than the transparent one; the difference is about 1/2 step on the Blue Scale.
P.R.170 and 5 are closely related as far as hue is concerned: 1/1 SD prints made from the bluish crystal modification (β-phase) resemble each other colouristically. The fact that P.R.5, depending on the depth of shade, is more lightfast by ½ to 1 step on the Blue Scale is somewhat compromised by its considerably inferior fastness in application and by its appreciably lower tinctorial strength. The slightly bluer P.R.8 is much less lightfast and solvent resistant than P.R.170; prints made from P.R.170 also have the advantage of being fast to butter, soap, alkali, and acid. Fastness to clear lacquer coatings and stability to sterilization are almost perfect (step 4–5). P.R.170 is also very heat stable; it retains its colour strength for 10 min at 200 °C or for 30 min at 180 °C, which makes it a valuable pigment for metal deco printing.
P.R.170 is also found in decorative printing inks for polyester films. It is almost completely inert to styrene monomer; in this respect, it matches step 4–5 on a 5 step scale. Gravure prints made from 8% pigment formulations almost equal step 7 on the Blue Scale, which indicates excellent lightfastness. There is no danger of plate-out during the production of laminated films (Section 1.6.4.1). Another area of application is in textile printing, in which the pigment shows very good fastness properties to a number of agents. It is almost completely fast to dry cleaning with tetrachloroethylene or white spirit, to washing with peroxide at 95 °C, and to alkali.
The plastics industry uses P.R.170 almost exclusively to colour rigid PVC. Transparent variations (0.1%) equal step 8 on the Blue Scale for lightfastness, while opaque types (1/1 to 1/25 SD) equal step 8 to step 6–7, depending on depth of shade and reduction with TiO2. In plasticized PVC, the pigment is also very lightfast, although it does tend to bleed. At low pigment concentrations, less than 0.01% pigment, P.R.170 may not be used in plasticized PVC. However, it is suitable for printing on films made of plasticized PVC or on PVC or PUR simulated leather, such as in automobiles.
P.R.170 is not always heat stable enough to allow application in polyolefins. In HDPE systems formulated at 1/3 SD, the pigment tolerates exposure to 220–240 °C for one minute. Its tinctorial strength, on the other hand, is excellent. P.R.170 is also occasionally used in polypropylene and polyacrylonitrile spin dyeing; in the latter medium, it satisfies the specifications of the clothing and home textiles industries. Besides, P.R.170 lends colour to viscose rayon and viscose cellulose; it is used for the mass colouration of semisynthetic fibres made of cellulose; last but not least, it colours yarns, fibres, and films made of secondary acetate.
P.R.170 is broad in scope. It is found in wood stains, including solvent-based stains; it is blended with carbon black and yellows to produce various interesting shades of brown. The colourations are fast to overcoating in these media and resist nitro and acid hardening varnishes and polyester coatings. Its lightfastness in these media equals step 7 on the Blue Scale.
2.6.4.3.9 Pigment Red 184
P.R.184 affords a red which is somewhat on the bluish side of P.R.146, to which it is closely related in terms of chemical constitution. Both products also behave very similarly in application. Their prints are fast to soap, butter, paraffin, dibutyl phthalate, white spirit, and toluene. P.R.184 produces a shade which matches that of the standard magenta for multicolour printing on the European Colour Scale CIE 12-66. This shade results from formulating an ink at 15% pigment concentration and printing the ink in a standard layer (1 µm).
P.R.184 is used especially in applications where P.R.57:1 fails the requirements for alkali, acid, or soap fastness, or where the pigment lake is not lightfast enough to satisfy the demand. Depending on the standard depth of shade, the lightfastness of P.R.184 exceeds that of P.R.57:1 by approximately one step on the Blue Scale. Excellent fastness to solvents makes P.R.184 a suitable candidate for letterpress and offset application, as well as for packaging gravure printing inks and flexo inks. Its metal deco prints tolerate clear lacquer coatings, but not sterilization. The pigment is heat stable up to 170 °C for 10 min or up to 160 °C for 30 min. P.R.184 is also employed in rubber.
2.6.4.3.10 Pigment Red 187
Two crystal modifications are known, which differ considerably in terms of shade and fastness properties. Only the bluish red form continues to be commercially available. Its high specific surface area (about 75 m2 g−1) makes it a transparent pigment.
The main market for P.R.187 is in the plastics field. The pigment does not migrate in plasticized PVC and shows very good lightfastness: depending on the depth of shade and on the amount of TiO2, it equals step 6 to step 8 on the Blue Scale. However, in PVC its white reductions are frequently replaced by the yellower and brighter benzimidazolone pigment P.R.208. P.R.187 exhibits high heat stability in polyolefins; in HDPE, 1/3 SD samples, reduced with 1% TiO2, retain their colour upon exposure to 250 °C for 5 min; in LDPE, the temperature may be raised to 270 °C, and the heat stability of 1/25 SD colourations is 10 °C higher. The shrinkage of the polymer is only slightly affected by the pigment. As a result, P.R.187 may be used in polyolefin extrusion products which do not show rotational symmetry, such as bottle crates. The pigment exhibits equally satisfactory lightfastness in polyolefins: at 1/3 SD, transparent and opaque versions equal step 7 on the Blue Scale.
Good thermostability is an asset in media like polystyrene. P.R.187 is a very interesting product for polypropylene spin dyeing, for which it is available as a special preparation. 1/3 SD colourations tolerate temperatures up to 290 °C for 5 min. The pigment is also very lightfast in polyacrylonitrile spin dyeing: 1/3 SD specimens equal step 6–7 on the Blue Scale. Dry and wet crocking fastness is satisfactory and makes it a suitable pigment for home textiles, such as upholstery and carpeting.
The printing industry uses P.R.187 inks for all printing techniques. Although its shade is somewhat more bluish, its lightfastness in print equals that of P.R.208, which is stronger and also much less transparent. The thermostability of P.R.187 is about 20 °C higher: it is stable at 220 °C for 10 min and at 200 °C for 30 min. The pigment is very fast to chemicals and to clear lacquer coatings and may be sterilized, which makes it a useful transparent product for metal deco printing. Likewise, P.R.187 offers some advantage in paper lamination, that is, in decorative prints. Fastness to light and other fastness properties in compression moulded melamine sheets are comparable. The pigment is also used to colour polyester films.
In paints, P.R.187 is fast to overcoating, even at high baking temperatures. This is another application in which high thermostability is an asset, which is especially true for coil coating (Section 1.8.2.2). The pigment lends colour to industrial paints in general; its high transparency facilitates application in transparent paints, such as in films or bicycle paints, and in metallic finishes. P.R.187 tolerates exposure to light very well; incorporated in an alkyd-melamine system, full shades equal step 7–8 on the Blue Scale, while 1:600 TiO2 reductions still reach step 6–7. Pigment weatherfastness is equally excellent, which makes P.R.187 a suitable candidate for automotive refinishes. The fact that the pigment is chemically inert makes it an interesting product for application in various powder coatings, such as amine accelerated epoxy powders. The list of applications also includes artists' colours, especially wax colours.
2.6.4.3.11 Pigment Red 188
P.R.188 is an intense yellowish red pigment with very good fastness properties.
Its main fields of application are in printing inks and paints; the pigment is suited to all printing techniques. 1/1 to 1/25 SD letterpress proof prints equal step 5–6 to step 5 on the Blue Scale for lightfastness. The pigment shows relatively poor tinctorial strength. However, the prints are very fast to organic solvents, fats, paraffin, soap, alkali, and acid. They are also fast to clear lacquer coatings and may be sterilized. Fastness to the DIN 16 524/1 solvent mixture, however, is not perfect. Regarding heat stability, P.R.188 prints tolerate exposure to up to 220 °C for 10 min or to 180 °C for 30 min. Similarly shaded but somewhat duller Toluidine Red pigments are less lightfast by one step on the Blue Scale, and they are considerably inferior in terms of solvent and heat stability, as well as other special application requirements.
The paints industry uses P.R.188 for its excellent fastness properties in high grade industrial finishes. Although some darkening is observed upon exposure to light, its full shades equal step 7 on the Blue Scale for lightfastness. TiO2 reductions up to 1:5 exhibit the same fastness to light. Pigment weatherfastness is equally excellent in full shade and related deep shades. The pigment does not bleed at baking temperatures of 160 °C or less, and it retains its colour in a coating up to 200 °C. P.R.188 is frequently found in decorative paints, an area in which it is used to a great extent in the USA.
A highly opaque P.R.188 grade has been introduced to the market. It provides brilliant, still yellower shades of red than the previously known types. It also shows improved lightfastness and weatherfastness, as well as very good rheological properties and high gloss, compared to these types. The pigment is mainly used to produce lead-free, clean shades of red in automotive and industrial paints.
In plasticized PVC systems, P.R.188 blooms at low concentrations, which is also true for rigid PVC. Its thermostability in polyolefins is only about 220 °C, which makes it an unsuitable pigment for most polymers. However, the pigment is used to some extent in PVC and PUR plastisols.
P.R.188 is also employed in paper mass colouration, paper surface colouration, paper pulp, and paper spread-coating formulations, as well as in wallpaper and wax crayons.
2.6.4.3.12 Pigment Red 210
P.R.210, a mixed coupling of P.R.170 and P.R.266, is used primarily in printing inks. Its hue is considerably bluer than that of P.R.170.
P.R.210 is inferior to P.R.170 in various aspects of pigment performance. 1/3 SD and 1/25 SD letterpress proof prints, for instance, score ½ to 1 step less on the Blue Scale for lightfastness. In contrast to P.R.170, which is almost entirely fast to clear lacquer coatings and sterilization, P.R.210 is very sensitive in this respect. The two pigments behave similarly in print; P.R.210 prints are equally fast to several organic solvents, paraffin, soap, alkali, and acid. They are also heat stable up to 200 °C.
Besides printing inks, P.R.210 is primarily found in aquarelle colours.
2.6.4.3.13 Pigment Red 212
A few years ago the pigment was withdrawn from the market. P.R.212 was rarely sold outside Japan. It produces considerably bluish shades of red, which might be considered pink. Compared to the similarly coloured quinacridone pigment P.R.122, P.R.212 is duller, weaker, and less fast. 1/3 SD letterpress proof prints, for instance, equal step 2–3 on the Blue Scale for lightfastness; while prints containing P.R.122 equal step 6–7. P.R.212 is also less lightfast than the somewhat more bluish and duller benzimidazolone pigment P.V.32, a colouristically related maroon; the difference is 2–3 steps on the Blue Scale.
P.R.212 was used throughout the graphics industry and for textile printing, as well as in specialized media, such as coloured pencils.
2.6.4.3.14 Pigment Red 213
P.R.213 is a product rarely encountered outside Japan.
The pigment affords very bluish shades of red, which are too much on the blue side to match the standard magenta for three- and four-colour printing. The commercially available type is considerably opaque but not very lightfast compared to other Naphthol AS pigments. 1/3 SD letterpress proof prints, for instance, equal only step 3–4 on the Blue Scale for lightfastness; while 1/25 SD specimens match step 3. P.R.150 is identical with P.R.213.
2.6.4.3.15 Pigment Red 222
Since recently the pigment is not offered to the market anymore, but it is still in use. It affords a bluish red shade, which may be considered magenta or ruby; it is very transparent.
Its main market was in printing inks, where it was used in three and four colour printing. P.R.222 is heat stable up to 180 °C, which made it a suitable candidate for metal deco printing. It also tolerates organic solvents well. Prints containing P.R.222 are fast to overcoating but may not be sterilized. Gravure prints on plasticized PVC films show a slight tendency to migrate.
The plastics industry uses P.R.222 primarily in polyurethane. The pigment exhibits average tinctorial strength. 1/3 SD colourations in HDPE, for instance (1% TiO2), are formulated at 0.23% pigment concentration.
P.R.222 is heat stable up to 240 °C; above this temperature, the colour shifts appreciably towards the bluish side of the spectrum. P.R.222 bleeds in plasticized PVC.
2.6.4.3.16 Pigment Red 223
P.R.223 used to have some commercial importance before it was withdrawn from the market. The pigment produces a bluish red shade, bluer even than P.R.170. Its main market was in high grade industrial finishes. Good lightfastness and durability in full shades and the fact that it met certain specifications made it suitable for use on public transportation vehicles. However, the pigment is not fast to overpainting.
2.6.4.3.17 Pigment Red 238
This pigment, which was introduced to the market a few years ago, enjoys only limited regional importance; its chemical constitution is identical with Pigment Red 269. It is recommended for printing inks and for industrial paints. Its shade in print is much bluer than the CIE 12-66 standard magenta for three colour printing on the European Colour Scale (Section 1.8.1.1). A pigment of low tinctorial strength, P.R.238 is neither fast to clear lacquer coatings, nor does it tolerate sterilization. In paints, P.R.238 is of average tinctorial strength and it bleeds.
The pigment is recommended for textile printing.
2.6.4.3.18 Pigment Red 245
P.R.245 is of little commercial significance and is rarely encountered in Europe. It provides bluish shades of red, ruby, and carmine. P.R.245 is used in printing inks, particularly in packaging inks. Suitable inks contain nitrocellulose polyamide-based polymers or VC/VAc copolymers; in these media, the pigment exhibits high tinctorial strength, but only average to poor lightfastness.
2.6.4.3.19 Pigment Red 253
P.R.253, which was introduced to the market a few years ago, provides medium red shades, which are considerably yellower than those of P.R.170. The commercial grade of P.R.253 exhibits good transparency. The pigment is recommended especially for use in paints.
Its fastness to organic solvents and chemicals corresponds to that of other representatives of group II Naphthol AS pigments (Section 2.6.2). Consequently, P.R.253 is almost completely fast to overpainting.
In full shade and in similarly deep shades, P.R.253 shows good lightfastness and weatherfastness with some darkening. White reductions with TiO2, however, exhibit noticeably less weatherfastness.
P.R.253 is used in packaging printing inks. For a pigment of its class, it shows medium tinctorial strength. The prints demonstrate good fastness to organic solvents, but they are not completely fast to the DIN 16524 solvent mixture (Section 1.6.2.1) (step 4–5). 1/1 SD letterpress proof prints show a lightfastness corresponding to step 5 on the Blue Scale.
2.6.4.3.20 Pigment Red 256
P.R.256 is used especially for industrial and decorative paints in applications where fastness to overcoating is not required. The pigment provides a yellowish red shade, a scarlet. Compared to other pigments covering the same range of shades and fastness properties, P.R.256 shows medium tinctorial strength. It exhibits good lightfastness; its full shade and similarly deep shades reach step 7 on the Blue Scale. Similar values are found for reduced shades with TiO2, up to a ratio of approximately 1 : 4.
P.R.256 is also recommended for paste, solvent and water based packaging inks, as well as for wall coverings. It exhibits limited migration- and heat-resistance and is therefore not recommended for tin printing.
2.6.4.3.21 Pigment Red 258
This pigment was reported to the Colour Index by a Japanese producer. The chemical constitution has been disclosed. P.R.258 has not yet been introduced into the market.
2.6.4.3.22 Pigment Red 261
A few years ago P.R.261, together with its chemical constitution, was reported to the Colour Index by an American manufacturer. It has not yet been met in the market.
2.6.4.3.23 Pigment Red 266
P.R.266 is a product affording a bluish red shade. The pigment is primarily used in packaging gravure and flexo printing inks, for which a special grade is offered. Its hue is considerably bluer than that of P.R.170 and bluer than that of P.R.210. In various aspects of pigment performance P.R.266 is inferior to P.R.170. So, for instance, the lightfastness of proof prints is ½ to 1 step less on the Blue Scale. It is sensitive to clear lacquer coatings and sterilization. In other application properties the commercially available grades of P.R.170 and P.R.210 behave very similar. Tinctorial strength, transparency and gloss of the prints of both pigments are comparable as well as the fastness to organic solvents used for printing inks, and to paraffin, soap, alkali and acid.
2.6.4.3.24 Pigment Red 267
The pigment provides slightly dull yellowish red shades. Commercially available grades of P.R.267 have as yet not been met on the market. In Japan the pigment is primarily recommended for the use in printing inks.
2.6.4.3.25 Pigment Red 268
The pigment is recommended in the USA for printing inks. It provides bluish shades of red.
2.6.4.3.26 Pigment Red 269
As for P.R.268 the pigment is recommended for printing inks. In prints it provides a very bluish red.
2.6.4.3.27 Pigment Orange 38
P.O.38, a clear yellowish red, is mainly used in printing inks and in plastics.
In printing inks, P.O.38 shows good fastness properties. 1/1 SD to 1/25 SD letterpress proof prints, for example, equal step 6 to step 5 on the Blue Scale for lightfastness, depending on the depth of shade. The prints are stable to the standard DIN 16 524 solvent mixture, to paraffin, fats, oils, soap, alkali, and acid; they also tolerate clear lacquer coatings and sterilization. They are heat stable up to 220 °C for 10 min or to 200 °C for 30 min. P.O.38 is therefore suitable for offset printing inks as well as for metal deco printing; it is used in special printing inks for substrates such as plasticized PVC and in various flexo inks.
Besides, P.O.38 lends colour to decorative inks for laminated sheets. Inks formulated at a pigment concentration of 8% and printed in 20 µm cell melamine sheets equal step 6–7 on the Blue Scale for lightfastness; at 40 µm, they match step 7. Laminated sheets do not exhibit plate-out.
P.O.38 is stable to styrene monomer, but not entirely fast to acetone, which occasionally leads to bleeding if the pigment is processed according to the diallyl phthalate method (Section 1.8.1.2). This is also true if the pigment is treated with a melamine resin solution.
The plastics industry uses P.O.38 primarily to colour PVC, polyolefins, and polystyrene. In plasticized PVC, the pigment does not bleed at concentrations up to about 0.3%. Its lightfastness is good. Transparent colourations in rigid PVC(0.1%) equal step 7–8 on the Blue Scale; while opaque versions (0.1% pigment + 0.5% TiO2) equal step 7.
P.O.38 is frequently employed in combination with P.Y.83 to provide brilliant and very lightfast shades of orange. P.O.38 is particularly useful to produce shades of brown in PVC and PUR imitation leather. 1/3 SD HDPE formulations containing 1% TiO2 withstand temperatures up to 240 °C for 5 min. At this temperature, the pigment does not noticeably affect the polymer shrinkage. Transparent specimens equal step 6 on the Blue Scale in terms of lightfastness; while opaque versions equal step 5–6.
P.O.38 is also used in spin dyeing, where it colours filament, fibres, and films made of secondary acetate. The pigment possesses excellent fastness properties in this medium.
P.O.38 is especially lightfast in unsaturated polyester resins. Transparent samples equal step 8 on the Blue Scale, opaque versions (0.1% pigment–0.5% TiO2) equal step 7; however, notably, the hardening of the resin is slowed down considerably.
As far as the paints field is concerned, P.O.38 is solvent resistant enough to qualify for application in high grade industrial paints wherever lightfastness and durability requirements are not too stringent. The lightfastness of close to full strength shades in an alkyd-melamine resin system, for instance, equals step 6–7 on the Blue Scale (1:1 TiO2); further addition of white pigment reduces the lightfastness to step 5–6. The pigment does not bloom; however, it ceases to be completely fast to overcoating as the baking temperatures rise. It is heat stable up to 180 °C.
P.O.38 is broad in scope. The list of applications includes special media, such as wax crayons, artists' colours, and wood stains, including those that are solvent based. The products are very lightfast (step 7 on the Blue Scale) and fast to overcoating. Blends of P.O.38 with yellow pigments, such as P.Y.83 or P.Y.120, or with carbon black produce useful shades of brown.
2.6.4.3.28 Pigment Orange 74
P.O.74 [76] is recommended for the paint industry. In full shades and in tints it exhibits clean reddish orange shades, with good tinctorial strength in white reductions. The commercial grade shows good hiding power. The viscosity of the mill base is rather high, P.O.74 tends to flocculate.
The pigment is not resistant to different organic solvents, thus coatings, for instance based on alkyd melamine resin systems, are not fast to overpainting.
In printing inks P.O.74 exhibits also a clean reddish orange shade with good light and weather fastness properties. The semi-transparent version is recommended for paste inks as well as for solvent- and water-based packaging gravure and flexographic printing inks. The high opaque version is recommended for printing on special substrates, for example Kraft paper or for packaging inks where very good light fastness properties are required.
2.6.4.3.29 Pigment Violet 13
The marketing of this pigment has recently been discontinued. P.V.13 produces clean shades of violet. Compared to other group I Naphthol AS pigments, P.V.13 shows poor general fastness properties. 1/1 SD letterpress proof prints only equal step 3 on the Blue Scale for lightfastness, while 1/3 SD specimens reach step 2. The prints tolerate acid but not alkali or soap. Similarly, they are not fast to overlacquering and may not be sterilized, which renders the pigment unsuitable for metal deco printing and for printing on PVC or polyolefin foils.
2.6.4.3.30 Pigment Violet 25
P.V.25 is only produced in Japan and enjoys limited regional significance. It is used in printing inks.
2.6.4.3.31 Pigment Violet 44
A Japanese product like P.V.50, the pigment is registered in the Colour Index as P.V.44. The two pigments are extremely similar, both colouristically and in terms of performance in application, even in detail. It is very likely that the products are chemically identical and listed twice in the Colour Index, although exhibiting different CAS numbers.
2.6.4.3.32 Pigment Violet 50
P.V.50, produced in Japan, is only of limited regional importance, since it fails to satisfy the increasingly stringent application requirements for organic pigments. Its fastness to light, for instance, is particularly poor: 1/3 SD and 1/1 SD letterpress proof prints equal only step 2 on the Blue Scale. Compared to P.V.23, Dioxazine Violet, P.V.50 is redder at greater depth of shade, while samples which are further reduced with TiO2 appear bluer and considerably duller. P.V.50 is tinctorially weaker than P.V.23, which in some media is as much as twice as strong as the former.
P.V.50 is used in printing inks and in office articles. Poor lightfastness and a strong tendency to migrate makes it an inadequate product for most plastics materials. Lack of lightfastness also precludes its use in paints (see also P.V.44).
2.6.4.3.33 Pigment Blue 25
P.Bl.25 is only produced in small volume in Europe, Japan, and the USA. It is used for the spin dyeing of secondary acetate, lends colour to rubber, and is found in inks for packaging purposes.
Its hue is a somewhat reddish navy blue, which varies considerably if the pigment is chemically modified.
P.Bl.25 is very fast in application; it is fast to fats, oils, soap, and paraffin, which makes it a suitable candidate for packaging inks. Its lightfastness, however, is not excellent. In natural rubber, P.Bl.25 tolerates curing very well, and it bleeds neither into the rubber nor into the fabric backing (Section 1.8.3.6). In rubber, the pigment is fast to cold and hot water, to soap, soda, and alkali solutions, and to acetic acid.
In spin dyed secondary acetate threads, fibres and films, P.Bl.25 exhibits good textile fastness properties; the only problem is a certain lack of fastness to bleaching with sodium hypochlorite (Section 1.6.2.4). Its fastness to light in 0.1% spin dyed specimens equals step 3–4 on the Blue Scale, while 1% samples equal step 5.
2.7 Red Hydrazone Pigment Lakes (Formerly Called Red Azo Pigment Lakes)
The term refers to hydrazone colourants bearing sulfonic and/or carboxylic acid functions, which are used as pigments after being rendered insoluble by conversion into insoluble alkali earth or manganese salts.
Red hydrazone pigment lakes may be classified according to the coupling component. There are four industrially important groups of coupling components:
- β-naphthol,
- 2-hydroxy-3-naphthoic acid (BONA),
- Naphthol AS derivatives,
- naphthalene sulfonic acid derivatives.
If β-naphthol is used as a coupling component, the diazo component must be substituted with sulfonic or carboxylic acid groups. Commercially important pigments are frequently produced by diazotization with sulfonic acid derivatives.
The red hydrazone pigment lakes have stimulated considerable technical interest. In contrast, the corresponding yellow pigments, which are based on acetoacetanilides or on pyrazolones (Sections 2.3.1.2 and 2.3.4), are less important.
2.7.1 β-Naphthol Pigment Lakes
The development of hydrazone pigment lakes was initiated by the discovery of ‘Lithol Red' by Julius (BASF) in 1899. Lithol Red, which is synthesized by an indirect diazotization procedure using 2-naphthylamine-1-sulfonic acid as a diazonium compound, was initially employed in the form of its calcium and barium salts, which were precipitated onto inorganic carrier materials. The pigment was used in its pure form after it became apparent that the carriers contribute very little to the application properties of the product. Lithol Red is one of the earliest colourants developed specifically for application as pigment.
Lithol Red was followed by Lake Red C pigments, which were discovered by Meister Lucius & Brüning, later Hoechst AG in 1902. β-Naphthol pigment lakes have lost much of their importance, although there are still some types that maintain a very important position in the market.
2.7.1.1 Chemistry, Manufacture and Crystal Structures
β-Naphthol-based red hydrazone pigment lakes are characterized by the following general structure:

In commercially important pigments, A typically stands for a benzene or a naphthalene ring, RD = Cl, CH3, C2H5, COOM, n = 0–2, and M is usually an alkaline earth metal, sometimes a manganese, aluminium, or sodium atom.
The pigments are manufactured by first diazotizing an aromatic aminosulfonic acid by the indirect method and subsequently coupling the product to a suspension of β-naphthol. The latter is prepared by precipitating the β-naphthol sodium salt with dilute acid, such as acetic acid. The coupling initially affords the usually water soluble sodium salt of the respective hydrazone dye. Addition of a suitable alkaline earth salt, such as calcium, barium, or strontium chloride, or manganese(II) sulfate, exchanges the metal ion, that is precipitates the insoluble alkaline earth or manganese salt as a pigment. The laking conditions must be precisely specified to afford the desired crystal modification and particle size distribution.
In some cases the water solubility of the sodium compounds is rather low. For this reason some of the sodium salts, such as P.R.49 or P.R.53 are even used as inexpensive pigments.
Studies of the metal-exchange process of P.R.49 (Na) to P.R.49:1 (Ba) [77] revealed that the process, apart from the temperature, is not only influenced by the crystal structure and the concentration of the barium ions but also by the amount of rosin soap. Colophony-based rosin which is assumed to act as a surfactant, is converted during the laking process into colourless insoluble rosinate salts. These salts are incorporated in the pigment till up to 30% by weight without a loss of tinctorial strength. Very often the colour strength is even increased, accompanied by a colour shift to more bluish reds.
The specific influence of the rosin is suggested to happen due to an increased solubility of the sodium compound by micellization and to the formation of less stable mixed crystal-type lakes [78].
In the past it used to be quite common for customers of the pigment industry, especially for printing ink manufacturers throughout Western Europe, to be supplied with the sodium salts of the sulfonated β-naphthol compounds. Pigment formation, for example precipitation was then typically carried out by the printing ink manufacturer.
Many laked pigments contain water molecules in their crystal lattice, that is, they are actually hydrates. The water molecules can be bonded very tightly, and the corresponding hydrates may be stable up to temperatures of more than 200 °C.
Laked pigments generally exhibit different crystal phases, which differ not only in the arrangement of molecules and ions in the solid state, but also in the number of water molecules in the lattice. Additionally, laked pigments may form solvates which contain solvent molecules (e.g. alcohols, DMSO, DMF, NMP) in the crystal lattice. In contrast to the hydrates, such solvates are not used commercially.
P.R.53:1, a barium lake, is usually sold in its red α-phase. The β-phase, which is of less importance, is considerably more yellowish [79]. For the strontium lake P.R.53:3 at least six different crystal phases are claimed by patents, whereof two modifications are described as red and two as yellowish-red [80]. The calcium lake, P.R.53:2 exists in at least 15 different crystal phases, but this number includes a few solvent-containing phases [81–83].
Crystal structure determinations of laked pigments frequently face the problem that single-crystals cannot be grown. Consequently, several structures were determined from X-ray powder data. Even electron diffraction was used: The crystal structure of the ζ-phase of P.R.53:2 was solved by T. Gorelik from electron diffraction data using crystals with a size of approximately only 100 nm (Figure 2.31) [84].

Figure 2.31 Crystal structure of the ζ-phase of P.R.53:2, determined by electron diffraction on crystals with sizes of approximately 100 nm only. (a) Coordination of the Ca2+ ion to two pigment molecules and to two sulfonate groups of neighbouring molecules; (b) crystal structure, showing the herringbone arrangement of molecules. Colour code: C grey, H white, N blue, O red, S yellow, Cl green and Ca light green.
For the different metal lakes of P.R.49, single crystals could be grown and the crystal structures were determined by X-ray structure analyses [85, 86].
In the crystal structure of the tetrahydrate of P.R.49:1 (Ba salt) the Ba2+ ion shows an eightfold coordination. Two pigment anions act as a bidentate ligand and coordinate via the SO3− and the CO groups, a third pigment anion coordinates via the SO3− group only. Additionally, the Ba2+ ion coordinates to three water molecules; the fourth one is connected via hydrogen bridges only (Figures 2.32 and 2.33).

Figure 2.32 Crystal structure of P.R.49:1 (tetrahydrate phase); coordination of the barium ion to three pigment anions (two acting as a bidentate ligand) and three water molecules.

Figure 2.33 Crystal structure of P.R.49:1 (tetrahydrate phase).
The calcium salt of P.R.49 exists in several phases. The determined crystal structure is that of the dihydrate (Figure 2.34).

Figure 2.34 Crystal structure of P.R.49:2.
The crystal structure analyses as well as optical spectroscopy studies of P.R.49 and its various metal salts showed that these lakes exist in solution as well as in the solid state in the ketohydrazone form [85–87].
The presence of the ketohydrazone form was also proven by crystal structure analyses of several non-commercial laked pigments [88].
2.7.1.2 Properties
β-Naphthol pigment lakes vary in shade from yellowish to bluish red; notably, however, these products tend to be yellower than the corresponding BONA pigment lakes based on the same diazonium component (Section 2.7.2). β-Naphthol pigment lakes produce pure shades. Most afford varying shades, depending on the synthetic method and also on the metal. The occurrence of various red shades in these pigments is also attributed to the influence of different crystal modifications.
As a result of their salt character, β-naphthol pigment lakes are faster to solvents and more resistant to migration than β-naphthol pigments, but also less light-fast. They are only moderately fast to alkaline agents. The strong intermolecular forces within laked pigments are responsible for their good heat stability.
2.7.1.3 Application
Good general fastness properties make β-naphthol pigment lakes suitable candidates primarily for the printing inks and plastics industry. Their primary area of application varies according to the type. β-Naphthol pigment lakes are also employed in paints and in emulsion paints, but to a lesser degree.
2.7.1.4 Commercially Available β-Naphthol Pigment Lakes
2.7.1.4.1 General
Table 2.10 lists the currently available β-Naphthol pigment lakes.
Table 2.10 Commercially available β-naphthol pigment lakes. The shade also depends on the polymorphic form.
| General chemical structure: | 
|
|||
| C.I. Name | C.I. Constitution Number | DC | M/2 | Shade |
| P.O.17 | 15510:1 | Ba | orange | |
| P.O.17:1 | 15510:2 | reddish orange | ||
| P.O.46 | 15602 |  |
Ba | yellowish red |
| P.R.49 | 15630 |  |
2 Na | yellowish red |
| P.R.49:1 | 15630:1 | Ba | yellowish red | |
| P.R.49:2 | 15630:2 | Ca | bluish red or maroon | |
| P.R.49:3 | 15630:3 | Sr | red | |
| P.R.50:1 | 15500:1 | Ba | scarlet | |
| P.R.51 | 15580 |  |
Ba | scarlet |
| P.R.53 | 15585 |  |
2 Na | scarlet |
| P.R.53:1 | 15585:1 | Ba | scarlet | |
| P.R.53:2 | 15585:2 | Ca | reddish orange | |
| P.R.53:3 | 15585:3 | Sr | yellowish red | |
| P.R.68 | 15525 |  |
Ca | yellowish red |
Pigment Red 53 has for some time dominated the β-naphthol pigment lake market in Europe and Japan and is still an important product. Pigment Red 49, employed especially as the barium and less frequently as the calcium salt, is mainly used in the USA. Other pigments of this type are less important.
A certain amount of emphasis is placed on Pigment Red 68, with its two acidic groups available for salt formation.
2.7.1.4.2 Pigment Orange 17
Besides being offered as a barium salt, P.O.17 is also available as a precipitate onto aluminium oxide hydrate. These types, especially in the USA, are referred to as Persian Orange. The products have been discontinued in Europe, but play a certain role in the Japanese market. P.O.17 affords brilliant, orange–red shades. Commercial types are tinctorially strong and highly transparent. They are used in inexpensive packaging prints, including metal deco printing. Varieties that contain a large amount of aluminium oxide hydrate often adversely affect the drying properties of oily media, such as offset printing inks. In a highly acidic binder, the pigment may lose its metal ion as it reverts to the free acid and consequently undergoes considerable colour shift. In the past, P.O.17 was also used in large volume to colour natural rubber.
2.7.1.4.3 Pigment Orange 17:1
Aluminium lakes of P.O.17:1 are available in the USA. Properties that are pertinent to the colouristics and application of the pigment parallel those of the barium lakes. P.O.17:1, an equally high strength pigment, also affords brilliant shades and is used in packaging prints, especially for paraffin-based wrapping paper for bread.
2.7.1.4.4 Pigment Orange 46
P.O.46, which is gaining commercial recognition in Europe, plays a certain role in the US market. The application properties of the pigment are quite similar to those of the Lake Red C types, P.R.53:1. P.O.46 produces a shade that is appreciably yellower than that of conventional P.R.53:1 types. Its shade is much more comparable to the colour of the second crystal modification of the barium salt of Lake Red C.
Commercially available P.O.46 types are usually quite transparent. They are employed primarily in packaging printing inks, also in offset and metal deco printing. Publication gravure inks, plastics, especially PVC, LDPE, and elastomers, as well as general industrial paints are suitable media for pigment application. P.O.46 is less solvent resistant than P.R.53:1, but it is faster to alkali and acid. In terms of lightfastness, P.O.46 performs poorly: 1/3 and 1/25 SD prints equal only step 1 on the Blue Scale.
2.7.1.4.5 Pigment Red 49 Types
Products of the Pigment Red 49 range differ considerably in their commercial importance, depending on the region in which they are marketed. Although of little importance in Europe and Japan, P.R.49 types play a major role in the USA. The pigments are supplied as sodium (P.R.49) or as calcium salts (P.R.49:2); but the largest fraction is sold as barium salts (P.R.49:1). The product line has been extended to include the strontium salt (P.R.49:3). The shade of the barium lake is yellower than that of P.R.57:1 and bluer than that of P.R.53:1. Calcium types are somewhat bluer than P.R.49:1, while the sodium salt is more yellowish.
Pigment Red 49 types are often referred to as Lithol Red pigments, especially in the USA. They are used in all types of printing inks. Throughout the USA, Pigment Red 49 grades are frequently employed to lend red shades to publication gravure printing inks targeted for three- or four-colour printing. P.R.49:1 is slightly too yellow to match the CIE 12-66 standard for process printing inks on the European Colour Scale for offset printing.
2.7.1.4.5.1 Pigment Red 49
P.R.49, sodium salt, is used to a limited extent. A pigment with high tinctorial strength, it is employed particularly in economical, solvent-based flexographic printing inks. Both as a pigment and in print, it is much less fast to various solvents than other metal salts. The same is true for its fastness to water, which precludes its use in aqueous printing inks. Moreover, P.R.49 is also less lightfast than other types.
2.7.1.4.5.2 Pigment Red 49:1 and 49:2
Both the barium lake P.R.49:1 and the calcium lake P.R.49:2 resemble Lake Red C pigments (P.R.53:1) in terms of fastness to organic solvents, to alkali and acid, and to fastness in application. However, P.R.49 grades are much less heat stable, which narrows their applicability in plastics. Areas of application, especially in the USA, include elastomers, to a lesser extent also inexpensive industrial paints, air drying and nitro paints. Their main market, however, is in printing inks, especially for publication gravure printing inks, packaging gravure and newsprint inks. Resinated grades are also available. These are typically more transparent, more brilliant, and show less of a tendency to bronze in print than resin-free varieties. Special-purpose types, on the strength of their good rheological properties, are supplied for use in water-based printing inks.
2.7.1.4.6 Pigment Red 50:1
P.R.50:1, patented as early as 1905 by Meister Lucius & Brüning, is only of regional importance today; its sale has been discontinued in Europe. Marketed first as a sodium lake (P.R.50) to be transformed into the barium salt by the consumer, the barium lake (P.R.50:1) is now exclusively used. This salt affords clean shades of scarlet.
P.R.50:1 is used primarily in packaging printing inks, which mostly afford bronzing prints with good fastness to organic solvents. Weak alkaline agents induce a colour shift to blue shades, while acids have a yellowing effect. In the presence of drying agents based on cobalt salts, the pigment undergoes a reformulation of its salt form to produce shades of brown. P.R.50:1 has accordingly lost its importance in offset inks, which used to be its major market. The pigment fails to meet modern requirements.
2.7.1.4.7 Pigment Red 51
P.R.51, a barium pigment lake, affords shades of scarlet similar to those of Pigment Red 53:1. P.R.51 performs like P.R.49 in terms of fastness, but it is particularly sensitive to alkaline agents. P.R.51, which used to be quite important, especially in printing inks and rubber, has largely been replaced by other products. It continues to be used in typewriter and printer ribbons for its shade.
2.7.1.4.8 Pigment Red 53 Types
Barium and sodium lakes are commercially available (P.R.53:1 and 53). Calcium salts (P.R.53:2) have only enjoyed commercial success for a limited time; they have largely vanished from the market; however, strontium lakes (P.R.53:3) are being used commercially.
2.7.1.4.8.1 Pigment Red 53
P.R.53, the sodium salt which used to be transformed into the barium salt by the customer, continues to be used occasionally for its very yellowish shade. Its use is restricted by poor solvent and application fastness properties.
2.7.1.4.8.2 Pigment Red 53:1
P.R.53:1, a barium lake, is one of the most important red pigments for use in printing inks. Its scarlet shade is much yellower than that of P.R.57:1, which is the DIN 16 539 standard magenta for three and four colour printing. This precludes its use for this purpose. P.R.53:1 has stimulated special interest as a so-called warm red. It has also become increasingly important as a replacement for P.R.49, although its importance varies considerably with the region. P.R.53:1 is used especially in disposable printed products, especially in sheet and Web offset, gravure, and flexographic printing inks. 1/1 SD letterpress proof prints equal step 3 on the Blue Scale for lightfastness, while 1/25 SD specimens reach only step 1. The Naphthol AS pigments P.R.9 and 10, which are somewhat yellower in equally deep shades, are more lightfast than the less expensive Lake Red C types. The difference comprises several steps on the Blue Scale; 1/25 SD samples, for instance, equal step 5 (P.R.9) and, respectively, step 4 (P.R.10). Both pigments are close in shade to P.R.53:1.
P.R.53:1 is a comparatively strong and brilliant pigment within its range of shades. Inks formulated at about 19 and 11% pigment concentration, respectively, suffice to print standard letterpress layers of 1/1 and 1/3 SD samples. For comparison, notably, the corresponding values for P.R.9 and 10 are approximately 20.5% and 13.5% pigment, respectively. Yellowish types of P.R.53:1 are usually less strong than bluish ones.
In print, P.R.53:1 shows good fastness to a series of organic solvents: it is almost completely stable to the DIN 16 524 solvent mixture. The pigment is entirely fast to clear lacquer coatings. As a result of the chemical constitution of the pigment, the prints are sensitive to alkali and acids. Moreover, there are some drawbacks to pigment performance in several special applications: the prints are, for instance, affected by agents such as soap or butter. Their heat stability, on the other hand, is excellent. P.R.53:1 prints tolerate exposure to more than 200 °C for 10 min. In contrast to the similarly coloured P.R.68, which is a member of the same class of pigments, P.R.53:1 is not fast to sterilization. The commercially available P.R.53:1 types differ considerably in their degree of transparency. Specific surface areas are around 16–50 m2 g−1. Today, resinated grades have somewhat less of a technical value.
P.R.53:1, which is used in large volume in aqueous flexographic printing inks, may present problems in terms of storage stability. Such basic printing inks tend to show a more or less pronounced viscosity increase, almost invariably combined with a shift to yellowish shades. The latter, however, is not very noticeable in deep shades. The lack of storage stability is attributed to interaction between pigment or, more precisely, between the alkaline-earth metal of the pigment on the one hand and the alkaline agent or the base of the printing ink on the other hand, which ultimately exchanges the metal.
P.R.53:1 is used for its excellent heat stability as a colourant for plastics. 1/3 SD samples in HDPE withstand exposure to about 260 °C for 5 min.
P.R.53:1 specimens exhibit medium tinctorial strength. P.R.68, a member of the same group of pigments, is somewhat yellower and even more heat stable than P.R.53:1. The dihydrazonepyrazolone pigments P.R.37 and 38 are, respectively, slightly and considerably bluer, but much less heat stable in PE (polyethylene). Under common processing conditions, up to 260 °C, P.R.53:1 has practically no effect on the shrinkage of injection-moulded polyolefins. Its lightfastness in PE is approximately between step 3 and step 1 on the Blue Scale, depending on the depth of shade and on the pigment concentration.
P.R.53:1 is also very strong in PVC. It is not very lightfast, but several disposable articles are coloured to advantage with P.R.53:1. However, it has a poor fastness to bleeding. For economic reasons, P.R.53:1 is also employed in polystyrene, in which it is heat stable up to 280 °C and moderately lightfast (step 1–2 on the Blue Scale).
P.R.53:1 may also be used in PUR foam products wherever the fastness requirements, especially regarding water, soap and solvents, are not too stringent.
The pigment also lends itself to application in elastomers, such as natural rubber blends. It is sufficiently migration fast to satisfy most specifications. P.R.53:1 is also completely bleed resistant in natural rubber, although some colour is transferred into the wet cotton cloth liner, the wrapper (Section 1.8.3.6). P.R.53:1 is lightfast enough for most applications. Products containing P.R.53:1 are not always entirely fast to hot water or alcohol.
Where it satisfies the fastness specifications, especially regarding lightfastness, P.R.53:1 may also be employed in paints. It is also found in applications outside the large pigment consuming industries. Less stable types lend colour to products such as cleaning agents and office articles, where P.R.53:1 is found in inexpensive colour pencils and watercolours. However, in several countries there are legal limits concerning the amount of soluble barium traces that are permissible in the pigment (Section 5.2.2).
A second crystal modification of P.R.53:1 (β phase) was introduced to the market much later, which is slightly weaker but considerably yellower than conventional barium or strontium lakes [89]. Both modifications perform similarly in application, and they are used in similar areas. Pigment mixtures afford a comparatively large variety of intermediate shades.
2.7.1.4.8.3 Pigment Red 53:2
The calcium lake is only of low commercial interest, due to its limited light fastness. The polymorphic forms γ and δ exhibit a bright orange shade and can be used for example for printing applications and for the colouration of seeds [81, 82].
2.7.1.4.8.4 Pigment Red 53:3
The strontium lake performs like barium lakes of P.R.53:1 and is equally fast to organic solvents, to alkali and acid. Both types are equally heat stable and lightfast, for instance in print. Coloristically, the strontium salt of P.R.53 is yellower, but it does not quite match the shade of the above-mentioned novel crystal modification of the barium lake. P.R.53:3 is generally being used for the same purposes as P.R.53:1.
2.7.1.4.9 Pigment Red 68
P.R.68 is supplied as a calcium salt. It produces a yellowish red shade, referred to as scarlet, which at equal depth of shade is slightly bluer than P.R.53:1, a member of the same class of pigments. P.R.68 has gained considerably less commercial recognition than the widely used Lake Red C pigments.
The main area of application for P.R.68 is in the colouration of plastics. The pigment, whose tinctorial strength is average, is fast to blooming but not quite bleed resistant in plasticized PVC. Its lightfastness, especially in white reduction, fails to satisfy more stringent requirements. 1/3 SD samples that contain 5% TiO2 equal step 4 on the Blue Scale for fastness to daylight, while 1/25 SD specimens are equal to step 3. P.R.68 is heat stable enough to be applied safely in polyolefins. Incorporated in HDPE, for instance, it is stable up to 300 °C. The pigment exhibits average tinctorial strength. P.R.68 does not affect the shrinkage of injection-moulded polymers. The lightfastness of 1/3 to 1/25 SD specimens equals between step 2–3 and step 3 on the Blue Scale. P.R.68 is also found in polystyrene.
The pigment is also used as a colourant for printing inks, but its importance in this area has diminished considerably. P.R.68 is somewhat weaker than P.R.53:1, but more lightfast. 1/3 SD letterpress proof prints equal step 4 (2) on the Blue Scale for lightfastness, while 1/25 SD specimens equal step 3 (1). The values in brackets represent the lightfastness of P.R.53:1. In print, P.R.68 shows excellent fastness to several organic solvents, including the standard DIN 16 524 solvent blend. Its resistance to soap, alkali and acid, however, is unsatisfactory. P.R.68, in contrast to P.R.53:1, is completely fast to sterilization. Since P.R.68 also provides excellent heat stability (1/3 SD samples withstand exposure to 220 °C for 10 min), it lends itself to metal deco printing, although it is not fast to clear lacquer coatings. As in other application media, insufficient fastness of P.R.68 inks to water may affect the results of offset printing.
P.R.68 is used in paints for applications where it performs well, especially in terms of lightfastness. Incorporated in an air drying alkyd resin vehicle, full shades equal step 5 on the Blue Scale for lightfastness. The 1:5 TiO2 reductions, however, only score as high as step 2. P.R.68 exhibits excellent resistance to numerous organic solvents, while it is quite sensitive to alkali and acid, as well as to water.
P.R.68 is also recommended for use in decorative cosmetics, such as nail polish, lipstick, powders and shading creams. For these purposes, types are supplied that fulfil legal purity standards and are tested accordingly.
2.7.2 BONA Pigment Lakes
These pigments derive their name from 2-hydroxy-3-naphthoic acid, which is used as a general coupling component for the entire group. The compound is also known as beta-oxynaphthoic acid (BONA, in German BONS).
The history of BONA pigment lakes parallels that of β-naphthol pigment lakes. Literary evidence of the use of 2-hydroxy-3-naphthoic acid, which was first synthesized in 1887 by Schmitt and Burkard as a coupling component, dates from 1893 (Kostanecki). However, it was not until 1902 that the compound began to be employed in dye synthesis by AGFA (aniline → BONA).
Pigment Red 57, discovered in 1903 by R. Gley and O. Siebert at AGFA, developed into one of the most important organic pigments in the market. It was first supplied in the form of its yellowish red sodium salt, to be converted into the calcium or barium salt by the consumer. Today the laking with Ca2+ is performed by the pigment producers.
Initially, these ‘lakes' were precipitated onto inorganic carrier materials. Interestingly, this group of colourants was originally used for pigments in paints. Application in the textiles market followed later. Today, it is mainly the calcium and barium salts, but also manganese and, less frequently, strontium salts, that have the largest sales volume.
Technically important pigments in this group – similar to β-naphthol pigment lakes – contain aminosulfonic acids as diazonium components. Therefore, these pigments contain two acidic groups for salt formation.
2.7.2.1 Chemistry, Manufacture and Crystal Structures
BONA pigment lakes are monohydrazone pigments, characterized primarily by the following general chemical structure:

In commercially important pigments, RD usually stands for hydrogen, chlorine or a methyl group. M typically represents a divalent metal atom from the alkaline earth series, including calcium, barium, strontium or manganese.
As a diazonium component, aniline sulfonic acid may occasionally be replaced by 2-aminonaphthalene-1-sulfonic acid or by aniline.
Aromatic aminosulfonic acids are synthesized by a sequence of important industrial processes, including sulfonation of benzene. This is followed, wherever necessary, by chlorination, nitration and reduction, or by aniline sulfonation, possibly involving subsequent ‘baking' [90, 91].
BONA pigment lakes are basically synthesized like β-naphthol pigment lakes.
Diazotization of the aminosulfonic acid and subsequent coupling onto the sodium salt of 2-hydroxy-3-naphthoic acid initially affords the monohydrazone compound in the form of its soluble sodium salt. Subsequent reaction with chlorides or sulfates of alkaline earth metals or with a manganese salt, frequently in the presence of a dispersion agent, or rosin or its derivatives, at elevated temperature yields the insoluble BONA pigment lake.
BONA pigments can exhibit polymorphism, and the individual phases may contain a different number of water molecules in their crystal lattice, as for β-naphthol lakes.
P.R.57:1 exists as a carmine-red trihydrate, which is formed during production. Careful drying leads to the monohydrate, which exhibits a magenta shade and is widely used for newspaper printing. The monohydrate is stable at temperatures of up to about 190 °C. Above this temperature it slowly releases water and transforms into an anhydrate with a dull dark magenta shade. Upon cooling in air, the monohydrate is formed again. In an atmosphere with high relative humidity (e.g. in tropical countries), the monohydrate slowly converts into the trihydrate, which results in a corresponding shift in the shade of the pigment. Solid-state high-resolution 13C NMR spectroscopy proved that P.R.57:1 exists in the usual keto-hydrazone form [92]. The crystal structures of the three phases were determined from X-ray powder data [93]. In all three phases the calcium ion is simultaneously coordinated to the sulfonate, keto and carboxyl groups of a pigment anion. In the trihydrate the calcium is additionally bound to the carboxylate group of a neighbouring anion, and to four water molecules, resulting in an eightfold coordination of the calcium ion (Figure 2.35a). In the monohydrate the calcium is additionally coordinated to two carboxylate groups (one acting as a bidentate ligand), one sulfonate group and one water molecule, and in the anhydrate additionally to two carboxylate groups (one acting again as a bidentate ligand) and one sulfonate group. The increasing coordination of the pigment anion to calcium cations may explain the colour shift from the carmine-red trihydrate via the magenta monohydrate to the dull dark magenta anhydrate. All three phases of P.R.57:1 exhibit a double-layer structure: The polar/ionic layer contains the Ca2+ ions, the SO3−, COO− and keto groups as well as the water molecules; this layer is dominated by ionic interactions, hydrogen bonds and other Coulomb interactions. The polar layers are separated by non-polar layers formed by the hydrocarbon groups, which interact through van der Waals forces only (Figure 2.36). Such double-layer structures are also found in other laked pigments, see for example P.R.53:2 (Figure 2.31) or P.R.49:2 (Figure 2.34).


Figure 2.35 Coordination of the Ca2+ ion in P.R.57:1. (a) α-phase (trihydrate), (b) β-phase (monohydrate) and (c) γ-phase (anhydrate). C black, H white, N blue, O red, S yellow and Ca green. Hydrogen bonds have been omitted for clarity.

Figure 2.36 Crystal structure of the commercial magenta-shaded β-phase of P.R.57:1. The polar layers contain the calcium coordination, the water molecules and the H-bond network. The non-polar layers consist of tolyl and naphthalene groups.
2.7.2.2 Properties
In terms of performance, BONA pigment lakes are most closely related to β-naphthol pigment lakes. BONA pigment lakes afford more bluish red shades (‘ruby', ‘maroon') than their β-naphthol counterparts and are also more lightfast. This is especially true for the manganese salts. Other aspects of pigment properties, however, such as fastness to alkali, soap, and acid, solvent and migration fastness, and heat stability, are very similar. BONA pigment lakes are products with high tinctorial strength.
2.7.2.3 Application
Most pigments within this group find extensive use in printing inks. There are also some products that are used primarily in paints. BONA pigment lakes play a role as colourants for plastics as well as for various other media.
2.7.2.4 Commercially Available BONA Pigment Lakes
2.7.2.4.1 General
As with β-naphthol pigment lakes, only a few BONA pigment lakes are marketed in large volume. Two of these pigments, however, maintain an important position within the pigments industry. Table 2.11 lists the currently available BONA pigment lakes.
Table 2.11 Commercially available BONA pigment lakes.

|
||||||
| C.I. Name | C.I. Constitution Number | M | Shade | |||
| P.R.48:1 | 15865:1 | CH3 | Cl | Ba | red | |
| P.R.48:2 | 15865:2 | CH3 | Cl | Ca | bluish red | |
| P.R.48:3 | 15865:3 | CH3 | Cl | Sr | red | |
| P.R.48:4 | 15865:4 | CH3 | Cl | Mn | bluish red | |
| P.R.48:5 | 15865:5 | CH3 | Cl | Mg | red | |
| P.R.52:1 | 15860:1 | Cl | CH3 | Ca | ruby | |
| P.R.52:2 | 15860:2 | Cl | CH3 | Mn | maroon | |
| P.R.57:1 | 15850:1 | CH3 | H | Ca | bluish red (magenta, ruby) | |
| P.R.58:2 | 15825:2 | H | Cl | Ca | bluish red | |
| P.R.58:4 | 15825:4 | H | Cl | Mn | medium red | |
| P.R.63:1 P.R.63.2 |
15880:1 15880:2 |
Ca Mn |
bordeaux bordeaux |
|||
| P.R.64 | 15800 | H | H | H | Ba/2 | red |
| P.R.64:1 | 15800:1 | H | H | H | Ca/2 | yellowish red |
| P.R.200 | 15867 | Cl | C2H5 | Ca | bluish red | |
| P.Br.5 | 15800:2 | H | H | H | Cu/2 | brown |
All pigments within this series, with the exception of P.R.64 and P.Br.5, contain two acidic functions: a sulfonic acid group and a carboxylic acid moiety. Precipitation is accomplished primarily with calcium or manganese, more rarely with barium, strontium or magnesium. The sulfonic acid function is found almost invariably in the ortho position relative to the hydrazone group. Commercially important pigments, including Pigment Red 48 and 57, also bear a methyl group in para position relative to the hydrazone bridge. Manganese lakes are always bluer than the strontium or barium lakes. They often appear even more bluish than the calcium salts.
2.7.2.4.2 Pigment Red 48 Types
Amongst the commercially available types are ‘lakes' with various metals, including barium (P.R.48:1), calcium (P.R.48:2), strontium (P.R.48:3), manganese (P.R.48:4) and magnesium salts (P.R.48:5). There are also types, such as barium/calcium salts, which are obtained by ‘mixed' laking. Commercial interest in each type depends on the area of application, but the pigments enjoy overall importance. Pigments of this type are known under various common names, especially in the English language. The best known designation, ‘2B toner', is clearly derived from the name under which the pigment was first marketed, namely as Permanent Red 2B, a sodium salt.
2.7.2.4.2.1 Pigment Red 48:1
P.R.48:1, the barium salt, is a colouristically versatile product. It affords light yellowish to medium red shades, depending on the specific surface area of the product. Fastness to several common organic solvents, such as esters, ketones, and aliphatic and aromatic hydrocarbons, is good. However, P.R.48:1 shows only poor resistance to soap, alkali and acid.
The main field of application for P.R.48:1 is in printing inks and plastics. To enhance the transparency in print and to reduce the strong tendency of the prints to bronze, pigments that are targeted for use in printing inks are frequently supplied in resinated form.
P.R.48:1 types show high tinctorial strength, but are less strong than P.R.53:1 grades. P.R.48:1 is considerably yellower than P.R.57:1 and noticeably bluer than P.R.53:1. Although the pigment has been recommended for use in all types of printing inks, the prints of many types lack resistance to the DIN 16 524 standard solvent mixture and to clear lacquer coatings and also lack fastness to sterilization. Paints of P.R.48:1 withstand exposure to 180 °C for 10 min or to 160 °C for 30 min. Barium lakes are much less lightfast than salts of other metals. Besides, a certain lack of storage stability may create problems in aqueous media: the printing inks tend to thicken.
Incorporated in plastics, P.R.48:1 exhibits medium tinctorial strength. Its fastness to migration in plasticized PVC is good. The pigment is stable to blooming and almost completely bleed resistant. Its lightfastness in full shades equals step 3 on the Blue Scale, while 1/3 SD specimens only reach step 1–2. In terms of heat stability, 1/3 SD formulations in PE withstand 200–240 °C for 5 min, depending on the type. Higher temperatures quickly shift the colour towards bluer, duller red shades. Transparent PE systems are less heat stable.
P.R.48:1 is recommended especially for use in LDPE. In addition, it also affords colourations of medium tinctorial strength in PE. Some 0.34% pigment is required to formulate 1/3 SD colourations with 1% TiO2. Comparative values are listed for several other pigments. Problems may occur during vulcanization with open steam in the presence of P.R.48:1. The pigment bleeds into the wrapper (Section 1.8.3.6).
Paint manufacturers frequently use P.R.48:1 in inexpensive industrial paints, in which the pigment exhibits good fastness to overpainting. The lightfastness in full shade equals step 5–6 on the Blue Scale, but the pigment loses its fastness to light rapidly as the TiO2 content increases. P.R.48:1 is not recommended for exterior application.
2.7.2.4.2.2 Pigment Red 48:2
In 1986, the Gravure Technical Association in the USA adopted P.R.48:2 as its standard process red for gravure packaging inks. P.R.48:2, the calcium salt, affords bluish red shades, referred to as ruby. P.R.48:2 is substantially bluer than P.R.48:1, distinctly bluer than P.R.48:4, and still noticeably yellower than P.R.57:1. In its fastness properties, for instance in terms of fastness to organic solvents, the pigment comes close to the corresponding barium salts. It is inferior to P.R.48:1 in terms of sterilization fastness in print, and the same is true for its fastness to soaps. On the other hand, P.R.48:2 is distinctly more lightfast than P.R.48:1. Exposed 1/1 SD P.R.48:2 letterpress proof prints, for instance, equal step 4–5 on the Blue Scale, while corresponding P.R.48:1 specimens only reach step 3–4.
The two types are used in almost exactly the same areas of application. P.R.48:2 is also found in printing inks, especially for packaging inks based on NC. It is employed, especially in the USA, in three- and four-colour printing for areas such as magazine covers, where P.R.49 varieties are not lightfast enough to satisfy the specifications. Resinated grades are more transparent and bronze less in print. The viscosity of aqueous printing inks often rapidly increases during storage, a problem that may lead to thickening.
P.R.48:2 is also used in plastics, where it affords good tinctorial strength. Some 0.21% pigment is, for instance, needed to produce 1/3 SD HDPE colourations containing 1% TiO2. Such colourations are heat stable up to 230 °C for 5 min, but higher temperatures shift the colour towards bluer, duller shades. At higher temperature, on the other hand, more reduced colourations (1/9 SD) shift the shade to yellower and cleaner hues. P.R.48:2 is used to an appreciable extent in LDPE, in which it is more lightfast than corresponding P.R.48:1 samples: the difference is about 2½ steps on the Blue Scale. Incorporated in plasticized PVC, P.R.48:2 is not entirely bleed resistant. The pigment may be used in PP spin dyeing only if polymers with a low melting point are used. To ensure sufficient lightfastness, the pigment is used to advantage in deep shades, that is, at high concentration.
P.R.48:2 is less commonly found in paints. In paints, like in other areas of application, the calcium salt performs like the barium lake. Both are, for instance, equally fast to overcoating. The list of suitable application areas for both pigments is the same. P.R.48:2 is also used in oven drying paints, nitro paints and in similar systems. In addition, it is also found in emulsion paints. While barium and calcium salts exhibit equal lightfastness in full shades, there is a considerable difference in white reductions. Increasing amounts of TiO2 render P.R.48:2 much more sensitive to light than P.R.48:1.
2.7.2.4.2.3 Pigment Red 48:3
P.R.48:3, the strontium salt, is distinctly bluer than P.R.48:1, noticeably yellower than P.R.48:2 and slightly yellower than P.R.48:4. The main field of application for P.R.48:3 is in plastics. Incorporated in plasticized PVC, P.R.48:3 is the most bleed resistant of all metal salts, although it does show a certain tendency to migrate. P.R.48:3 is quite often more lightfast than other P.R.48 types. Transparent samples, for instance, formulated at a 0.2% pigment concentration, equal step 6 on the Blue Scale. This is 3 steps higher than the lightfastness of corresponding dispersions of P.R.48:1 and ½ to 1 step higher than that of P.R.48:2 and 48:4 samples. 1/3 SD colourations containing 5% TiO2 equal step 4 on the Blue Scale. Only white reductions of P.R.48:4 score one step higher.
P.R.48:3 is of average tinctorial strength. 1/3 SD HDPE colourations containing 1% TiO2, for instance, are produced by using about 0.25% pigment. In terms of heat stability, P.R.48:3 withstands up to 240 °C. At temperatures in excess of this value P.R.48:3 rapidly undergoes a colour shift towards bluer and duller shades. The pigment is used primarily in LDPE, but also in PP spin dyeing if polymers with a low melting point are processed. The list of application media also includes polystyrene, polyurethane and elastomers.
P.R.48:3 is also recommended for the colouration of printing inks, especially for packaging purposes, and also for the paint field. It is recommended for use in architectural paints and general industrial finishes. The pigment demonstrates overall fastness properties that parallel those of P.R.48:1 and 48:2.
2.7.2.4.2.4 Pigment Red 48:4
P.R.48:4, the manganese salt, affords red shades, which are noticeably on the bluish side of P.R.48:3 and yellower than P.R.48:2. The pigment is used in various applications, especially in paints. To produce opaque shades of red, P.R.48:4 is frequently combined with Molybdate Orange. The pigment is considerably more lightfast and durable than other P.R.48 types, especially in full shades. In oven drying and air drying systems, for instance, P.R.48:4 equals step 7 on the Blue Scale, while its weatherfastness after one year of exposure matches step 4–5 on the Grey Scale. Its shade darkens upon weathering. In such deep shades the pigment satisfies more stringent requirements and may even be used in applications such as automobile refinishes.
In terms of solvent fastness, however, P.R.48:4 performs less well than other P.R.48 types. It is noticeably less acid and alkali resistant and less fast to lime. Its fastness to overcoating, on the other hand, is satisfactory. Since the pigment contains manganese metal, it may accelerate the drying process in oxidatively drying paint systems. The plastics industry uses P.R.48:4 primarily in PVC and in polyolefins. For a pigment with good tinctorial strength in PE, only 0.18% P.R.48:4 is needed to formulate a 1/3 SD sample containing 1% TiO2. Such colourations are heat stable up to 200–290 °C for 5 min, depending on the type. Higher temperatures shift the colour of less heat stable varieties partly reversibly towards yellower shades and also make for dull colours. However, economic considerations frequently make it necessary to compromise between colouristic perfection and price.
Incorporated in PP, P.R.48:4 often causes ageing, which makes the plastic brittle and precludes the use of P.R.48:4 in this medium. The effect on PE is less noticeable. The shrinkage of PE extrusion products at higher processing temperatures is practically unaffected by P.R.48:4.
P.R.48:4 does not bloom in plasticized PVC and is almost completely fast to bleeding. Its tinctorial strength in this medium is equally good. Desirable dielectric properties make P.R.48:4 a suitable candidate for use in PVC cable insulations. The pigment is also used for mass coloured secondary acetate threads, fibres and films wherever it meets the requirements for application.
In printing inks, P.R.48:4, due to its manganese content, may accelerate the drying process in oxidative drying systems, for instance in offset printing inks. This effect must be taken into consideration in calculating the siccative content of such inks. In print, the pigment is insufficiently fast to alkali, acid and soap, and it also fails to tolerate clear lacquer coatings and sterilization. P.R.48:4 does, however, afford good tinctorial strength in print. Some 18% pigment suffices to produce 1/1 SD letterpress proof prints, printed in films of standardized thickness. 1/3 SD prints, on the other hand, are produced using about 9% pigment. The prints equal step 4 on the Blue Scale for lightfastness. Exposure of the sample to light causes a certain yellow shift, the extent of which depends on the depth of shade and on the printed substrate. This colouristic change is frequently neglected in the evaluation of lightfastness: samples consequently appear several steps higher on the Blue Scale than their actual lightfastness warrants.
P.R.48:4 is also found in packaging gravure printing inks and in flexographic inks. Notably, however, the manganese content in the pigment may oxidatively destroy the resin in polyamide vehicle systems, rapidly causing the printed sheets to stick together and producing an unpleasant odour. Problems may also occur as P.R.48:4 is used in different binder systems to be printed on PE films. In PE mass colouration, these materials may become brittle, that is, age prematurely. P.R.48:4 is also supplied in the form of resinated grades.
2.7.2.4.2.5 Pigment Red 48:5
P.R.48:5, a magnesium salt, was a comparatively recent product but its manufacture has already been discontinued. It is considerably yellower and at the same time more brilliant than P.R.48:4. The pigment is also much weaker in white reductions. Compared to the manganese version, P.R.48:5 does not perform as well if exposed to light and/or weather, and it darkens in full shades. Its primary field of application is the pigmentation of polyolefins, but it is also employed to colour PVC and polystyrene. Its application properties and fastness properties in print and in paints largely parallel those of P.R.48:2.
2.7.2.4.3 Pigment Red 52 Types
Both calcium and manganese salts are commercially available. The US market also offers strontium salts and mixed barium/calcium lakes. Although they are important pigments in the USA, P.R.52 types generally have little commercial importance in Europe. These types are referred to in the USA as IBB toners.
2.7.2.4.3.1 Pigment Red 52:1
P.R.52:1, the calcium salt, covers the same range of shades as P.R.57:1. The pigment is somewhat bluer than P.R.48:2 and also duller. Both grades are very similar in terms of fastness to organic solvents. P.R.52:1 is used for printing inks, especially for solvent-based packaging gravure and flexographic printing inks. The pigment is tinctorially strong in these media. P.R.52:1 prints are only poorly to moderately fast to alkali and acid and are particularly sensitive to soap and sterilization. However, some grades are fast to alkaline agents and are almost completely acid resistant.
Resinated types are more transparent and, as a result of smaller particle sizes, are considerably bluer. In the USA they are often used instead of P.R.57:1 to produce standardized process inks for offset printing. In terms of lightfastness, P.R.52:1 does not perform as well as P.R.48:2 and 57:1. The difference is 1 to 2 steps on the Blue Scale in the first case and roughly 1 step in the latter, depending on the standard depth of shade. Various P.R.52:1 types, in contrast to P.R.57:1, have the advantage of being non-thickening in aqueous flexographic inks.
P.R.52:1 exhibits equally poor lightfastness in paints. Full shades, like those of P.R.57:1, match step 4–5 on the Blue Scale, a value that decreases considerably in white reduction. P.R.52:1 is of little interest for the colouration of plastics.
2.7.2.4.3.2 Pigment Red 52:2
P.R.52:2, the manganese lake, affords shades of maroon. Its main area of application is in oven drying systems, in which full and related shades exhibit good lightfastness and weatherfastness. In this respect, the pigment is much superior to P.R.52:1 and P.R.57:1, but does not quite reach the qualities of the yellower manganese lake P.R.48:4. Like the latter, P.R.52:2 may accelerate the drying process in oxidatively drying systems. Despite the fact that the pigment is only moderately fast to common solvents, numerous paint systems containing P.R.52:2 exhibit excellent fastness to overpainting; however, its fastness to acid and alkali is very poor. P.R.52:2 is therefore unsuitable for use in acid- or amine-cured systems. The pigment is recommended particularly for use in blends with Molybdate Red pigments.
2.7.2.4.4 Pigment Red 57:1
P.R.57:1 is known under various names, the most common amongst which is 4B toner. P.R.57:1, the calcium salt, ranks high amongst organic pigments in production volume and use. The pigment is used primarily in printing inks. Its shade matches the standard magenta of several colour scales for three- and four-colour printing, including the CIE 12-66 European Colour Scale (Section 1.8.1.1). Even the printed edition of this book is printed with P.R.57:1.
There are enough commercially available grades of P.R.57:1 to cover a comparatively wide range of shades. Adding particular mixed coupling components, for instance to the diazonium component, will produce any desired hue within this range. A similar effect is achieved by varying the particle size distribution. If Tobias acid (2-aminonaphthalene-1-sulfonic acid) is added as diazo component, the resulting molecules of P.R.63:1 are incorporated in the crystal lattice of P.R.57:1. The resulting blue shift is attributed to an expansion of the crystal lattice through the comparatively large diazo compound. The rate of crystal growth is also inhibited, which also contributes to the colour shift.
P.R.57:1 is a pigment with high tinctorial strength. Approximately 19% pigment is needed to formulate offset and letterpress inks, which produce 1/1 SD proof prints at standard film thickness. 1/3 SD prints, on the other hand, require 7.8% pigment, and 1/25 SD specimens are made with 2.1% pigment. Comparative values are listed under the respective pigments. Inks for prints that satisfy the specifications of the so-called European Standard are prepared under standardized conditions at pigment concentrations of about 14%.
The lightfastness of 1/1 SD P.R.57:1 letterpress proof prints equals step 4–5 on the Blue Scale, while 1/3 and 1/25 SD specimens match step 4 and step 3, respectively. In print, P.R.57:1 is fast to many of the most common organic solvents, including the DIN 16 524 standard solvent mixture, but it performs poorly in terms of soap, alkali and acid resistance. The pigment is equally sensitive to clear lacquer coatings. Wherever prints are expected to be fast to such media, P.R.57:1 is frequently replaced by the colouristically closely related Naphthol AS pigment P.R.184, which has the added advantage of being more lightfast. However, prints made from P.R.184 are as sensitive to sterilization as those obtained from P.R.57:1. P.R.57:1 is heat stable enough to withstand exposure to 180 °C for 10 min or to 160 °C for 30 min.
Several resinated grades are produced to provide higher transparency and to optimize other aspects of pigment properties in application. For reasons connected with process engineering, the resin is typically incorporated as a metal (calcium) resinate. In the past, types of P.R.57:1 additionally contained certain amounts of barium sulfate.
P.R.57:1 is found in all types of printing inks. Like with other pigment lakes, however, there is a certain possibility that storage problems may arise, a fault that is independent of the resination of a pigment. The viscosity of an alkaline printing ink often increases more or less upon storage, that is, the ink thickens. This problem is largely attributed to the interaction between the pigment lake and the cation of the base and leads to an exchange of the metal ion. Reprecipitation also plays a certain role. This phenomenon is based on the fact that the pigment lake, being a salt, is somewhat soluble in water. In an aqueous alcoholic medium, the binder converts dissociated metal ions (calcium ions) into similarly sparingly soluble metal/binder salts, forming the partially soluble sodium pigment lake. The process is repeated as more of the calcium lake dissolves, until the solubility product is reached. In the face of these problems, P.R.184 is also a suitable alternative to P.R.57:1.
Temperatures of up to 100 °C and higher may be observed as P.R.57:1 is dispersed in offset printing inks, especially in Web offset inks by means of modern dispersion equipment. This is especially true for agitating pearl mills. Printing inks and prints may undergo colour shifts if they are exposed to these conditions. The process, which is reversible, is caused by release of the water of crystallization from the pigment.
The different grades of P.R.57:1 vary considerably, not only in their colouristics but also in their rheological behaviour. Individual grades are frequently optimized for special printing inks, for instance for publication gravure inks. In contrast to corresponding diarylide yellow pigments, amine treated types of P.R.57:1 are not available for publication gravure printing inks.
The plastics industry uses P.R.57:1 primarily wherever performance in application is a minor concern, especially with respect to lightfastness. In plasticized PVC, for instance, transparent colouration (0.1% pigment) of P.R.57:1 equals only step 2 on the Blue Scale, while samples containing TiO2 never score higher than step 3. The exact value depends on the standard depth of shade and on the pigment concentration. P.R.57:1 is not suitable for use in concentrations below approximately 0.03%.
P.R.57:1 is employed in cable insulations because of its good dielectric properties. It is much more lightfast in rigid PVC: transparent colourations (0.1% pigment) equal step 6 on the Blue Scale, while white reductions with TiO2 match between step 4 and step 2, depending on the standard depth of shade and on the TiO2 content.
P.R.57:1 is heat stable up to about 250 °C, which makes it a suitable candidate for use in polyethylene, particularly in LDPE types. Incorporated in LDPE, the pigment only slightly affects the shrinkage of extruded articles (Section 1.6.4.3). However, the lightfastness of such products is below expectation, which considerably restricts the versatility of the pigment. The same is true for PP spin dyeing. Apart from being used in these areas, P.R.57:1 also lends colour to polystyrene.
P.R.57:1 is also used for PUR foam products. Problems arise with steam vulcanization, because some colour is transferred to the wrapper as the pigment is worked into rubber (Section 1.8.3.6). The pigmented rubber articles are not completely fast to a number of organic solvents, to soap and sodium carbonate solutions, and to acids and SO2 (Section 1.6.2.2).
Poor lightfastness considerably restricts the use of P.R.57:1 in coatings and emulsion paints. The pigment is, however, occasionally found in industrial paints.
P.R.57:1 is a useful colourant for various special purpose media, including coloured pencils and crayons. Several countries have legally defined purity regulations concerning the use in decorative cosmetics articles, such as face powder and lipstick. The same is true for cheese casings [94]. Suitable grades are commercially available, they are registered in the USA as D & C Red 7, in Japan as Red No. 202.
2.7.2.4.5 Pigment Red 58 Types
The following salts have been produced: the sodium salt (P.R.58), the barium salt (P.R.58:1), the calcium salt (P.R.58:2), the strontium salt (P.R.58:3) and the manganese lake (P.R.58:4). The commercial success of each type varies considerably by region but has generally decreased considerably as the pigments have been replaced by other lakes. P.R.58:4 is unique in that it is the only representative to have remained in the European market.
2.7.2.4.5.1 Pigment Red 58:2
East Asia is the only part of the world where P.R.58:2, a calcium lake, continues to be used. The pigment affords bluish red shades, somewhere between those of P.R.53:1 and 57:1. Its main field of application is in printing inks, where its lightfastness equals that of P.R.57:1; the two pigments also exhibit similar tinctorial strength.
2.7.2.4.5.2 Pigment Red 58:4
P.R.58:4 is primarily used in general industrial paints, where its full shade and related shades are of interest. This is also true for other manganese pigment lakes. In full shade, P.R.58:4 affords deep carmine shades, but white reductions provide only very dull, bluish shades of red. The pigment is fast to organic solvents and shows good overspraying fastness, but its resistance, especially to alkali, soap and lime, is poor. Even in full shades, it remains inferior to P.R.48:4 and 52:2 in terms of lightfastness and weatherfastness. The difference is even more noticeable in white reductions. P.R.58:4 also exhibits moderate heat stability.
Like other manganese lakes, P.R.58:4 accelerates the drying process of oxidatively drying systems, which rapidly thickens the paint. This clearly precludes its use in such systems.
2.7.2.4.6 Pigment Red 63 Types
The following cations have been used for salt formation: sodium, calcium, barium and manganese, as well as blends of calcium and manganese. European manufacturers continue to supply the calcium and manganese lakes, while the Japanese market also provides a barium lake. The latter, however, is of limited regional importance.
2.7.2.4.6.1 Pigment Red 63:1
The use of P.R.63:1, the calcium lake, is steadily declining, even in the USA, where the pigment used to be very important until the mid-1950s. P.R.63:1 produces a deep, bluish bordeaux shade and shows good fastness to organic solvents, including aliphatic and chlorinated hydrocarbons and plasticizers. It bleeds slightly into alcohols, ketones and aromatic hydrocarbons (Section 1.6.2.1). Its primary area of application is in low-cost industrial and trade sales paints, and it also lends colour to leather finishes. The pigment is not acid or alkali resistant and it is sensitive to lime. Full shades approximately equal step 4 on the Blue Scale for lightfastness, while 1/3 SD samples in white reductions match approximately step 2. The pigment is therefore not recommended for exterior use.
2.7.2.4.6.2 Pigment Red 63:2
P.R.63:2, the manganese lake, affords a dark maroon shade. It is a pigment with high tinctorial strength. P.R.63:2 is much less fast to organic solvents than the corresponding calcium lake, P.R.63:1. The pigment equals step 3 on the 5 step stability scale (Section 1.6.2.1), due to its bleeding into alcohols, ketones, and aliphatic and chlorinated hydrocarbons. The pigment is even less stable to aromatic hydrocarbons and esters; it is consequently not fast to overspraying. Its acid and alkali fastness are even inferior to that of P.R.63:1.
Like other manganese lakes, P.R.63:2 may accelerate the drying process of oxidatively drying resin systems. Its full shade lightfastness, which matches step 6–7 on the Blue Scale, is good. The pigment reaches step 4–5 in white reductions (1/3 SD), which is considerably better than that of the corresponding calcium lake. P.R.63:2 is also considerably more durable than P.R.63:1. The pigment is used regionally to afford low-cost shades of red in industrial finishes, and it is also recommended for use in printing inks.
2.7.2.4.7 Pigment Red 64 Types
Several metal lakes have been prepared from this parent structure of all β-oxynaphthoic acid pigments. The list includes the barium salt (P.R.64), the calcium salt (P.R.64:1) and the copper lake, which is registered as Pigment Brown 5. The pigments are rarely used in Europe, and their impact on the market in Japan and the USA has also decreased considerably.
2.7.2.4.7.1 Pigment Red 64:1
P.R.64:1, a low-cost calcium lake, exhibits good lightfastness. It provides brilliant, yellowish shades of red, which are referred to as scarlet. P.R.64:1 is registered in the USA as D&C Red No. 31. It is used in cosmetics.
2.7.2.4.8 Pigment Red 200
P.R.200, which was first introduced in the USA, is as yet only supplied in the form of its calcium salt. It produces a clean bluish shade of red, which is close to that of P.R.57:1. The two pigments are also similar in their resistance to organic solvents. P.R.200 shows average fastness to overcoating. It is recommended for use in air drying and heat drying systems, in which it only exhibits moderate lightfastness. P.R.200 is also found in various different types of printing inks, especially those for offset and gravure printing. The pigment is used to lend colour to plastics wherever lightfastness is a minor issue. The pigment is not fast to acid, alkali or soap.
2.7.2.4.9 Pigment Brown 5
Production of P.Br.5 seems to be discontinued. It enjoyed only limited regional importance. The pigment is the poorest performer of all pigments within its class. P.Br.5 is used in special applications, such as flexographic printing inks, textile inks and wood stains.
2.7.3 Naphthol AS Pigment Lakes
2.7.3.1 Chemistry, Manufacture, Crystal Structures, Properties and Applications
Naphthol AS pigment lakes form a comparatively small group of pigments, all of which feature one or two sulfonic acid functions. These groups may be introduced into the pigment molecule either through the diazo component or through the arylide moiety of the coupling component. The sulfo groups, apart from providing a site for salt formation to form the insoluble pigment, also contribute to the performance of their host by noticeably improving the solvent and migration fastness of Naphthol AS pigments.
Pigment synthesis follows the typical route to hydrazone pigment lakes: the aniline derivative or the aniline sulfonic acid is diazotized with sodium nitrite in an acidic medium (hydrochloric acid), followed by coupling on the Naphthol AS derivative, which is initially dissolved in an alkaline solution and then precipitated by adding inorganic or acetic acid. If a Naphthol AS sulfonic acid is used as a coupling component, it must be neutralized with alkali for dissolution and coupled directly.
The resulting alkali salts (usually sodium salts) of the sulfonated Naphthol AS pigments are treated as described in Section 2.7.1.1 and reacted with calcium or barium salts. Crystal structures of Naphthol AS pigment lakes have not been published.
Properties and applications of the individual Naphthol AS pigments are described in section 2.7.3.2.
2.7.3.2 Commercially Available Naphthol AS Pigment Lakes
2.7.3.2.1 General
There is no common structural principle to these pigments beyond the basic Naphthol AS pigment skeleton. Table 2.12 lists the commercially available Naphthol AS pigment lakes.
Table 2.12 Commercially available Naphthol AS pigment lakes.

|
||||||||
| C.I. Name | C.I. Constitution Number | M | Shade | |||||
| P.R.151 | 15892 | H | H | H | Ba | red | ||
| P.R.211 | — | CH3 | Cl | OCH3 | H | Ca/2 | scarlet red | |
| P.R.237a) | — | — | — | — | — | — | — | yellowish red |
| P.R.239 | — | — | — | — | — | — | — | bluish red |
| P.R.240 | — | — | — | — | — | — | — | maroon |
| P.R.243 | 15910 | CH3 | Cl | OCH3 | H | Ba/2 | red | |
| P.R.247 | 15915 | CH3 | H | CONHC6H4 |
H | OCH3 | Ca/2 | bluish red/redb) |
| a) Apparently identical with P.R.243, see text. b) Different crystal modifications. |
||||||||
2.7.3.2.2 Pigment Red 151
P.R.151, a barium lake, which is produced in Japan, affords a bluish red colour that may be referred to as carmine. Of medium tinctorial strength, the pigment is used primarily in plastics.
P.R.151 is completely migration resistant in plasticized PVC. Transparent colourations (0.1% pigment) equal step 6 on the Blue Scale for lightfastness, while the pigment only matches step 3–4 in white reduction (0.01% pigment + 0.5% TiO2). Pigmented rigid PVC systems are appreciably more lightfast, the corresponding values are 7 and 6, respectively. P.R.151 is frequently used for the colouration of synthetic leather made of PVC. However, the pigment is not fast to acid or alkali. Good dielectric properties make it a suitable candidate for PVC cable insulations.
Incorporated in PE, P.R.151 shows only average tinctorial strength. Some 0.24% pigment is required to produce 1/3 SD colourations in PE containing 1% TiO2. In HDPE, the pigment is heat stable up to 290 °C, but this advantage is somewhat compromised by the fact that it affects the shrinkage to a considerable extent. Caution should therefore be exercised if P.R.151 is to be used in larger, non-symmetrical injection-moulded objects. The lightfastness of P.R.151 in PE equals that in rigid PVC.
One of the primary fields of application for P.R.151 is in polystyrene, although there is a slight colour change at temperatures above 260 °C, at which the pigment partially dissolves. It is also used to a considerable extent in ABS. Cast resins based on methyl methacrylate and unsaturated polyesters are also frequently coloured with P.R.151, which is resistant to the peroxide catalysts that are used to harden the plastic. The lightfastness in these media is good – it equals step 6–7 on the Blue Scale.
2.7.3.2.3 Pigment Red 211
P.R.211 is offered only in the Japanese market. It is the calcium salt of a monohydrazone pigment. P.R.211 affords shades of scarlet that resemble those provided by Lake Red C types (P.R.53:1). The list of suitable media includes plastics and printing inks. Incorporated in these media, P.R.211 presents application and fastness properties that are very close to those of P.R.53:1. HDPE samples containing P.R.211, for instance, like those based on P.R.53:1, are heat stable up to approx. 260 °C. Both pigments are fast to bleeding in plasticized PVC. In prints, however, the commercial grade of P.R.211 is tinctorially weaker than similarly coloured Lake Red C types. Depending on the application medium, P.R.211 is weaker by 5–50%.
2.7.3.2.4 Pigment Red 237
P.R.237, introduced some years ago, enjoys only limited regional importance. Its chemical constitution has not yet been published but several test results indicate chemical identity with P.R.243, C.I. No. 15910. The pigment affords a yellowish red shade, referred to as scarlet. P.R.237 is particularly recommended as a colourant for PVC, in which it shows good bleed resistance. Its colouristic properties in this medium parallel those of the β-naphthol pigment lake P.R.68. P.R.237 is only of average tinctorial strength: 0.36% pigment is needed to formulate a 1/3 SD sample (1% TiO2). The pigment is heat stable up to 260 °C. Good fastness to overlacquering makes it a suitable candidate for industrial finishes.
2.7.3.2.5 Pigment Red 239
P.R.239 enjoys limited regional impact and is used very little in Europe. Its exact chemical constitution remains to be published. P.R.239 affords dull, bluish shades of red. Its main application media are plastics. The pigment is bleed resistant in plasticized PVC. Its shade in this medium equals that of P.R.247, a member of the same class of pigments, which, however, has the advantage of providing a much cleaner shade. Incorporated in HDPE, P.R.239 is of average tinctorial strength and is heat stable up to 270 °C.
2.7.3.2.6 Pigment Red 240
The exact chemical constitution of this pigment, which was introduced to the Japanese market some years ago, has not yet been published. P.R.240 enjoys only limited regional importance. Its dull bluish shades of red are referred to as maroon. P.R.240 is recommended for use in industrial finishes for its good lightfastness and weatherfastness as well as for its bleed resistance. The commercial type is quite transparent and lacks tinctorial strength. P.R.240 is equally weak in plastics: 0.42% pigment is needed, for instance, to afford 1/3 SD HDPE samples (1% TiO2). In this medium the pigment withstands temperatures up to 300 °C.
2.7.3.2.7 Pigment Red 243
P.R.243, the barium salt of a Naphthol AS pigment, affords a dull, yellowish to medium red. The pigment is somewhat sensitive to solvents. P.R.243 is recommended for use in plastics. It is almost completely bleed resistant in plasticized PVC, but fails to satisfy more stringent lightfastness requirements. Transparent samples (0.1% pigment) equal merely step 4 on the Blue Scale, while opaque formulations (1/3 SD) match only step 3. Incorporated in HDPE, the pigment affects the shrinkage of the polymer. It is recommended specifically for use in LDPE.
2.7.3.2.8 Pigment Red 247
The primary area of application for P.R.247 is in plastics, in which it produces medium to bluish, brilliant and opaques shades or red. Its tinctorial strength is moderate: 0.28% pigment is needed, for instance, to formulate 1/3 SD HDPE samples containing 1% TiO2. Such samples equal step 6–7 on the Blue Scale for lightfastness.
P.R.247 exhibits excellent heat stability as it withstands temperatures up to 300 °C. The shrinkage of such partially crystalline plastics is not noticeably affected. P.R.247 is not completely bleed resistant in plasticized PVC, but it shows good lightfastness. Transparent (0.1% pigment) and opaque colourations (0.01% pigment + 0.5% TiO2) equal step 6–7 on the Blue Scale for lightfastness. The same values apply if the pigment is incorporated in rigid PVC. P.R.247 is also used to colour PS, ABS and polyacetal. Excellent heat stability makes it a suitable candidate for PP spin dyeing.
Some time ago a different crystal modification [95] of the same chemical structure became commercially available. Originally listed in the Colour Index with the C.I. Generic Name Pigment Red 247:1, this name has now been changed to P.R.247. The new designation is in accordance with other pigments equally existing in different crystal modifications, such as the quinacridone pigment P.V.19, but are listed under the same C.I. Generic Name and C.I. Constitution Number.
The main application area of the new β-crystal modification is also in plastics. It affords a much yellower shade than the other α-crystal phase and shows similar tinctorial strength. 1/3 SD HDPE samples (1% TiO2), for instance, are formulated with 0.32% pigment. These samples equal step 5–6 on the Blue Scale for lightfastness, which is about one step less than the other modification. The heat stability is excellent, the new type equally withstands temperatures up to 300 °C in HDPE. Partially crystalline plastics show no noticeable shrinkage through the incorporation of this pigment. It is almost completely bleed resistant and in this respect superior to the other modification. The pigment exhibits good lightfastness but is not quite as lightfast as the first type.
This more yellowish grade is also recommended for use in rigid PVC, PS and ABS. In polycarbonate 0.64% pigment is needed to afford 1/3 SD (1% TiO2). These samples withstand temperatures up to 310 °C and equal step 5–6 on the Blue Scale for lightfastness. In PP spin dyeing this more yellowish modification is also heat stable up to 300 °C. The lightfastness of colourations containing 0.3% pigment equals step 4–5 on the Blue Scale, while those with 2% pigment equal step 6.
2.7.4 Naphthalene Sulfonic Acid Pigment Lakes
2.7.4.1 Chemistry, Manufacture, Crystal Structures, Properties and Applications
Naphthalene sulfonic acid pigment lakes are monohydrazone pigment lakes, obtained by using a naphthalene derivative bearing one or two sulfo groups as a coupling component. Typical diazonium compounds are monosubstituted anilines carrying either another SO3H function or a COOH group.
Metal cations are Ba, Na or Al. Some grades are precipitated onto aluminium oxide hydrate.
The pigments are synthesized by diazotizing the aniline derivative or the aniline sulfonic acid with sodium nitrite in hydrochloric acid and subsequently coupling onto the naphthalene sulfonic acid derivative, which is previously dissolved by neutralizing with a sodium hydroxide solution, producing the corresponding hydrazine dye solution.
The pigment is then laked according to the procedure described for β-naphthol pigments (Section 2.7.1.1). Aluminium lakes are an exception. A soluble aluminium salt is first converted into aluminium oxide hydrate, which is washed to remove salt. The moist product is then combined with the dye solution, while a more soluble aluminium salt is added simultaneously. The insoluble pigment is finally washed salt-free and dried. Crystal structures of naphthalene sulfonic acid pigment lakes have not been published.
The properties and applications of these pigments are described in Section 2.7.4.2.
2.7.4.2 Commercially Available Naphthalene Sulfonic Acid Pigment Lakes
2.7.4.2.1 General
These pigments have the following general structures:

Table 2.13 shows the monohydrazone pigment lakes that are based on naphthalene sulfonic acid.
Table 2.13 Commercially available naphthalene sulfonic acid pigment lakes.
| (a) Coupling at the 1-position: | |||||||||

|
|||||||||
| C.I. Name | C.I. Constitution Number | m | M | Shade | |||||
| P.Y.104 | 15985:1 | H | H | H | H | 3 | 2 Al | reddish yellow | |
| P.O.19 | 15990 | Cl | H | H | H | H | 2 | Ba | orange |
| P.O.79 | — | H | COO− | H | H | H | 1 | Sr | orange [96] |
| P.R.60 | 16105 | COO− | H | H | H | 2 | 3 Ba | bluish red | |
| P.R.273 | 16035:1 | OCH3 | CH3 | H | H | 3 | 2 Al | yellowish red | |
| P.R.274 | 16255:1 | DC: 1-amino naphthalene-4-sulfonate | H | 1 | Al | bright red | |||
| P.R.276 | — | Solid solution of: | scarlet | ||||||
| Cl | C2H5 | H | H | 1 | Sr | ||||
| CH3 | Cl | H | H | 1 | Sr | ||||
| P.R.277 | — | Solid solution of: | bluish red [97] | ||||||
| Cl | C2H5 | H | H | 1 | Sr | ||||
| DC: 2-amino naphthalene-1-sulfonate | H | H | 1 | Sr | |||||
| P.V.52 | — | OCH3 | H | H | H | 1 | Sr | violet [98] | |
| (b) Coupling at the 7-position: | |||||||||

|
|||||||||
| C.I. Name | C.I. Constitution Number | X | m | M | Shade | ||||
| P.R.66 | 18000:1 | H | CH3 | H | 2 | Ba, 2Na | red | ||
| P.R.67 | 18025:1 | OCH3 | H | Cl | 2 | Ba, 2Na | bluish red | ||
2.7.4.2.2 Pigment Yellow 1043)
P.Y.104, an aluminium salt, has been approved for use in food, pharmaceuticals and cosmetics, and appropriate purity standards have been developed. The pigment is known under the designation E 110 throughout the European Community and as FD&C Yellow No. 6 in the USA. It produces reddish shades of yellow. Like several other aluminium lakes of simple dyes, P.Y.104 exhibits poor fastness to organic solvents and limited bleeding fastness. Similarly, it is not fast to soap and alkali. P.Y.104 exhibits very little tinctorial strength in various media and is not lightfast.
2.7.4.2.3 Pigment Orange 19
P.O.19 has stimulated only limited industrial interest and is rarely used in Europe. It provides a shade that in white reductions resembles that of P.O.34, but which is much duller. Its full shade is noticeably yellower than that of P.O.34. P.O.19, incorporated in medium-oil alkyd resin systems, is less lightfast, both in full shade and in white reductions, than opaque P.O.34 types. The commercially available type exhibits poor rheological behaviour.
2.7.4.2.4 Pigment Orange 79
Pigment Orange 79, CAS 250640, is a bright orange pigment of the class of hydrazone naphthalene sulfonic acid pigment lakes (strontium lake) [99].
This pigment has been adopted as the preferred orange pigment for food packaging and containers due to its extremely low warpage and shrinkage. It is heat stable up to 285 °C and is compatible with a wide variety of polymers including ABS, PET, PC, polyolefins and styrenics. This product has been in the market since 1995 and in that time has replaced other oranges for food packaging applications. Pigment Orange 79 complies with the FDA requirements for food contact under the conditions of use (A) through (H) as described in Table 2 of 21 CFR Part 196.170(c) where this pigment is allowed to be used at levels up to 1% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR Part 178.3297.
P.O.79 has exhibited very low distortion and warpage characteristics when moulded in polyolefins.
2.7.4.2.5 Pigment Red 60
P.R.60, which was patented as early as 1902 by Meister Lucius & Brüning, has lost much of its importance in recent years. Today, it is mostly used throughout the USA and Japan. P.R.60 affords bright bluish red shades, which are referred to as shades of scarlet. Its tinctorial strength is poor compared to that of other pigment lakes with similar shades. P.R.60 shows limited fastness to acid, alkali and soap, and exhibits poor lightfastness. Several commercial types, which feature the pigment as a barium salt laked onto aluminium oxide hydrate, provide improved colouristic and fastness properties. Zinc oxide, added as the pigment precipitates, affects only the colouristic properties of the pigment. The exact chemical composition of such types is therefore very complex.
P.R.60 is used throughout the printing ink industry, especially for inexpensive packaging and metal deco printing inks. It does not bronze. Since the pigment is sensitive to water, problems may occur in offset printing. P.R.60 is used increasingly as a colourant for plastics, such as PVC, polyethylene, and especially for LDPE and polystyrene. Its lightfastness is satisfactory in these media.
P.R.60 is also used for emulsion paints and in paper mass colouration.
2.7.4.2.6 Pigment Red 66
P.R.66, a barium salt, is sold only in the USA. The pigment is also available as an aluminium oxide hydrate precipitate. Its shade is considered a brilliant medium red, which is somewhat yellower than that of the chemically related P.R.67. Commercial types of P.R.66 are very transparent. The pigment is highly sensitive to acid, alkali and soap. Its fastness to organic solvents is poor, as it its fastness to overcoating. P.R.66 exhibits limited lightfastness. Its main application is in metal deco printing.
2.7.4.2.7 Pigment Red 67
P.R.67, a barium salt, is also available in the form of an aluminium oxide hydrate precipitate. Its shade is bluer than the chemically related P.R.66; it is referred to as a bright bluish red. Commercial types are transparent and tinctorially strong. P.R.67 is used especially in metal deco printing. The prints do not tolerate acid, alkali or soap. They show only limited fastness to organic solvents and to clear lacquer coatings. P.R.67 prints are not fast to sterilization and only poorly lightfast. The pigment is also used for the colouration of rubber. It exhibits good resistance to common oxidants and does not tend to migrate.
2.7.4.2.8 Pigment Red 276
This is a solid solution of two pigments (CAS No. 197566-90-8 and CAS No. 197566-87-3) [100]. P.R.276 exhibits a bright scarlet shade and is predominantly useful in plastic applications that do not require exterior weatherfastness.
Pigment Red 276 is FDA compliant under 21 CFR Part 176.170(c) at 2% by weight of the polymer subject to provisions and definitions described in Title 21 CFR Part 178.3297. It is primarily used in food packaging applications and in mouldings when its superior warpage characteristics to DPP chemistry is needed. The heat stability of P.R.276 approaches 285 °C in masstone and all tint levels. Although the pigment is of modest colour strength in light tints, it has steadily gained in acceptance since its inception in the mid-1990s due to its excellent value in the above applications.
2.7.4.2.9 Pigment Red 277
Like P.R.276, this is a solid solution of two pigments (CAS No. 197566-90-8 and CAS No. 250639-69-1) [101]. The bluish red pigment was developed in mid-1990s as a heat stable pigment specifically for the plastics packaging market. Pigment Red 277 has very little use in applications where weatherability is needed, but fills an essential position in plastic packaging and other interior plastic durable goods. The pigment is heat stable up to 285 °C in masstone and tint, and although of modest colour strength in light tints, it delivers good economic value in the masstone and near masstone levels.
P.R.277 is FDA compliant under 21 CFR Part 176.170(c) at 2% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR Part 178.3297. By virtue of this compliance, P.R.277 finds its way into many food-packaging applications. In moulding, P.R.277 has desirable low-warpage characteristics.
2.7.4.2.10 Pigment Violet 52 [102]
This laked violet hydrazone pigment has excellent heat stability of above 310 °C. P.V.52 has been developed for the plastics industry where it brings good value to the packaging and interior household durables segments. Tinctorially it is quite strong and similar in colour to quinacridone violet and can be used as cost-effective diluent, but its use is restricted to the interior due to its low fastness properties. Pigment Violet 52 is redder than Pigment Violet 51 (Section 4.4.9) and is in compliance with the FDA requirements for food contact under the conditions of use, (A) through (H) as described in Table 2 of 21 CFR Part 176.170(c), where the pigments are allowed to be used at levels of up to 2% by weight of the polymer subject to the provisions and definitions described in Title 21 CFR part 178.3297. The pigment exhibits very low warpage characteristics in polyolefin mouldings.
2.8 Benzimidazolone Pigments
Benzimidazolone pigments [103, 104] derive their name from the 5-(carbonylamino)benzimidazolone group (22), which is common to all pigments within this group:

Although, strictly speaking, it would be more precise to refer to such compounds as benzimidazolone hydrazone pigments, the convention of listing them as benzimidazolone pigments will be followed.
Basically, there are four ways of improving the solvent and migration fastness of an organic pigment (see Section 1.4.4):
- enlarging the pigment molecule, as with diaryl pigments or dihydrazone condensation pigments;
- introducing substituents into the pigment molecule that reduce the solubility of their host structure;
- laking,
- formation of metal complexes.
Benzimidazolone pigments correspond to (b).
Historically, option (c) (laking) is the oldest. Already in the nineteenth century it was observed that colourants could be rendered insoluble in organic media if they were converted into polar structures: molecules containing sulfo or carboxylic acid groups form insoluble salts with alkaline earth metals or manganese (‘lakes', Section 2.7).
Diaryl pigments (option (a)) were invented in 1911 and entered the market in 1935. In parallel option (b) was applied, by introducing groups to improve the hydrophilicity of the parent structure to a certain extent, but not enough to render it soluble in water. Best results were achieved by the carbonamide function. The resulting Naphthol AS pigments were first synthesized in 1911. Additional introduction of several such groups, for instance into Naphthol AS pigments, resulted in very solvent-fast and migration resistant pigments (Section 2.6.2).
Later, five- and six-membered heterocyclic rings were introduced into the pigment molecule. The most effective amongst these are benzimidazolone (5), tetrahydroquinazoline-2,4-dione (23) and tetrahydroquinoxaline-2,3-dione (24):

Benzimidazolone pigments appeared on the market in around 1970. The latest developments are pigments based on tetrahydroquinoxaline-2,3-dione (24), with the first (and hitherto only) pigment of this class being produced since about 2000 (P.Y.213, by Clariant, see Section 2.8.5). Pigments based on tetrahydroquinazoline-2,4-dione (23) have been described in patents, but they have not yet been produced industrially. In all cases it is most advantageous to introduce the heterocyclic ring as part of the coupling component. Amongst them, benzimidazolone pigments are of the highest commercial interest.
The most important coupling component for pigments in the yellow range is 5-acetoacetylamino-benzimidazolone, also called ‘acetolone' (25), while red pigments are derived primarily from 5-(2′-hydroxy-3′-naphthoyl)-aminobenzimidazolone, also called ‘naphtholone' (26):

Compound (25) parallels the acetoacetarylides of the monohydrazone yellow pigment series. Red and brown pigments, on the other hand, which are obtained with (26), find their counterpart in the corresponding Naphthol AS pigments.
Introducing a benzimidazolone moiety into a monohydrazone yellow or Naphthol AS pigment structure is exceptionally useful in improving the properties of the parent pigments. This is especially true not only for the solvent and migration fastness but also for the lightfastness and weatherfastness of these ‘host' pigments. It is on the basis of their improved fastness properties that benzimidazolone pigments are suitable for applications with more stringent fastness requirements. Benzimidazolone pigments are amongst the products with the highest fastness standards in the hydrazone range.
2.8.1 Chemistry, Manufacture and Crystal Structures
Various synthetic pathways have been proposed to prepare the benzimidazolone skeleton, from which all pigments in this series are derived. The 5-amino derivative should be mentioned in particular. It is prepared advantageously from 4-nitro-1,2-diaminobenzene as a starting material, which reacts with phosgene or with urea in the melt to form 5-nitrobenzimidazolone. Subsequent reduction yields 5-aminobenzimidazolone:
The chemistry and manufacture of the coupling components for yellow/orange and red benzimidazolone pigments are treated separately.
2.8.1.1 Coupling Components for Yellow and Orange Benzimidazolone Pigments
Yellow and orange benzimidazolone pigments are derived from the following general structure:

RD: for example is Cl, Br, F, CF3, CH3, NO2, OCH3, OC2H5, COOH, COOAlkyl, CONH2, CONHC6H5, SO2NHAlkyl, SO2NHC6H5; m = 0–3.
The synthesis of the coupling component 5-acetoacetylaminobenzimidazolone (25) corresponds to the preparation of acetoacetarylides (Section 2.1.2) from 5-aminobenzimidazolone by reaction with diketene or acetoacetic ester:

2.8.1.2 Coupling Components for Red Benzimidazolone Pigments
Red benzimidazolone pigments, which cover the entire range of shades in the red and brown part of the spectrum, are based on the following general structure:

RD and m represent the same atoms, groups and numbers as in the corresponding yellow pigments (Section 2.8.1.1).
The coupling component, naphtholone (26), is prepared like a Naphthol AS derivative. 5-Aminobenzimidazolone is treated with 2-hydroxy-3-naphthoic acid chloride or with 2-hydroxy-3-naphthoic acid and phosphorus trichloride in an organic solvent (Section 2.1.2):

2.8.1.3 Pigment Synthesis and Aftertreatment
A benzimidazolone pigment is prepared by diazotizing the corresponding aromatic amine and subsequently coupling the diazo component onto a suspension of the coupling component. 5-Acetoacetylaminobenzimidazolone or 5-(2′-hydroxy-3′-naphthoyl)aminobenzimidazolone is dissolved in an alkaline solution. It is necessary to reprecipitate the product with acid (usually with acetic or hydrochloric acid) in the presence of a surfactant, since coupling reactions in alkaline media do not afford uniform products. The suspended coupling component now consists of particles small enough to make it react in a coupling reaction.
Coupling reactions, which are commonly carried out in aqueous media, afford benzimidazolone pigments, usually in the form of hard particles. Thermal aftertreatment is necessary to adapt these crude products to the demands of technical application (Section 2.2.3).
Subsequent finishing of the crude product typically involves heating the aqueous pigment suspension, frequently to temperatures of 100–150 °C. The crude pigment slurry is thus heated under pressure. This technique may be varied to a certain extent. It is possible, for instance, to add or to exclusively use either water-soluble or water-immiscible organic solvents or to add surface-active non-ionic, anionic or cationic agents.
2.8.1.4 Crystal Structures
Single crystals of yellow and a red benzimidazolone pigment were already studied by X-ray diffraction analysis around 1972, including a non-commercial yellow pigment (27) as well as Pigment Red 208 (28) [105–107]:

Scheme 2.6 Molecular structure of P.R.208
The molecular structures exhibit the same features as most other hydrazone pigments:
- presence of the ketohydrazone form;
- largest possible number of intramolecular hydrogen bonds;
- hydrogen bonds connecting three atoms (bifurcated H bonds);
- almost entirely planar molecules.
In contrast to many ‘simple' monohydrazone yellow, diaryl, β-Naphthol and Naphthol AS pigments, the molecules show not only intramolecular hydrogen bonds but also intermolecular hydrogen bonds. Hence the molecules are connected to their neighbouring molecules not only by van der Waals and Coulomb interactions, but also by hydrogen bonds. The hydrogen bonds can hardly be broken by organic solvents or any other organic medium such as polymers. This explains the excellent solvent and migration fastness that is typical of all benzimidazolone pigments.
In compounds 27 and 28 the molecules are connected to two neighbouring molecules by two hydrogen bonds each, leading to molecular chains (29, 30):


In P.R.208 (30) the chains arrange in layers. The n-butyl group, which is an unusual substituent for organic pigments, since it generally enhances the solubility, is apparently necessary in P.R.208 to fill the space between the molecules (Figure 2.37).
In 2009 a series of crystal structures of yellow benzimidazolone pigments were determined from X-ray powder data [108]. The investigations revealed a large variety of H-bond motifs: In P.O.36, P.Y.154 and the non-commercial compound 27 two molecules are connected to pairs via an eight-membered hydrogen-bonded OCNH![]() OCNH ring. The second NH group of the benzimidazolone fragment forms a hydrogen bond to the acetyl group of a neighbouring molecule, leading to a double-chain (Figure 2.38). These double chains arrange in layers for P.O.36 (Figure 2.39), in wavy layers for P.Y.154 (Figure 2.40), and in a herringbone pattern for 27 (Figure 2.41). In P.O.62 the benzimidazolone moiety forms a hydrogen bond with the nitro group of a neighbouring molecule (Figure 2.42). In P.Y.151 the eight-membered hydrogen-bond ring is not formed by two benzimidazolone groups, but by a benzimidazolone and a COOH group; consequently, the pairs of molecules are held together not only by two but by four hydrogen bonds. The pairs again are connected into chains (Figure 2.43). In P.Y.194 the benzimidazolone moieties do not form an eight-membered hydrogen-bond ring, but arrange in a spiral (Figure 2.44). In the β-phase of P.Y.181 these spirals are supported by hydrogen bonds of the two additional CONH moieties on the diazonium component, resulting in a complicated two-dimensional hydrogen bond network (Figure 2.45). Apparently the good application properties of benzimidazolone pigments do not depend on a singular type of hydrogen-bond motif, but on the formation of a high number of intermolecular hydrogen bonds in combination with an effective, dense packing of molecules [108].
OCNH ring. The second NH group of the benzimidazolone fragment forms a hydrogen bond to the acetyl group of a neighbouring molecule, leading to a double-chain (Figure 2.38). These double chains arrange in layers for P.O.36 (Figure 2.39), in wavy layers for P.Y.154 (Figure 2.40), and in a herringbone pattern for 27 (Figure 2.41). In P.O.62 the benzimidazolone moiety forms a hydrogen bond with the nitro group of a neighbouring molecule (Figure 2.42). In P.Y.151 the eight-membered hydrogen-bond ring is not formed by two benzimidazolone groups, but by a benzimidazolone and a COOH group; consequently, the pairs of molecules are held together not only by two but by four hydrogen bonds. The pairs again are connected into chains (Figure 2.43). In P.Y.194 the benzimidazolone moieties do not form an eight-membered hydrogen-bond ring, but arrange in a spiral (Figure 2.44). In the β-phase of P.Y.181 these spirals are supported by hydrogen bonds of the two additional CONH moieties on the diazonium component, resulting in a complicated two-dimensional hydrogen bond network (Figure 2.45). Apparently the good application properties of benzimidazolone pigments do not depend on a singular type of hydrogen-bond motif, but on the formation of a high number of intermolecular hydrogen bonds in combination with an effective, dense packing of molecules [108].

Figure 2.37 P.R.208. Chains of molecules (vertical), arranged in a layer. On the right-hand side, only half a chain is drawn.

Figure 2.38 Double-chain of molecules in P.O.36.

Figure 2.39 Layer structure of P.O.36.

Figure 2.40 Wavy layer structure of P.Y.154.

Figure 2.41 Herringbone arrangement of double-chains in the non-commercial compound 27 (structure determined from single-crystal data).

Figure 2.42 Crystal structure of P.O.62 with the nitro group acting as hydrogen-bond acceptor.

Figure 2.43 Crystal structure of P.Y.151. The benzimidazolone moiety forms a mixed eight-membered hydrogen-bonded ring with the COOH group of a neighbouring molecule.

Figure 2.44 P.Y.194, exhibiting a spiral of benzimidazolone moieties (indicated by the arrow).

Figure 2.45 Hydrogen bond pattern in P.Y.181. The spiral of the benzimidazolone units is indicated by an arrow.
The crystal structure of P.O.72 could not be determined hitherto, because the compound is completely insoluble in all solvents and resists any recrystallization attempts. The pigment is of inherently low crystallinity, causing broad peaks in the powder diffractogram; consequently, none of the powder diagrams can be indexed and the structure could not be solved from X-ray powder data either. Modelling the crystal structure by lattice-energy minimization [109] suggests that the commercial orange β-phase exhibits a two-dimensional hydrogen bond network with six intermolecular hydrogen bonds per molecule, which would explain the observed insolubility, thermal stability and solvent and migration fastness.
2.8.2 Properties
Pigments obtained by using 5-acetoacetylaminobenzimidazolone as a coupling component cover the range from very greenish yellow to orange shades. Products derived from 5-(2′-hydroxy-3′-naphthoylamino)benzimidazolone, on the other hand, extend this range towards the yellowish red region to include all major shades of red, including bordeaux, maroon and carmine. Technically important brown pigments are also obtained by using 5-(2′-hydroxy-3′-naphthoylamino)benzimidazolone as a coupling component.
Benzimidazolone pigments vary considerably in their tinctorial strength. The different physical characteristics of the various types, especially the wide spectrum of particle size distributions, contribute appreciably to the differences in tinctorial strength. Several pigments produce relatively strong colourations, while others are comparatively weak. Formulating 1/3 SD samples with 5% TiO2 in plasticized PVC, for instance, requires between 3.6% (of the weakest type) and 0.4% pigment (of the strongest representative). Benzimidazolone pigments in the yellow and orange series are somewhat comparable to monohydrazone yellow pigments in terms of tinctorial strength, while the red types parallel Naphthol AS pigments in strength.
The benzimidazolone moiety, a fundamental feature of all benzimidazolone pigments, is responsible for the high fastness of these pigments to the solvents that are typically found in application media. In this respect, benzimidazolone pigments perform much better than their counterparts in the monohydrazone yellow and Naphthol AS pigment series. Excellent fastness to solvents and chemicals is accompanied by good migration fastness. Benzimidazolone pigments do not bloom, and most of them show good and some even excellent bleed fastness and fastness to overcoating. All benzimidazolone pigments, with one exception (P.Y.151), are inert to alkali and acid. Most of them disperse easily in the common application media.
A considerable number of benzimidazolone pigments meet the major heat stability standards for practical application, while some are even amongst the most heat stable of organic pigments known. Moreover, benzimidazolone pigments as a group are characterized by excellent lightfastness, especially those covering the yellow and orange range, and a high degree of weatherfastness.
Different types are available as standard products featuring optimized physical parameters to satisfy the demands of particular applications. A custom-made product may be adapted to the customer's demand regarding transparency or opacity, flow behaviour, or certain fastness properties.
Polymorphism is a common phenomenon in benzimidazolone pigments. However, each pigment is marketed in only one of its crystal modifications. The polymorphic form can have a strong effect on the properties. For example, the α-phase of P.O.36 is brown, whereas the β-phase is orange. P.O.72 even exhibits four crystal phases with bright orange, dull red and dark brown shades.
2.8.3 Application
Representatives of the benzimidazolone pigment series are used on a broad scale in almost all areas of pigment application, primarily for their excellent fastness properties. Benzimidazolone pigments satisfy higher or even very high specifications in application, especially regarding lightfastness and weatherfastness, heat stability, chemical inertness and migration fastness.
Benzimidazolone pigments are used throughout the paint industry to lend colour to all types of industrial finishes.
Numerous representatives fulfil the standards for use in automotive refinishes. Some even satisfy highest durability specifications and are used in original automotive finishes, sometimes even as shading pigments, or in metallic finishes. Areas of application include finishes for commercial vehicles, agricultural machinery and implements, as well as other high grade industrial finishes.
Certain commercial varieties with particularly high transparency provide transparent or metallic effects in finishes. Other grades are optimized in terms of high opacity and may, for instance, offer a technical alternative to inorganic Chrome Yellow or Molybdate Red pigments. Excellent rheological properties make it possible to increase the pigment concentration in a paint without affecting the gloss. The hiding power of the paint is thus open to further improvement. Such high opacity products are frequently used in combination with inorganic pigments, such as Chrome or Nickel Titanium Yellow, or iron oxide pigments. Opaque grades show excellent lightfastness and weatherfastness over a range of shades. However, in several pigments these fastness properties deteriorate rapidly as the concentration of white pigment increases. Pigment application frequently requires fastness to overcoating, a specification that at common baking temperatures is satisfied by several benzimidazolone pigments in many systems.
Several benzimidazolone pigments are suitable candidates for use in powder coatings based on polyester, acrylic or polyurethane resin. These pigments satisfy the heat requirements of this application and do not plate out in these media (Section 1.6.4.1). Various benzimidazolone pigments even meet the particularly high thermal standards of coil coating. These pigments are also suitable for use in architectural and emulsion paints.
Benzimidazolone pigments, especially those covering the red range of the spectrum, were originally developed and used mostly for plastics. None of them were found to adversely affect the physical characteristics of their host medium. Benzimidazolone pigments do not bloom in plasticized PVC and other polymers. They are usually bleed resistant under typical application conditions.
In PVC, most benzimidazolone pigments are heat stable up to 220 °C. They have excellent to outstanding lightfastness. Some representatives are very weatherfast in impact resistant PVC types and in rigid PVC and even withstand long-term weathering. Various benzimidazolone pigments are used, for instance by the automobile industry, in PVC plastisols to lend colour to synthetic leather.
Incorporated in polyolefins, benzimidazolone pigments vary considerably in terms of heat stability. These pigments may tolerate temperatures from less than 200 to 300 °C. Specific grades are available for use in various types of polyolefins, depending on the respective heat requirements. Pigments are thus custom-made for use in HDPE, LDPE or PP. There are many varieties that do not affect the shrinkage of polyolefins in injection moulding. Benzimidazolone pigments are therefore used to advantage in thick-walled, large, non-symmetrical injection-moulded articles, such as bottle crates.
Benzimidazolone pigments are also used to colour polystyrene, ABS and other polymers that are processed at high temperature. Several grades show excellent lightfastness and also satisfy the heat requirements for use in unsaturated polyester without affecting the hardening of the polymer.
Various benzimidazolone pigments are heat stable enough to be used in polypropylene spin dyeing. Several types find extensive use in the spin dyeing of other fibres, such as polyacrylonitrile, viscose rayon, and viscose cellulose, or secondary acetate.
The printing ink industry employs benzimidazolone pigments to colour high grade printing inks. The transparent grades have stimulated particular interest in connection with this area. Benzimidazolone pigments usually perform well in print, most of them are fast to clear lacquer coatings and may safely be sterilized. More heat stable grades withstand exposure to up to 220 °C for 30 min and satisfy the stringent requirements of metal deco printing. In print, like in other applications, most grades are very lightfast. It is their lightfastness that makes them suitable colourants for long-term products, such as posters or other advertising items. Benzimidazolone pigments are frequently selected for their good solvent fastness, fastness to plasticizers and migration fastness in printing inks for PVC films. Some products are particularly interesting as colourants in decorative printing inks for plastic laminates.
Commercial types of benzimidazolone pigments are also sold for use in solvent-based wood stains and other special-purpose media not been mentioned previously.
2.8.4 Commercially Available Benzimidazolone Pigments
Table 2.14 lists commercially available benzimidazolone pigments. Notably, pigments in the red range are often derived from a diazonium component carrying a methoxy group at the ortho position to the hydrazone group.
Table 2.14 Commercially available benzimidazolone pigments.
| Yellow and orange series | 
|
||||||
| C.I. Name | C.I. Constitution Number | Shade | |||||
| P.Y.120 | 11783 | H | COOCH3 | H | COOCH3 | yellow | |
| P.Y.151 | 13980 | COOH | H | H | H | greenish yellow | |
| P.Y.154 | 11781 | CF3 | H | H | H | greenish yellow | |
| P.Y.175 | 11784 | COOCH3 | H | H | COOCH3 | very greenish yellow | |
| P.Y.180 | 21290 | (a) | H | H | H | greenish yellow | |
| P.Y.181 | 11777 | H | H | (b) | H | reddish yellow | |
| P.Y.194 | 11785 | OCH3 | H | H | H | yellow | |
| P.O.36 | 11780 | NO2 | H | Cl | H | orange | |
| P.O.60 | 11782 | Cl | H | H | CF3 | reddish yellow | |
| P.O.62 | 11775 | H | H | NO2 | H | yellowish orange | |
| P.O.72 | 211095 | See formula (c) | orange | ||||
 |
|||||||
 |
|||||||
 |
|||||||
| Red and brown series | 
|
||||||
| C.I. Name | C.I. Constitution Number | Shade | |||||
| P.R.171 | 12512 | OCH3 | NO2 | H | maroon | ||
| P.R.175 | 12513 | COOCH3 | H | H | bluish red | ||
| P.R.176 | 12515 | OCH3 | H | CONHC6H5 | carmine | ||
| P.R.185 | 12516 | OCH3 | SO2NHCH3 | CH3 | carmine | ||
| P.R.208 | 12514 | COOC4H9(n) | H | H | red | ||
| P.V.32 | 12517 | OCH3 | SO2NHCH3 | OCH3 | bordeaux | ||
| P.Br.25 | 12510 | Cl | H | Cl | brown | ||
2.8.4.1 The Yellow and Orange Series
2.8.4.1.1 Pigment Yellow 120
P.Y.120 affords a medium yellow shade. The pigment powder shows good fastness to solvents, in which it is similar to other yellow pigments within this class.
P.Y.120 is primarily applied in plastics, especially in PVC. A pigment preparation is available for this purpose. P.Y.120 is very bleed resistant in plasticized PVC and has excellent lightfastness in both plasticized and rigid PVC. 1/1 to 1/25 SD samples (5% TiO2) as well as transparent formulations equal step 8 on the Blue Scale for fastness to daylight. The pigment, however, is much less weatherfast in lead/cadmium stabilized rigid PVC than other representatives of its class, which precludes its use in exterior application. P.Y.120 is heat stable enough in HDPE for 1/3 SD samples (1% TiO2) to tolerate exposure to 270 °C for 5 min. The shrinkage of the polymer is not affected. Incorporated in LDPE, P.Y.120 withstands approximately 220 °C, in polystyrene 240 °C. Transparent HDPE colourations (0.1%) equal step 8 on the Blue Scale for lightfastness, while opaque samples (0.01% pigment + 0.5% TiO2) match step 6–7.
The printing ink industry uses P.Y.120 primarily for decorative printing inks on laminated melamine and polyester resin sheets. In terms of lightfastness, 8% gravure prints in 20 and 40 µm cells on such plates correspond to step 8 on the Blue Scale. Plate-out is not observed. P.Y.120 is insoluble in monostyrene and acetone (Section 1.8.1.2).
P.Y.120 lends itself to all printing methods. Its lightfastness in letterpress and offset prints is also very good. Depending on the depth of shade, prints equal step 7 to step 6 on the Blue Scale. P.Y.120 thus scores approximately 1 step higher than the similarly coloured P.Y.97.
P.Y.120 is used primarily to colour high grade gravure inks for posters, metal sheets and packaging purposes. The pigment has gained particular recognition in gravure inks for plasticized PVC. The prints are completely resistant to the DIN 16524 standard solvent mixture, as well as to various organic solvents and media, including toluene, mineral spirits, methyl ethyl ketone, ethyl acetate, paraffin, butter, soap, alkali, and acid. Moreover, the prints are fast to clear lacquer coatings and may safely be sterilized. They are heat stable up to 200 °C for 30 min.
P.Y.120 has less of an impact on the paint industry. In contrast to the similarly coloured P.Y.97, P.Y.120 is fast to overcoating. Moreover, it exhibits noticeably higher durability. White reductions, reduced 1:1 to about 1:5, are approximately as durable as P.Y.151 systems. P.Y.120 is recommended for general industrial finishes, including automotive refinishes, and it is also suitable for use in architectural paints. P.Y.120 is completely fast to alkali.
2.8.4.1.2 Pigment Yellow 151
P.Y.151, available since 1971, affords a clean greenish yellow shade. Its hue is somewhat greener than that of P.Y.154 and distinctly redder than that of P.Y.175. The type which features a specific surface area of less than 20 m2 g−1 provides good hiding power.
P.Y.151 maintains an important position throughout the pigment industry. Its main area of application is in the paint industry, which uses P.Y.151 particularly for high grade industrial finishes. Good rheological properties make it possible to incorporate up to approximately 30% pigment in a paint without affecting the gloss of the coating. The percentage value is given relative to the amount of solid binder. For comparison, full shade paints containing other pigments are usually restricted to a pigment concentration of 10–15%. P.Y.151 is frequently used in combination with TiO2 and/or inorganic yellow pigments. Very lightfast and weatherfast P.Y.151/phthalocyanine green pigment combinations are equally important; they are sometimes used in conjunction with the above-mentioned inorganic pigments. As a rule, P.Y.151 is employed to produce deeper shades, which makes it an interesting product for manufacturers of automobile (O.E.M) finishes and automotive refinishes and paints for commercial vehicles.
Coatings containing P.Y.151 are very lightfast and durable. Systems based on acrylic melamine resin, for instance, were exposed to the Florida climate for one year and then evaluated. 1:1 reductions with TiO2 equalled step 5 on the Grey Scale for weatherfastness, while 1:3 TiO2 reductions matched step 4, and 1:35 reduced samples coincided with step 3–4. Comparative values for P.Y.154 and 175 are listed for the respective pigments. P.Y.151 is fast to overcoating up to 160 °C.
P.Y.151 is heat stable up to a maximum of 200 °C and withstands acid, but is affected by alkali under certain test conditions. Although the pigment tolerates weak bases, it will undergo a distinct colour change towards reddish yellow shades if it is exposed to strong alkali. P.Y.151 is also affected by lime (Section 1.6.2.2), which almost entirely precludes its use in emulsion paints.
The plastics industry uses P.Y.151 to colour PVC, polyolefins, and other polymers. The pigment shows excellent migration fastness in plasticized PVC. Plasticized PVC samples up to 1/25 SD equal step 8 on the Blue Scale for lightfastness. The pigment exhibits good weatherability in rigid PVC, but performs distinctly poorer in this respect than P.Y.154 and 175, and it is therefore usually unacceptable for long-term exposure. 1/3 SD P.Y.151 samples in HDPE (with 1% TiO2) show a heat stability of 260 °C for 5 min. Temperatures in excess of this value shift the colour towards the reddish side of the spectrum and decrease its brightness. The shrinkage of the polymer is only slightly affected at processing temperatures between 220 and 280 °C. P.Y.151 is equally heat stable in polystyrene, as long as the processing temperature does not exceed 260–280 °C. The pigment is very lightfast in this medium (step 8).
P.Y.151 is employed for printing inks wherever lightfastness is a prime consideration. 1/1 SD letterpress proof prints equal step 7 on the Blue Scale for lightfastness, while 1/3 SD prints match step 6–7. The prints are fast to soap but not sufficiently alkali resistant. They are fast to clear lacquer coatings, but not fast to sterilization. P.Y.151 finds extensive use in offset and letterpress applications, as well as in packaging gravure inks for PVC substrates. It is equally suitable for decorative printing inks for laminated plastic sheets based on polyester resin. P.Y.151 is insoluble in monostyrene and acetone. In terms of lightfastness, 8% gravure prints (etching depth 20 and 40 µm) equal step 8 on the Blue Scale. P.Y.151 should not be used for laminated melamine resin sheets. It is not compatible with aqueous melamine resin solutions, in which it dissolves to a certain extent.
2.8.4.1.3 Pigment Yellow 154
P.Y.154, which was introduced in the mid-1970s, affords a somewhat greenish yellow shade of very high lightfastness and weatherfastness. Its shade is distinctly redder than that of P.Y.175 and noticeably redder than that of P.Y.151, both of which are also members of the benzimidazolone series. P.Y.154 is completely or at least almost completely resistant to the major organic solvents. The list includes alcohols, esters, such as butyl acetate, aliphatic and aromatic hydrocarbons, such as mineral spirits or xylene, and dibutyl phthalate.
P.Y.154 is primarily applied in paints, in which it is one of the most weatherfast organic yellow pigments. Incorporated in the same system and tested and evaluated under the same conditions as P.Y.151, for instance, after one year of outdoor exposure in Florida, TiO2 reductions up to 1:3 were found to equal step 5 on the Grey Scale, while 1:30 TiO2 reductions matched step 4–5.
P.Y.154 is thus also a suitable shading component for other hues and may be used to tone reduced clean yellow and green shades.
Irrespective of the concentration, P.Y.154 is recommended for all high grade industrial paints, including automobile (O.E.M.) finishes. Incorporated in baking enamels, it may safely be overcoated up to 130 °C. Temperatures in excess of this value will increase its tendency to bleed, which is only slightly noticeable at 140 °C. The pigment is heat stable up to 160 °C. Its good rheology makes it possible to increase the pigment concentration in a paint without affecting the gloss of the coating. P.Y.154 disperses easily. Areas of application also include other common media throughout the coatings and paints industry, such as architectural paints and emulsion paints. P.Y.154 is employed wherever high lightfastness and weatherfastness are required.
Its principal application within the plastics area is in PVC. P.Y.154 reigns supreme in terms of lightfastness and durability in rigid PVC and in impact resistant PVC types, which makes it a suitable product for exterior use. P.Y.154 also has excellent lightfastness and weatherfastness in PVC plastisols which are coil coated onto steel. Systems of this kind are frequently applied to the exterior of houses. P.Y.154, however, exhibits inferior tinctorial strength. 2.5% pigment is required to formulate 1/3 SD colourations with 5% TiO2 in plasticized PVC. Only 1.4% are needed of the slightly redder P.Y.120, which is also a member of the benzimidazolone pigment series. P.Y.154 has excellent bleeding fastness in plasticized PVC. The heat stability of 1/3 SD samples in HDPE (with 1% TiO2) is only up to 210 °C for 5 min, which practically precludes its use in this polymer. The pigment is equally unsuitable for polypropylene spin dyeing.
P.Y.154 is a useful pigment for the printing ink industry wherever high lightfastness is required. Letterpress proof prints up to 1/25 SD equal step 6–7 on the Blue Scale for lightfastness, which is at least 1 1/2 to 2 steps above that of similarly coloured diarylide yellow pigments or representatives of the monohydrazone yellow pigment series. In print, however, like in plastics, P.Y.154 lacks tinctorial strength. 1/3 SD standardized letterpress proof prints are prepared from inks which contain 25% pigment. For comparison, equally deep samples are prepared from inks which contain 3.5% P.Y.13 or 8.8% P.Y.1, respectively. P.Y.154 prints are not fast to clear lacquer coatings or to sterilization.
The list of applications also includes other media, such as oil colours for artists, in which P.Y.154 is used for its high lightfastness.
2.8.4.1.4 Pigment Yellow 175
P.Y.175, a clean, very greenish yellow pigment, is a comparatively recent product. It has only gained limited commercial recognition. The shade of the commercially available modification is somewhat greener than that of other benzimidazolone pigments and greener than corresponding pigments of other types, such as P.Y.109, 128 or 138.
P.Y.175 is primarily used in the paint field, where it is used especially to colour high grade systems, such as automobile (O.E.M.) and automotive refinishes. The products are used for brilliant yellow or green shades. Shades of green are accessible, for instance, by combining P.Y.175 with phthalocyanine pigments. Despite its comparatively low specific surface area (approx. 20 m2 g−1), the commercially available type exhibits good transparency. However, it is not weatherfast enough to be used in metallic finishes. On the other hand, both lightfastness and durability are excellent in solid shades. In medium white reductions with TiO2, P.Y.175 shows excellent weatherfastness, but this fastness deteriorates rapidly as more white pigment is added. In this respect, P.Y.175 is inferior to the somewhat redder P.Y.154, which is also a member of the benzimidazolone pigment series. Incorporated in an acrylic melamine resin system and exposed to the Florida weather for one year, for instance, P.Y.175 samples which are reduced 1:1 with TiO2 equal step 5 on the Grey Scale, while 1:2.5 samples coincide with step 4, and 1:30 reductions match step 3–4.
P.Y.175 does not bloom. It may safely be overcoated up to 140 °C. At higher temperatures, bleeding is observed to a small extent in various systems. The pigment is heat stable up to 180 °C. The commercially available type exhibits good rheological properties in paints and may therefore be used at higher concentrations.
P.Y.175 is of interest to the plastics industry wherever high lightfastness and weatherfastness are a prime concern. It is, for instance, frequently used for long-term exposure in rigid PVC in white reductions containing little TiO2. The pigment is, however, less durable than P.Y.154. 1/3 SD HDPE samples (with 1% TiO2) are heat stable up to 270 °C. The shrinkage of the polymer is not affected at processing temperatures between 220 and 270 °C (Section 1.6.4.3). However, P.Y.175 is comparatively weak. 0.55% pigment is needed to produce a 1/3 SD sample with 1% TiO2, while only 0.13% is required of the less lightfast, still somewhat greener diarylide yellow pigment P.Y.17. 0.27% of the somewhat redder P.Y.16, on the other hand, is sufficient to achieve the same purpose, although the two latter pigments are considerably less heat stable and lightfast than P.Y.175.
The printing ink industry uses P.Y.175 only for high grade products. Up to 1/25 SD, its lightfastness equals step 6–7 on the Blue Scale. Its limited tinctorial strength, however, is somewhat of a disadvantage. The prints are fast to paraffin, butter, soap, dibutyl phthalate, toluene, mineral spirit, and other media. They are also fast to clear lacquer coatings but may not be sterilized. Prints containing P.Y.175 are heat stable up to 200 °C for 10 min or up to 180 °C for 30 min.
2.8.4.1.5 Pigment Yellow 180
P.Y.180, a greenish to medium yellow shade pigment, was introduced a few years ago. It is a dihydrazone yellow pigment and is of particular interest to the plastics industry and for laser printing.
In HDPE, P.Y.180 is heat stable up to 290 °C. P.Y.180, like P.Y.181, does not affect the shrinkage of the plastics, so that there is no limitation to its use in injection-moulded articles. It is, however, less fast to light than the redder P.Y.181. 1/3 SD colourations (1% TiO2), for instance, equal step 6 on the Blue Scale. 1/3 SD specimens are formulated at a pigment concentration of 0.15%, which indicates good tinctorial strength. P.Y.180 is also used for PP spin dyeing, since it has excellent heat stability and affords clean hues. A special pigment preparation is available for this purpose. Redder shades are accessible through combination with the very reddish P.Y.181. Deep colours have excellent lightfastness: 1/1 to 1/3 SD samples equal step 7 on the Blue Scale. The fastness to light decreases rapidly, however, as more white pigment is added: 1/25 SD specimens match only step 5 on the Blue Scale. P.Y.180 performs excellently in terms of textile fastness properties.
P.Y.180 is becoming increasingly important and is utilized in printing inks to suit particular applications where diarylide yellow pigments cannot be used. Diarylide yellow pigments decompose at temperatures in excess of 200 °C (Section 2.4.1.3), which precludes their use in certain inks for metal deco which are baked at temperatures above 200 °C.
P.Y.180 is an equally important pigment for PVC. It does not migrate in plasticized PVC. Depending on the standard depth of shade and on the TiO2 content, its lightfastness in rigid and in plasticized PVC equals step 5 (0.01% pigment + 0.5% TiO2) to step 6–7 on the Blue Scale (0.1% pigment + 0.5% TiO2). P.Y.180 is also used to colour other plastics which are processed at high temperature. Technical polymers, such as polycarbonate, PS, ABS, and polyester, are particularly common media for P.Y.180. The pigment is also used to colour injection-moulded and extrusion-made polyamide – in contrast to P.Y.181, which is not suitable for this purpose. These plastics melts are slightly basic and have a reducing effect, in contrast to other polymer melts which are neutral.
A special grade is also commercially available which is recommended for the colouration of solvent and water based packaging gravure and flexo printing inks, respectively, and for metal deco printing as well. The grade exhibits good dispersibility and flocculation stability and is especially being employed where the use of diarylide yellow pigments is limited due to their thermal decomposition above 200 °C (Section 2.4.1.3).
2.8.4.1.6 Pigment Yellow 181
P.Y.181, a reddish yellow pigment, was introduced to the market a few years ago. Its main area of application is in plastics, especially in polyolefins. In these media, P.Y.181 is heat stable up to 300 °C and very lightfast. 1/3 SD HDPE samples (1% TiO2), for instance, equal step 8 on the Blue Scale. The pigment does not affect the shrinkage of the partially crystalline polymer. Its tinctorial strength, however, is poor.
P.Y.181 does not migrate in plasticized PVC. Besides, it shows excellent lightfastness in this medium. Both transparent samples (0.1% pigment) and white reduced samples (0.1% pigment + 0.5% TiO2) equal step 8 on the Blue Scale. Even plasticized and rigid PVC samples which contain a higher concentration of white pigment (0.01% pigment + 0.5% TiO2) still match step 7–8 on the Blue Scale.
P.Y.181 is extremely heat stable, which makes it a suitable pigment for other polymers which are processed at high or very high temperature. The list includes PS, ABS, polyester, polyacetal, and various other technical plastics. The pigment is equally interesting in connection with PP spin dyeing. The 0.3% and 3% PP samples equal step 7 and step 7–8 on the Blue Scale, respectively. A special pigment preparation is available for this purpose. P.Y.181 also frequently colours spin dyed viscose rayon and viscose cellulose. In these media, the pigment satisfies the particularly stringent specifications regarding lightfastness and weatherfastness for use in automobile interiors. This is a purpose to which only very few organic yellow pigments are suited.
P.Y.181 is also recommended for use in paints, but in this area it is in direct competition with numerous similarly shaded pigments within the same class as well as from different pigment classes. Inferior tinctorial strength makes P.Y.181 a less important product in this field.
2.8.4.1.7 Pigment Yellow 194
The pigment affords medium yellow shades. Regarding shades and performance it competes with some other pigments, such as the monohydrazone pigment P.Y.97 and the bisacetoacetarylide pigment P.Y.16.
P.Y.194 is gaining increasing importance in liquid industrial coatings and in powder coatings as well. Owing to its solvent fastness in baking enamels it is fast to overpainting at 120 °C, shows a slight bleeding at 140 °C (after 30 min baking time) and is heat stable up to 200 °C. High hiding power and correspondingly a specific surface area of 20 m2 g−1 favour its use for lead free coatings.
The pigment is also employed in the plastics field. A special grade features good tinctorial strength and particularly a good dispersibility. Pigmentation of HDPE in 1/3 SD (1% TiO2) requires 0.17% of pigment. The colourations are heat stable up to 240 °C and the lightfastness corresponds with step 5 on the Blue Scale. The pigment is preferentially used in LDPE.
It is bleeding in plasticized PVC. Dependent on the standard depth of shade in this medium the lightfastness equals step 5–6 on the Blue Scale. The pigment is equally employed for cable insulation, since it influences the specific electrical resistance only negligibly.
2.8.4.1.8 Pigment Orange 36
P.O.36 is a reddish, somewhat dull orange pigment which provides very good lightfastness and weatherfastness. It is a highly important pigment. The commercially available types differ considerably in their hiding power/transparency. Different types vary even in terms of shade – the opaque version is redder and noticeably cleaner – and other properties, as well as fastness properties.
In the production, P.O.36 is obtained upon coupling as a brown α-phase. A subsequent finishing process is needed to gain the desired orange β-phase.
P.O.36 is broad in scope, its main area of application is in paints. Masstone and similar deep shades have excellent lightfastness. The opaque variety, which was introduced in the mid-1970s, performs much better in this respect than the standard grade. In deep shades, P.O.36 meets the requirements for use in automobile (O.E.M.) finishes. The opaque version is the current standard grade in this range of shades for applications which require lead free pigmentation. To suit this purpose, P.O.36 is frequently used in combination with quinacridone pigments to produce paints which feature, for instance, lightfast and durable RAL shades 3000 (fire engine red), 3002 (carmine), 3003 (ruby), and 3013 (tomato red). Small amounts of TiO2 may be added. Very good rheological behaviour in paints makes it possible to increase the concentration of opaque pigment without affecting the gloss. The opaque version of P.O.36 is also used in combination with chromate pigments. Coatings which are pigmented in full and similarly deep shades do not darken as they are exposed to light and weather. The list of applications also includes automotive repair finishes, paints for commercial vehicles and agricultural machines, as well as for general industrial finishes. P.O.36 is used extensively in these areas.
P.O.36 is completely fast to overcoating. Bleeding into a white overcoat is only observed at baking temperatures above 160 °C. P.O.36 is heat stable up to 160 °C. In dispersing the opaque type, it is important to avoid excessive shearing forces and to closely monitor the temperature, especially if agitated ball mills are used. Faulty temperature control may lead to a dull colour.
P.O.36 is used throughout the printing ink industry for letterpress and offset inks, all types of packaging gravure and flexographic inks, and for metal deco printing. The prints are very lightfast. Depending on the grade and on the standard depth of shade, letterpress proof prints up to 1/25 SD equal step 6–7 to step 5 on the Blue Scale. The prints are excellently fast to chemicals and resistant to solvents such as toluene, mineral spirit, methyl ethyl ketone, and to the DIN 16 524 standard solvent blend. They withstand clear lacquer coatings and may safely be sterilized. Prints made from P.O.36 tolerate exposure to 220 °C for 30 min. At equal standard depth, their shade is noticeably duller than that of the somewhat redder P.O.34 and also duller than that of other less fast pigments covering the same range of shades.
The plastics industry uses P.O.36 to colour PVC. 1/3 to 1/25 SD plasticized PVC samples containing 5% TiO2 equal step 8 and step 7, respectively, for lightfastness, while transparent specimens (0.1% pigment) equal step 8 on the Blue Scale. The pigment does not migrate, for example, it is practically resistant to bleeding and blooming. Combinations with carbon black are frequently used to create shades of brown for furniture films. Suitable media include PVC plastisols.
Incorporated in rigid PVC, P.O.36 lacks lightfastness at low concentrations. Barium/cadmium stabilized 1/3 SD colourations in rigid PVC show good durability but are frequently not weatherfast enough to satisfy the requirements for plastics materials in respect of long-term exposure. Heat stability, tested in HDPE at 1/3 SD, is to 220 °C. The pigment does not affect the shrinkage of the plastic. P.O.36 is normally, however, not heat stable enough to meet the standards of practical pigment application in polyolefins.
P.O.36 is also used to colour unsaturated polyester resins. Both transparent and opaque samples exhibit a lightfastness in these media that equals step 7 on the Blue Scale. The pigment does not affect the shrinkage of the plastic.
2.8.4.1.9 Pigment Orange 60
Introduced some years ago the production of this pigment has been discontinued. P.O.60 is a yellowish orange pigment with excellent lightfastness and durability.
Its main area of application was in paints, in which it was used primarily to colour high grade industrial finishes, such as automotive finishes, especially automobile O.E.M. finishes. P.O.60 was used to advantage in white reductions and as a shading pigment. Its response to accelerated weathering under a xenon arc lamp is much inferior to its actual weatherfastness in outdoor exposure, for instance in Florida. The commercially available grade was transparent and exhibited a low specific surface area (less than 20 m2 g−1). This is what made P.O.60 a suitable candidate to produce orange shades in metallic finishes. There is a certain disadvantage to its lack of tinctorial strength, which is attributed to the low specific surface area.
P.O.60 is one of the organic orange pigments with the highest fastness standards. Its durability – even in metallic finishes – equals that of P.Y.154. After one year of outdoor exposure in Florida, acrylic melamine resin systems containing up to 1:25 TiO2 equal step 5 on the Grey Scale for durability. Metallic finishes containing 60% pigment and 40% aluminium paste (65%) and also 40:60 blends equal step 4–5 on the same scale. Comparative values are listed with some other representatives of this class.
P.O.60 is heat stable up to 160 °C. Baking temperatures in excess of this value will in some paint systems cause a decrease in fastness to overcoating.
P.O.60 was basically also used in printing inks and plastics, provided the requirements concerning lightfastness and durability were high. Adding small amounts of TiO2 confers long-term weatherfastness on the pigment, although this advantage is compromised by its poor tinctorial strength. Wherever the requirements are appropriate, P.O.60 was also employed in other application media, such as artists' colours, poster colours and so on.
2.8.4.1.10 Pigment Orange 62
P.O.62 affords a clean, very yellowish shade of orange. The commercially available grade exhibits a low specific surface area of approximately 12 m2 g−1 and has, accordingly, coarse particles that make for good hiding power.
P.O.62 is utilized mostly to colour paints, especially to produce very clean yellowish shades of red or reddish shades of yellow in full and similarly deep shades. Good rheological properties make it possible to employ the pigment at higher concentrations without affecting the gloss. The pigment concentration may reach up to 30% relative to the content of solid binder. Deep shades are very lightfast and weatherfast. Full and related shades of P.O.62 are also recommended for use in automotive finishes. The list of applications includes automotive refinishes, commercial vehicles, agricultural machinery, and other high grade and general industrial paints. This is especially true for areas where intense shades of red are required and Molybdate Red is to be avoided or its use restricted. At higher baking temperatures (160 °C), P.O.62 is not completely fast to overcoating. The pigment is heat stable up to 180 °C.
P.O.62 is frequently used in the printing inks field to produce lightfast offset and aqueous flexographic inks. Its lightfastness in these media equals step 5 to step 6 on the Blue Scale, depending on the standard depth of shade. The prints are not completely fast to alkali and do not tolerate clear lacquer coatings and sterilization.
P.O.62 is interesting to the plastics field because it is very lightfast and durable in rigid PVC. However, it fails to meet higher standards regarding long-term weathering.
P.O.62 also lends itself to polypropylene spin dyeing, especially at temperatures up to approximately 230 °C. This makes it a suitable colourant for PP types that exhibit good flow behaviour.
2.8.4.1.11 Pigment Orange 72
P.O.72 is polymorphic: the red α-phase is obtained upon synthesis. During finishing in solvents it transforms into an orange β-phase. Additionally, two brown phases (γ and δ) are known. The commercial product contains only the orange β-phase.
Providing a bright yellowish orange, P.O.72 is particularly useful for the colouration of plastics. Incorporation in HDPE in 1/3 SD (1% TiO2) requires 0.2% of pigment – the respective heat stability reaches 290 °C and the lightfastness corresponds with step 8 on the Blue Scale. The pigment does not affect the shrinkage of this partially crystalline polymer and can therefore be used in injection moulding without restriction.
P.O.72 is as well suitable for the application in polystyrene and ABS. Colorations of PS in 1/3 SD, requiring only 0.15% pigment, exhibit a heat stability of 280 °C, colourations of ABS are stable up to 290 °C. In these media the lightfastness equals step 8 on the Blue Scale. Pigment concentration of less than 0.1% in polyoxymethylene (POM, polyacetals) may lead to coating of the manufacturing equipment (see plate out, Section 1.6.4.1).
P.O.72 is used for melt spin dyeing of polypropylene, in which 1/3 SD (1% TiO2) affords 0.2% pigment. The lightfastness of PP colourations with 0.3% pigment equals step 7, such with 2% pigment step 7–8 on the Blue Scale. Owing to its heat stability the pigment is equally suitable for powder coatings.
P.O.72 is also recommended for paste- and liquid packaging inks as well as for decorative inks. It exhibits a clean yellowish orange shade, excellent fastness properties and very high heat resistance.
2.8.4.2 The Red and Brown Series
2.8.4.2.1 Pigment Red 171
P.R.171 affords a dull, very bluish red, referred to as maroon. The pigment generally provides good fastness properties. The commercially available types are highly transparent.
P.R.171 is used in plastics and in paints. Its lightfastness in PVC equals step 7 to step 8 on the Blue Scale, depending on the exact composition of the tested system, the pigment concentration and the TiO2 content. When incorporated in plasticized PVC, P.O.171 is migration resistant and heat stable up to 180 °C. It is used in conjunction with organic yellow pigments, frequently also with iron oxides, to produce shades of brown. Shades of bordeaux are accessible in deep transparent colourations.
P.R.171 exhibits excellent tinctorial strength. Only 0.46% pigment is required to formulate 1/3 SD samples containing 5% TiO2. 1/3 SD samples (with 1% TiO2) in PE are heat stable up to approximately 240 °C. P.R.171 application is accordingly restricted to LDPE, which is processed at low temperature. Transparent samples (0.1% pigment) equal step 6–7 on the Blue Scale for lightfastness, while reduced colourations (0.01% pigment + 0.5% TiO2) match step 6. Used in polyacrylonitrile spin dyeing, P.R.171 affords very lightfast products. In the concentration range between 0.1% and 3%, its lightfastness equals step 7–8 on the Blue Scale. The products are completely fast to dry rubbing but do not entirely withstand wet rubbing. Incorporated in unsaturated polyester resins, P.R.171 accelerates the hardening of the plastic. It shows very good lightfastness in this medium, too.
Paints containing P.R.171 may be safely oversprayed and exhibit excellent lightfastness and weatherfastness. Its good fastness properties make P.R.171 a suitable candidate for high grade industrial paints, including automotive repair finishes. Its high transparency in paint films is used to advantage in a host of applications, such as in transparent foil coatings and metallic finishes.
P.R.171 lends maroon shades to printing inks wherever it suits the required fastness standards.
2.8.4.2.2 Pigment Red 175
P.R.175, a somewhat dull red pigment, exhibits very good fastness properties. It is completely or almost completely insoluble in common organic solvents. The commercially available types exhibit high specific surface areas and are therefore very transparent.
The paint industry uses P.R.175 primarily to colour industrial paints and also automobile repair finishes. High transparency makes the pigment an important product for transparent and metallic effect finishes. The pigment is suited to two-coat metallic automobile (O.E.M.) finishes, also referred to as base coat/clear coat finishes, especially if the clear coat contains UV absorbants. Its lightfastness and weatherfastness are excellent. P.R.175 does not bloom, is completely fast to overpainting and is heat stable up to 200 °C.
In plastics, P.R.175 is also very lightfast and weatherfast. Its lightfastness in plasticized and rigid PVC, for instance, equals step 7 to step 8 on the Blue Scale, depending on the composition of the polymer, the type of stabilization, the depth of shade and possibly on the TiO2 content. In these media, P.R.175 is frequently applied in conjunction with carbon black to afford shades of brown for furniture laminates. The pigment is migration resistant in plasticized PVC – bleeding or blooming is practically never observed. P.R.175 is also applied in PVC and PUR plastisols for synthetic leather, which is used in markets such as automobile interiors. The pigment is very durable. P.R.175, combined with carbon black but containing no TiO2, is used in window frames made of impact resistant PVC.
In white reductions, P.R.175 does not quite satisfy the requirements of long-term outdoor exposure. PVC plastisols, coil coated onto steel plates, also provide good fastness to light and weather. 1/3 SD HDPE samples (with 1% TiO2) tolerate exposure to up to 270 °C for 5 min. The pigment only affects the shrinkage of HDPE to a negligible extent. Transparent (0.1% pigment) and opaque colourations (0.01% pigment + 0.5% TiO2) equal step 6–7 on the Blue Scale for lightfastness.
P.R.175 is also used in polypropylene spin dyeing, where it satisfies the lightfastness requirements. The pigment is an interesting colourant for polystyrene and for polyester (PETP). These systems are utilized to make bottles and other products.
P.R.175 has stimulated interest throughout the printing ink industry wherever high lightfastness, excellent solvent fastness, fastness to sterilization and very high heat stability (up to 220 °C) are required.
2.8.4.2.3 Pigment Red 176
P.R.176 provides a bluish red shade, which is somewhat bluer than those of P.R.187 and 208 and somewhat yellower than that of P.R.185.
P.R.176 is primarily applied in plastics and in laminated papers. The pigment exhibits very good migration fastness in plasticized PVC. 1/3 SD samples containing 5% TiO2 equal step 6–7 on the Blue Scale for lightfastness, 1/25 SD specimens match step 6, and transparent colourations (0.1% pigment) equal step 7. Transparent rigid PVC colourations match step 7–8. P.R.176 is heat stable up to 200 °C, and it is a suitable colourant for PVC cable insulations and synthetic leather. The pigment is also used in polyolefins and in polystyrene, where its lightfastness is equally good. Transparent polystyrene samples (0.1%) are heat stable up to 280 °C, addition of large amounts of TiO2 reduces this stability to 220 °C.
The list of applications includes decorative prints for laminated plastic sheets. P.R.176, like P.R.187 and 208, is insoluble in styrene monomer and acetone, exhibits no plate-out onto the plastic sheets, and does not bleed if it is soaked with a melamine resin solution. This is what makes P.R.176 an interesting pigment for both melamine and polyester resin sheets. Its lightfastness is inferior to that of these other pigments covering the same colour range; it scores one step less on the Blue Scale. P.R.176 affords a shade that closely approaches the standard magenta for three- and four-colour printing.
P.R.176 provides very lightfast polyacrylonitrile spin dyeing products. The samples equal step 6–7 on the Blue Scale. Dry and wet crocking may affect the objects to a certain extent. P.R.176 is also used in polypropylene spin dyeing, especially for coarse textiles, such as carpet fibres, split fibres, filaments, bristles or tape, and also for finer denier yarns. A special pigment preparation for this purpose is commercially available. 1/3 SD samples tolerate exposure to up to 300 °C for 1 min or up to 290 °C for 5 min. In terms of lightfastness, 0.1% colourations equal step 5–6 on the Blue Scale, while 2% samples match step 7.
2.8.4.2.4 Pigment Red 185
The commercially available types of this polymorphous pigment afford very clean, bluish shades of red. P.R.185 is completely or almost completely insoluble in common solvents.
Its main area of application is in graphics printing and in the mass colouration of plastics.
The printing ink industry uses P.R.185 for all printing techniques. The prints show very good solvent fastness, for instance towards the standard DIN 16524 solvent mixture and towards clear lacquer coatings. They may safely be sterilized. P.R.185 prints are heat stable up to 220 °C for 10 min or to 200 °C for 30 min, which makes the pigment a suitable product for metal deco printing. In terms of lightfastness, 1/1 SD prints equal step 6–7 on the Blue Scale, while 1/3 to 1/25 SD specimens match step 5. P.R.185 affords a colour that closely approaches the DIN 16524 standard magenta for three- and four-colour printing (Section 1.8.1.1). Consequently, the pigment is used in process colours wherever P.R.57:1 and 184 are not fast enough to meet the standards. This is true for applications such as metal deco printing. Moreover, P.R.185 also lends itself to use in laminated polyester products.
P.R.185 is employed throughout the plastics industry to colour PVC, poly(vinylidene) chloride, and polyolefins. In plasticized PVC, the pigment is migration resistant at concentrations down to 0.005%. Under standardized conditions, the extent of bleeding into a white plasticized PVC sheet that is in contact with a 1/3 SD coloured plasticized PVC sheet (Section 1.6.3.2) is only 0.2 CIELAB units. 1/3 SD samples (5% TiO2) equal step 6–7 on the Blue Scale for lightfastness, while 1/25 SD samples match step 6. Similar values are measured for rigid PVC. In deeper shades, P.R.185 is also found in several types of synthetic leather, including materials based on PVC and PUR for use in automobiles. In terms of heat stability, 1/3 SD P.R.185 samples in PE (1% TiO2) withstand less than 200 °C, which restricts its use to low temperature LDPE. P.R.185 is also well suited to polypropylene spin dyeing, provided the polymer can be processed at low temperature. P.R.185 is used by the paint industry to colour general industrial finishes.
2.8.4.2.5 Pigment Red 208
Incorporated in its application medium, P.R.208 affords medium shades of red. The pigment exhibits good fastness to chemicals and solvents. Its main area of application is in the mass colouration of plastics and in packaging gravure printing inks.
P.R.208 worked into PVC provides medium shades of red. Pigment blends with compounds such as P.Y.83 or with carbon black are frequently employed to produce shades of brown. P.Y.208 is an interesting product for synthetic leather based on PVC, which is targeted for use in automobiles. The pigment does not bloom in plasticized PVC, provided it is used at concentrations above 0.005%. It shows excellent bleed resistance. Samples in the range between 1/3 and 1/25 SD (with 5% TiO2) equal step 6–7 on the Blue Scale for lightfastness. The pigment is heat stable up to approximately 200 °C and exhibits high tinctorial strength. Some 0.6% pigment is required to formulate 1/3 SD PVC samples with 5% TiO2. Good dielectric properties make the pigment a suitable candidate for use in PVC cable insulations.
In white reductions, P.R.208/polyolefin systems only withstand temperatures below 200 °C, while transparent specimens (0.1%) are stable up to approximately 240 °C. Thus the pigment is a suitable and economical candidate for polypropylene spin dyeing, provided the temperature is kept below 200 °C. It is also possible to apply higher temperatures if a colour shift towards more yellowish shades is acceptable. In terms of lightfastness, P.R.208 meets the common standards for interior application.
P.R.208 is also used in polyacrylonitrile spin dyeing. It exhibits excellent textile fastness properties and shows good lightfastness. Full shades (3% pigment concentration) equal step 7 on the Blue Scale, while very light (0.1% pigment) red specimens match step 5. The list of applications includes secondary acetate spin dyeing and mass colouration of polyurethane foam and elastomers. P.R.208 is inert to peroxides.
P.R.208 is recommended for all printing methods and is very lightfast in print. The prints exhibit very good resistance to solvents and clear lacquer coatings and may safely be sterilized. Moreover, they are heat stable up to 200 °C for 10 min or 180 °C for 30 min. For comparison, notably, the cleaner, somewhat yellower P.R.112 is less lightfast, less fast to solvents and chemicals, and less heat stable. Shades of brown are accessible by blending P.R.208 with yellow pigments and with carbon black, frequently in printing inks based on vinyl chloride/acetate mixed polymer. Such materials are used especially to produce wood imitations. P.R.208 is particularly suited to use in decorative printing inks. The lightfastness of 8% gravure prints at 20 and 40 µm cell depth in laminated melamine sheets equals step 8 on the Blue Scale. Plate-out, that is colouration of the printing plates, is not observed. The pigment does not bleed if the print is soaked with a melamine resin solution. It is also completely inert to styrene monomer and acetone, which makes it an equally interesting candidate for polyester sheets.
The paint industry recommends P.R.208 primarily for general industrial finishes, in which it is used to a limited extent. Its lightfastness and weatherfastness do not satisfy the standards of several applications.
P.R.208 is also applied in various speciality media besides the three main groups mentioned, such as in crayons and laundry inks, as well as in solvent-based wood stains. Applied onto a surface, these products may safely be overcoated and are resistant towards nitro varnish, acid hardening varnish and polyester varnish. The lightfastness in these media equals step 7 on the Blue Scale, which is excellent.
2.8.4.2.6 Pigment Violet 32
P.V.32 is a somewhat dull, very bluish red pigment, referred to as a bordeaux. It is completely fast to numerous organic solvents and is used in paints, plastics and printing inks, as well as in spin dyeing.
Paints containing P.V.32 are totally resistant to overpainting. However, the pigment exhibits only moderate lightfastness. Full shade samples in an alkyd-melamine resin equal step 5–6 on the Blue Scale and darken, while 1:10 TiO2 reductions match step 5–6 and 1:100 reductions coincide with step 3–4. The pigment is utilized in general industrial finishes wherever its lightfastness and weatherfastness satisfy the requirements. The similarly coloured P.R.12, on the other hand, a Naphthol AS pigment, bleeds and blooms in baking enamels.
High transparency makes P.V.32 an interesting colourant for transparent foil coatings and similar purposes. Like P.R.171 and 175, P.V.32 shows excellent heat stability, even in long-term exposure. Incorporated in a silicone resin coating, even more reduced colourations (1:50 TiO2) withstand up to 200 °C for 120 h and 180 °C for 1000 h. No colour change is observed. However, the lightfastness deteriorates during exposure by approximately ½ step on the Blue Scale.
Transparent P.V.32 colourations in PVC equal step 7–8 on the Blue Scale for lightfastness, while opaque types (1/3 SD; 5% TiO2) match step 6–7. The pigment is completely bleed resistant in plasticized PVC. P.V.32 exhibits high tinctorial strength. Only 0.44% pigment is required to produce 1/3 SD colouration in PVC (5% TiO2). The similarly coloured but not bleed resistant tetrachlorothioindigo pigment P.R.88 is only about half as strong (Section 3.6.2.3). P.V.32 is frequently used in combination with Molybdate Red. Its heat stability in polyolefins is somewhat below 200 °C, although the colour change, as in many other cases, is not so noticeable as to absolutely preclude pigment application at a higher temperature. Therefore, P.V.32 is generally recommended only for use in LDPE at low processing temperatures. It is also suitable for spin dyeing, for instance for secondary acetate, viscose rayon or viscose cellulose. Its lightfastness in these media is good: 1/1 SD samples equal step 7 on the Blue Scale and 1/12 SD specimens match step 6. The textile fastnesses are excellent, although the pigment is not entirely fast to wet crocking and only poorly vat resistant.
Incorporated in printing inks, P.V.32 affords clean, highly transparent bluish shades of bordeaux. The pigment is used to produce high grade printing inks wherever fastness to calendering and other fastness properties are required. Moreover, P.V.32 is employed in laminated plastic sheets. Prints made from 8% gravure printing inks in 20 µm cells correspond to step 7 on the Blue Scale for lightfastness. The pigment is suitable both for polyester and for melamine sheets. Bleeding, however, may occur to a very slight extent if prints are soaked with a melamine resin solution. Notably, the pigment is not entirely fast to acetone (Section 1.8.1.2). P.V.32 is frequently used in printing inks based on vinyl chloride/vinyl acetate copolymer.
Apart from these areas of application, P.V.32 is also used in solvent-based wood stains. The lightfastness of the products equals step 6 on the Blue Scale, which is very good. These systems may safely be overcoated. Pigment blends with yellow pigments, such as with P.Y.83, and blends with black provide interesting shades of brown.
2.8.4.2.7 Pigment Brown 25
The pigment affords a reddish shade of brown. The commercially available types feature a high specific surface area of approximately 80 m2 g−1 and are therefore highly transparent. P.Br.25 is somewhat less fast to some solvents than other pigments of the same class. It is used in paints, plastics, and printing inks and is in these areas in direct competition with the colouristically closely related but somewhat yellower and more opaque P.Br.23.
The paint industry uses P.Br.25 primarily as a colourant for high grade industrial paints, such as automobile O.E.M. and repair finishes. High transparency makes it a suitable product wherever transparent or metallic effects are to be created, especially in two-coat (base coat/clear coat) metallic automotive finishes. For these purposes, P.Br.25 is frequently used in pigment blends with transparent iron oxides or with carbon black to afford shades of brown or maroon that are not accessible by using iron oxide pigments alone. P.Br.25 is completely fast to overcoating and is heat stable up to 200 °C. Its lightfastness and weatherfastness are equally excellent. Even in white reductions, alkyd-melamine resins systems reduced 1:300 with TiO2 equal step 7–8 on the Blue Scale. White reductions of acrylic/melamine resin, reduced 1:1 with TiO2 and exposed to Florida weather for one year, match step 5 on the Grey Scale for durability, while 1:6 reductions equal step 4–5 and 1:60 specimens correspond to step 4 on the Grey Scale.
P.Br.25 is a frequently used pigment in plastics. It exhibits very good but not perfect migration fastness in plasticized PVC. At concentrations down to 0.005%, P.Br.25 is heat stable up to 200 °C. It exhibits high tinctorial strength: only 0.77% pigment is required to produce 1/3 SD colourations in PVC with 5% TiO2. Its lightfastness in PVC equals step 8 on the Blue Scale, and step 7–8 if considerable amounts of white pigment are added. P.Br.25 shows excellent weatherfastness in rigid PVC and in impact resistant PVC, which is also true for reductions with TiO2. The pigment even satisfies the requirements of long-term exposure. It is used, for instance, in window frames made of impact resistant PVC. PVC plastisols that are coil coated onto steel plates exhibit excellent lightfastness and durability. P.Br.25 is also found in PVC and PUR leather targeted for use in automobiles.
1/3 SD HDPE colourations containing 1% TiO2 are heat stable up to 290 °C. The shrinkage of injection-moulded articles is noticeably affected in the direction of flow if the processing temperature is low (220 °C). This influence, however, is already very slight at 260 °C.
P.Br.25 also shows excellent lightfastness in polyolefins. Transparent polystyrene samples are heat stable up to 280 °C, while specimens reduced considerably with TiO2 withstand up to 240 °C. P.Br.25 is also used in polyester, in which it provides an interesting raw material for the manufacture of bottles.
Excellent fastness properties make P.Br.25 a suitable product for polypropylene spin dyeing. It is also very lightfast in polyacrylonitrile spin dyeing: 1/3 SD samples equal step 7 on the Blue Scale for lightfastness. Pigment weatherfastness is equally excellent, which qualifies P.Br.25 for use in awnings and so on. The pigment is completely fast to dry rubbing and almost completely fast to wet crocking. Moreover, P.Br.25 is also interesting as a pigment for polyurethane foam.
The printing ink industry uses P.Br.25 for all printing methods. The prints show excellent lightfastness. 1/1 to 1/25 SD letterpress proof prints, for instance, equal step 7 to step 6–7 on the Blue Scale. Prints made from P.Br.25 are fast to the DIN 16 524 standard solvent mixture, to paraffin, butter, soap and acid, but they are not entirely fast to alkali. The products are fast to clear lacquer coatings and may safely be sterilized. The temperature stability is up to 240 °C for 10 min or 220 °C for up to 30 min, which makes P.Br.25 a suitable candidate for metal deco printing inks. It is also frequently applied in printing inks for PVC.
Moreover, P.Br.25 is used in various speciality media, for instance in oil colours for artists and in watercolours. It lends itself to solvent-based wood stains. The products are very lightfast (step 7) and fast to overcoating.
2.8.5 Quinoxalinedione Pigments
Quinoxalinedione pigments are named after the tetrahydroquinoxaline-2,3-dione group (24), which can be regarded as homologue of a benzimidazolone moiety, which is extended by one CO unit:

The tetrahydroquinoxaline-2,3-dione group has a similar effect as a benzimidazolone group: due to the additional hydrogen bonds in the crystal, solubility is reduced and solvent and migration fastness are increased. Hitherto, the quinoxalinedione group has been used commercially only in acetoacetyl hydrazone pigments. However, it could be applied in Naphthol AS-like pigments as well.
2.8.5.1 Chemistry, Manufacture and Crystal Structures
The acetoacetylated 6-amino-tetrahydroquinoxaline-2,3-dione is prepared in a similar way as the corresponding benzimidazolone derivative:

At present there is only one quinoxalinedione pigment on the market, which is listed in the Colour Index as P.Y.213 with the constitution number 11875:

Scheme 2.7 Molecular structure of P.Y.213.
P.Y.213 exists in two different polymorphic forms. A nanocrystalline brown β-phase emerges from the synthesis. Heating in organic solvents to 150–200 °C leads to the desired greenish-yellow α-phase.
The crystal structure of the α-phase of P.Y.213 was determined by a combination of electron diffraction and X-ray powder diffraction [110]. The molecules show the usual hydrazone-tautomeric form with two intramolecular hydrogen bonds from the NH groups to the keto groups of the acetoacetyl fragment. In P.Y.213 these hydrogen bonds are bifurcated and involve the COOCH3 and the OCH3 substituents, too (Figure 2.46). As for the benzimidazolone group, the quinoxaline-dione moiety donates two hydrogen bonds to neighbouring molecules. In P.Y.213 the quinoxalinedione groups of neighbouring molecules form an eight-membered ring via a double CO![]() HN bond. The second NH group of the quinoxaline moiety bonds to a COOCH3 group of another neighbouring molecule, so that a double-chain of molecules is formed. These double-chains are planar and arrange in layers (Figure 2.47). Within the planes, the molecules fill the space in an almost perfect way.
HN bond. The second NH group of the quinoxaline moiety bonds to a COOCH3 group of another neighbouring molecule, so that a double-chain of molecules is formed. These double-chains are planar and arrange in layers (Figure 2.47). Within the planes, the molecules fill the space in an almost perfect way.

Figure 2.46 Molecular double-chain in P.Y.213 (α-phase).

Figure 2.47 Crystal structure of P.Y.213 (α-phase); view along the layers.
The combination of hydrogen bonds and the efficient molecular packing explains the insolubility and the outstanding fastness properties of the α-phase of P.Y.213.
The crystal structure of the nanocrystalline β-phase was investigated by pair-distribution function analysis [110]. The analysis showed that the molecules are arranged in stacks (like the α-phase). The domain size (size of coherently scattering regions) of the β-phase is always very small, only about ten molecules in the stacking direction, and only about ten stacks in the lateral directions. The pigment particles in total are much larger; they are built from an aggregation of these domains, and probably contain amorphous regions as well.
2.8.5.2 Properties and Applications
P.Y.213 is a greenish yellow pigment with good hiding power and outstanding light and weather fastness, better than that of P.Y.154. The product is completely resistant to solvents used in paint applications. P.Y.213 is primarily recommended for paints. It shows distinct advantages over P.Y.154 in waterborne base coat systems when fastness to co-solvents like butyl-glycol or NMP is important. After 2 years of Florida exposure even in pastel shades (1:50 TiO2) its fastness was rated 5 on the grey scale. Irrespective of the concentration P.Y.213 is recommended for all high grade industrial paints including automobile finishes – both solvent and water-borne. Fastness to overpainting is excellent up to baking temperatures of 180 °C. Owing to the excellent heat stability, the pigment can be used in powder and coil coating applications without restrictions, including for outdoors use. Areas of application also include other common media throughout the coatings and paint industry, such as architectural and emulsion paints. P.Y.213 is employed wherever high light and weather fastness are required.
2.9 Dihydrazone Condensation Pigments (Formerly Called Disazo Condensation Pigments)
There are various techniques that make it possible to confer higher solvent and migration resistance on a Naphthol AS pigment:
- salt formation, that is, formation of pigment lakes (Section 2.7);
- introducing additional carbonamide moieties into the pigment molecule (Section 2.8);
- enlarging the pigment molecule.
In the early 1950s, Ciba research succeeded in synthesizing red dihydrazone compounds of relatively high molecular weight. These products were known as ‘disazo condensation pigments' [111, 112]. However, the correct name is ‘dihydrazone (or: bishydrazone) condensation pigments', because these pigments, like all other ‘azo' pigments, do not contain azo groups (NN) but, instead, hydrazone moieties (NHN).
Dihydrazone condensation pigments are formally composed of two monohydrazone units, which are attached to each other by an aromatic diamino carbonamide bridge:
- D: diazo component,
- Z: β-naphthol or acetoacetyl fragment,
- Ar: bifunctional aromatic group.
The number of carbonamide groups is thus doubled in relation to the monohydrazone pigment.
Yellow as well as red dihydrazone pigments are derivatives of this parent structure. Both types are prepared by essentially the same but slightly modified route.
A monohydrazone yellow pigment obtained from acetoacetarylide (Section 2.3) constitutes the monohydrazone portion in a typical yellow product. Red types, on the other hand, are derived from a β-naphthol derivative (Section 2.5) or a BONA pigment using the carboxylic function. Bisacetoacetarylide pigments, although featuring a simpler structure, are also technically considered constituents of the yellow series (Section 2.4.2). Notably, however, the term dihydrazone condensation pigment, as it is used in this context, refers to somewhat more complex compounds. The presence of additional carbonamide functions in the diazo component produces a marked influence on the fastness of these pigments to solvents and migration. In contrast to bisacetoacetylarylide pigments (Section 2.4.2) it is not possible to synthesize dihydrazone condensation pigments by traditional methods. Max Schmid at Ciba (not the co-author of this book) discovered a suitable pathway and thus laid the foundation for the industrial manufacture of dihydrazone condensation pigments [111].
2.9.1 Chemistry, Manufacture and Crystal Structures
The formal structure is exemplified by the red dihydrazone condensation pigments. The structure may be visualized as resulting from the dimerization of two monohydrazone pigments of the Naphthol AS type:
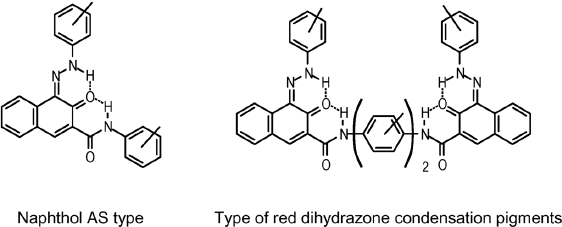
Red dihydrazone condensation pigments therefore have the following general structure:

RD stands for the usual substituents for diazo components (see, for instance, Section 2.6.1). However, it may also consist of additional carbonamide groups, connected via phenyl moieties. In commercially important pigments, A commonly represents a phenylene or a diphenylene group, and n = 1–3. This general structure also covers orange and brown pigments.
Two possible chemical structures have been found for yellow dihydrazone condensation pigments. The two monohydrazone units may be connected either via the coupling component (type 1) or via the diazo moiety (type 2):

Scheme 2.8 Molecular structures of yellow dihydrazone condensation pigments.
Derivatives in this series have been prepared in which rings B through E in types 1 and 2 carry substituents such as CH3, OCH3, OC2H5, Cl, NO2, COOCH3, CF3 or OC6H5.
There are basically two routes leading to dihydrazone condensation pigments. The two schemes will be exemplified by the synthesis of red pigments.
Using amine, 2-hydroxy-3-naphthoic acid, and aromatic diamine as starting materials, there are two pathways, I and II, that lead to the desired products:

Pathway I is the method that first comes to mind but presents something of a problem. It is not possible to synthesize the product in a straightforward two-step sequence by simply coupling two equivalents of diazotized amine with the bifunctional coupling component. This reaction does not afford a definitive product, since the monohydrazone compound is frequently insoluble enough to precipitate and is thus eliminated from further reaction.
Although difficulties of this kind may be obviated, the necessary procedures are comparatively demanding. Applying physical action, such as thoroughly milling the starting materials, vigorously stirring the reaction mixture, or using a mixing jet and working with an excess of dinaphthol are amongst the techniques used to facilitate this route. An alternative scheme involves diazotizing a mixture of amine and dinaphthol in an organic solvent with alkyl nitrite. Another suitable starting material is the diazoamino compound of the amine to be coupled. It may be treated with dinaphthol in an organic solvent in the presence of glacial acetic acid. All these techniques present serious disadvantages, since they are not generally applicable and do not always afford a uniform product.
The decisive step towards a practical synthesis was taken by performing the reverse reaction II: coupling preceding condensation.
This method proceeds via the carboxylic acid of the monohydrazone dye, which is obtained from diazotized amine and 2-hydroxy-3-naphthoic acid in an alkaline medium. The prepared intermediate is then dried (azeotropically) in an organic solvent, such as mono or dichlorobenzene, with a phosphorus halogenide or with thionyl chloride to form the acid chloride. Instead of using chlorinated solvents the synthesis can be carried out in 1,2,3-trimethylbenzene or anisole as solvent [113]. This step only proceeds uniformly and under very mild conditions in the presence of catalytic amounts of N,N-dimethylformamide (DMF) to afford the desired product [114]. Subsequent condensation of two equivalents of the monohydrazone dye acid chloride with one equivalent of diamine H2N-A-NH2 is achieved in an organic solvent. This is the step that has given these pigments their name. Some publications also recommend adding an acid scavenger, such as sodium acetate or a tertiary organic base. Since the reaction is performed in an organic solvent system, the pigment is most efficiently separated in a closed filtering apparatus, preferably by using a suction filter.
The technique of having the coupling step precede the condensation is similarly applied to the two types of yellow pigments. Type 1 (see Scheme 2.8) is synthesized by coupling two equivalents of diazotized aminobenzoic acid onto bisacetoacetylated aromatic diamine, especially diaminobenzene. Conversion into the dihydrazone acid chloride, followed by condensation with two equivalents of usually carbonamide substituted amine, finally affords the desired pigment.
Type 2 (see Scheme 2.8) is obtained by coupling diazotized aminobenzoic acid onto acetoacetarylide. The resulting acid is converted into the acid chloride and condensed with an aromatic diamine.
The condensation principle makes it possible to synthesize a wide range of dihydrazone condensation pigments.
Crystal structures of dihydrazone condensation pigments have not yet been determined, but it can safely be assumed that they exhibit the same features as their mono-counterparts, that is, as monohydrazone yellow and mono-Naphthol AS hydrazone pigments.
2.9.2 Properties
Dihydrazone condensation pigments provide various shades ranging from very greenish yellow to orange to bluish red and violet. Brown pigments are also available. The commercial types exhibit good-to-average tinctorial strength. It is especially the yellow representatives that, although members of the dihydrazone series, are tinctorially weak.
The doubling of the molecule and, in some grades, larger pigment particles enhance the fastness properties of these products, especially their resistance to various organic solvents. Pigments in the yellow range, for instance, are fast to alcohols and aliphatic and aromatic hydrocarbons, but show some bleeding in ketones and esters. The extent of bleeding is a function of the substitution pattern of the molecule. Some of the Naphthol AS type dihydrazone condensation pigments are somewhat less fast to solvents. Tested relative to conventional standards (Section 1.6.2.1), these pigments have shown to be not entirely resistant to alcohols and aromatic hydrocarbons. As a rule, however, good overall solvent fastness makes this variety of dihydrazone condensation pigments largely migration resistant. The pigments do not bloom, and many exhibit good-to-excellent bleed resistance. The same is true for fastness to overcoating. Moreover, dihydrazone condensation pigments are also fast to acid and alkali. Easy dispersion in various media, especially in plastics, is attributed to the fact that the pigments are prepared in organic media. Numerous representatives provide excellent lightfastness; some exhibit good-to-very good weatherfastness. Besides, dihydrazone condensation pigments often possess excellent heat stability.
2.9.3 Application
Dihydrazone condensation pigments, whose manufacture is somewhat more demanding than that of diarylide yellow and Naphthol AS pigments, are correspondingly expensive.
As a result of their higher price, due to the demanding synthesis, these pigments are used primarily in high grade media and in quality products. Dihydrazone condensation pigments are broad in scope, they are found in various types of plastics, in spin dyeing products, printing inks, industrial finishes and paints, as well as in special media.
The pigments are primarily applied by the plastics industry, which uses many of these pigments as colourants for PVC and PO. Large-sized molecules make these pigments very migration resistant in plasticized PVC. The yellow types are typically more bleed resistant than the red types. Dihydrazone condensation pigments are heat stable enough to satisfy the requirements for use in plasticized and rigid PVC. Some of the yellow types are tinctorially weak. Approximately 0.7–2% of a yellow dihydrazone condensation pigment is needed to produce 1/3 SD plasticized PVC systems with 5% TiO2 on a two-roll mill. Similarly coloured diarylide yellow pigments, on the other hand, afford the same result at a concentration of approximately 0.3–1.0% pigment. This advantage, however, is compromised by the fact that diarylide yellow pigments are generally less fast. The corresponding concentration range for red dihydrazone condensation pigments is approximately between 0.5% and 1.4% pigment. In plasticized PVC, dihydrazone condensation pigments satisfy almost any requirement regarding lightfastness, but this is not entirely true for their weatherfastness. Incorporated in rigid PVC, for instance, dihydrazone condensation pigments (with the exception of Pigment Brown 23) do not meet the demands of long-term exposure.
Some of these pigments are also used to an appreciable extent in polyolefins. The tinctorial strength may vary considerably between pigments. Between 0.18% and 0.44% pigment is required to produce a 1/3 SD HDPE sample containing 1% TiO2, depending on the choice of pigment. A tinctorially weak representative may thus differ from a pigment with high tinctorial strength by a factor of 2.5. 1/3 SD formulations are heat stable enough to withstand temperatures between 250 and 300 °C, the exact value depending on the type of pigment. Several dihydrazone condensation pigments considerably affect the shrinkage of polyethylene (Section 1.8.3.2). Colouring larger symmetrical articles made from polyolefins or other partially crystalline polymers with dihydrazone condensation pigments may therefore result in distortion. Other application media, such as elastomers, polystyrene or PUR, are subject to similar distortion.
As a result of their good heat fastness, various dihydrazone condensation pigments are used to advantage in polypropylene spin dyeing. Like other classes of pigments, dihydrazone condensation pigments are therefore also supplied in the form of special-purpose preparations that contain predispersed pigment. Several grades are also used to an appreciable extent in polyacrylonitrile spin dyeing. The pigments demonstrate good textile fastness in this medium.
The paint industry is interested in individual representatives of this class, such as P.Y.128. These are used to colour high grade paints, such as automobile (O.E.M) finishes and automotive refinishes. Other types are used in general industrial paints. As a rule, the fastness of dihydrazone condensation pigments to overcoating is good-to-excellent in some media. The pigments are also used in architectural paints, sometimes also in emulsion paints.
Dihydrazone condensation pigments have stimulated interest throughout the printing ink industry, they are suited to all types of printing techniques. Their main market in this area, however, is in high grade packaging inks. Good migration and solvent fastnesses make these pigments suitable candidates for special-purpose ketone/ester-based gravure inks intended for use on PVC films. Dihydrazone condensation pigments are also used in printing inks targeted for other substrates. Special pigment preparations are available for this purpose.
In print, most pigments in this series are completely fast to many of the packaged goods: butter, cheese and soap. Prints made from dihydrazone condensation pigments are also acid and alkali resistant and fast to overcoating, calendering and sterilization. The prints are completely heat stable up to 160 °C for 60 min. Exposure to 200 °C for 15 min results in minor colour changes. It is their high heat stability that makes these pigments suitable candidates for metal deco printing. As a rule, the prints provide excellent lightfastness.
Various dihydrazone condensation pigments are utilized in decorative printing on laminated plastic sheets. They are also applied in several special-purpose media outside the above-mentioned groups, such as oil colours for artists and crayons.
2.9.4 Commercially Available Dihydrazone Condensation Pigments
2.9.4.1 General
Amongst yellow dihydrazone condensation pigments, it is especially the ones that are based on the type I structure that enjoy widespread use. These pigments cover the spectral range from very greenish to reddish shades of yellow. Members of the Naphthol series produce colours from orange to red and violet as well as shades of brown.
The product lines offered by pigment manufacturers include a considerable number of commercially interesting dihydrazone condensation pigments. The most important representatives in the yellow range are Pigment Yellow 93, 94, 95 and 128. Orange, red and brown shades are covered particularly by Pigment Orange 31, Pigment Red 144, 166, 214, 220, 221, 242, 248 and Pigment Brown 23. Table 2.15 lists the commercially available dihydrazone condensation pigments.
Table 2.15 Commercially available dihydrazone condensation pigments.
| Yellow series | 
|
||||
| C.I. Name | C.I. Constitution Number | Structure | Shade | Reference | |
| A | B | ||||
| P.Y.93 | 20710 |  |
 |
yellow | [115, 116] |
| P.Y.94 | 20038 |  |
 |
greenishyellow | [117] |
| P.Y.95 | 20034 |  |
 |
reddish yellow | [115, 118] |
| P.Y.128 | 20037 |  |
 |
greenishyellow | |
| P.Y.166 | 20035 |  |
 |
yellow | |
| Orange, red and brown series | 
|
||||
| C.I. Name | C.I. Constitution Number | Structure | Shade | Reference | |
| A | B | ||||
| P.O.31 | 20050 |  |
orange | [115] | |
| P.R.144 | 20735 |  |
bluishred | [115, 119] | |
| P.R.166 | 20730 | yellowishred | [115] | ||
| P.R.214 | 200660 |  |
bluish red | [120] | |
| P.R.220 | 20055 |  |
 |
bluish red | |
| P.R.221 | 20065 |  |
 |
bluish red | |
| P.R.242 | 20067 |  |
reddish orange (scarlet) | [120, 121] | |
| P.R.248 |  |
 |
bluish red | ||
| P.R.262 |  |
bluish red | [122] | ||
| P.Br.23 | 20060 |  |
 |
reddishbrown | |
| P.Br.41 |  |
 |
yellowishbrown | ||
| P.Br.42 |  |
 |
yellowishbrown | [122] | |
2.9.4.2 Yellow Series
2.9.4.2.1 Pigment Yellow 93
P.Y.93 affords a light greenish to medium yellow shade similar to that of P.Y.16.
The pigment is primarily used to colour plastics, including PP spin dyeing. Incorporated in plasticized PVC, P.Y.93 shows good-to-average tinctorial strength and good bleed resistance. Approximately 0.85% pigment is required to formulate a 1/3 SD samples with 5% TiO2. P.Y.93 demonstrates very good lightfastness. In PVC, it is as lightfast as P.Y.94 and in this respect even slightly superior to P.Y.95, but it is less stable to light than the greener P.Y.128.
1/3 SD pigment formulations in rigid PVC and in PVC plastisols that are intended for coil coating lose weatherfastness much more rapidly as more TiO2 is added than do P.Y.94 and 128. There is no difference in the response of the respective pigmented systems to weather, as long as they contain only 1% TiO2, while a TiO2 content of 5% renders the P.Y.93 system considerably less durable than its counterparts.
1/3 SD P.Y.93 formulations in HDPE (1% TiO2) withstand exposure to up to 290 °C for 1 min or up to 270 °C for 5 min. The pigment causes distortion at processing temperatures between 220 and 280 °C. This effect, however, is so minor as to be tolerable for most purposes. Distortion phenomena therefore rarely restrict the extent of pigment application. In terms of lightfastness, 1/3 to 1/25 SD samples (1% TiO2) equal step 7 on the Blue Scale. Good heat fastness makes P.Y.93 a suitable candidate for polypropylene spin dyeing. The pigment is also very lightfast in this medium. P.Y.93 shows good-to-excellent textile fastnesses in textile printing.
P.Y.93 has no importance in the paint industry. The graphic ink field uses P.Y.93 only where fastness standards are high, such as in high grade packaging and metal deco inks.
2.9.4.2.2 Pigment Yellow 94
P.Y.94 is the greenest one of all available yellow dihydrazone condensation pigments. It was only recently withdrawn from the market. With approximately equal cleanness, it is somewhat greener than P.Y.128, which is a member of the same class of pigments. However, P.Y.94 is only about half as strong tinctorially. Some 0.44% P.Y.94 is required to produce a 1/3 SD HDPE sample containing 1% TiO2, while the same end result is achieved by using only either 0.22% P.Y.128 or 0.2% of the somewhat redder P.Y.93.
P.Y.94 shows excellent heat stability. 1/3 SD to 1/25 SD samples in HDPE (1% TiO2) withstand up to 300 °C. The shrinkage of the plastic, however, is considerably affected. P.Y.94 is equally weak in PVC. Some 2.1% pigment is needed to formulate 1/3 SD samples containing 5% TiO2. P.Y.94 exhibits excellent lightfastness and weatherfastness, which is also true for PVC plastisols targeted for coil coating. The pigment is completely fast to migration, even in highly plasticized PVC. Its excellent heat stability makes it a suitable candidate for PP spin dyeing. In addition, P.Y.94 is also used in polyacrylonitrile spin dyeing.
The printing ink industry uses P.Y.94, like other dihydrazone condensation pigments, only for high grade printed products, especially in metal deco printing. Economical pigment formulation is compromised by somewhat poor tinctorial strength. The commercial type, which features a comparatively low specific surface area of approximately 35 m2 g−1, is consequently less transparent than other products in this class of pigments. P.Y.94 is noticeably less lightfast than P.Y.128, which has a finer particle size; the difference is approximately one step on the Blue Scale. In this respect, P.Y.94 resembles the diarylide yellow pigment P.Y.113, which is somewhat greener and distinctly stronger tinctorially. P.Y.94 exhibits plate-out on the metal plates if it is used in decorative printing, that is, on laminated papers for laminated plastic sheets (Section 1.6.4.1).
2.9.4.2.3 Pigment Yellow 95
P.Y.95 affords reddish shades of yellow. At 1/3 SD, its shade may be located somewhere between those of the diarylide yellow pigments P.Y.13 and 83.
Its main field of application is in plastics and in spin dyeing, which is also true for other members of this class of pigments. P.Y.95 is a pigment with high tinctorial strength, it is the strongest yellow dihydrazone condensation pigment. Some 0.7% pigment is required to formulate a 1/3 SD sample in plasticized PVC containing 5% TiO2. For comparison, 0.8% is needed of the greener P.Y.93 and 2.1% of P.Y.94, which is even greener at 1/3 SD.
Some 0.35% must be added of the similarly shaded, but generally less fast P.Y.13 to achieve the same result. P.Y.95 is very lightfast. However, incorporated in plasticized or in rigid PVC, P.Y.95 is less lightfast than P.Y.93.
The pigment shows excellent heat stability in polyolefins. 1/3 SD samples may safely be exposed to 290 °C, while 1/25 SD specimens (1% TiO2) withstand 270 °C. Incorporated in polyolefins, P.Y.95 demonstrates much higher tinctorial strength than most other yellow pigments within its class. Tested in HDPE, the pigment was found to influence the shrinkage of the polymer in injection moulding only to a very negligible extent. In terms of lightfastness, 1/3 SD colourations (1% TiO2) of this medium equal step 6 on the Blue Scale.
P.Y.95 is also used for PUR as well as for polypropylene spin dyeing, where it is employed for its good heat stability and its high tinctorial strength. However, the pigment is not lightfast and durable enough to meet the standards for application in polyacrylonitrile spin dyeing products, such as canvas awnings (Section 1.8.3.8). P.Y.95 is also utilized in textile printing.
Like other representatives of its class, P.Y.95 has only limited impact on the printing ink industry. It is only used in special-purpose printing inks for high grade prints. Its lightfastness in offset and in metal deco printing corresponds to that of P.Y.93. P.Y.95 exhibits good heat stability. The prints are fast to overcoating and sterilization and in most cases show excellent fastness in special applications (Section 1.6.2). P.Y.95 therefore lends itself to metal deco printing and packaging gravure printing inks, that is, printing inks based on nitrocellulose, polyamide and vinyl chloride copolymers.
2.9.4.2.4 Pigment Yellow 128
At medium tinctorial strength, P.Y.128, after P.Y.94, is the second greenest pigment in the dihydrazone condensation range, and it exhibits very good general fastness properties. The pigment is completely or at least almost completely fast to almost all organic solvents that are used in colouristically important media. The pigment possesses excellent lightfastness and weatherfastness, and it is completely fast to overcoating. This makes it a suitable product for automotive paints, even for original automotive (O.E.M) finishes. The commercial type features a relatively high specific surface area of approximately 70 m2 g−1 and is correspondingly transparent. It is mainly used in high grade industrial paints, on which it confers good tinctorial strength and high gloss, and also in architectural paints, for instance in green shades. P.Y.128 is, however, also used in emulsion paints.
The plastics industry uses P.Y.128 mainly in PVC, in which the pigment exhibits average tinctorial strength. 1/3 SD colourations containing 5% TiO2, for instance, are formulated at 1.35% pigment. The lightfastness is very good and equals step 7–8 on the Blue Scale for 1/3 SD samples. Pigment durability is equally very good in this medium and also in PVC plastisols for coil coating. P.Y.128 also lends colour to elastomers. 1/3 to 1/25 SD colourations in HDPE (1% TiO2) withstand exposure to 250 °C for 5 min. The shrinkage of polyethylene is only slightly affected. P.Y.128 can also be used for polyacrylonitrile spin dyeing, an area in which it exhibits very good lightfastness and performs excellently in terms of the more important textile fastnesses. Its weatherfastness, however, does not satisfy the requirements for outdoor application. P.Y.128 is therefore used particularly in home textiles such as upholstery.
The printing ink industry uses P.Y.128 particularly in high quality products. The resulting prints, like those made from other dihydrazone condensation pigments, are fast to calendering and sterilization. P.Y.128 lends itself particularly to metal deco printing. It shows good lightfastness. In this respect, it scores approximately one step higher under comparable conditions than the greener P.Y.94 and the somewhat redder P.Y.93, which are members of the same class of pigments.
2.9.4.2.5 Pigment Yellow 166
P.Y.166 is available in Japan. The pigment has generally a better performance as compared with P.Y. 93, 94 or 95. P.Y.166 is used for the colouration of PVC and polyolefines.
2.9.4.3 Orange, Red and Brown Pigments
2.9.4.3.1 Pigment Orange 31
This pigment is no longer listed as a sales product. It affords a reddish, somewhat dull orange shade. It was recommended particularly for brown shades.
P.O.31 was a useful colourant for plastics and was employed especially in polypropylene spin dyeing. Compared to other members of its class, the pigment exhibits good-to-average tinctorial strength.
P.O.31 is not completely fast to bleeding in plasticized PVC. Its lightfastness in this medium is only satisfactory at higher pigment concentrations, but the pigment possesses excellent heat stability. 1/3 SD HDPE samples (1% TiO2) are fast up to 300 °C. P.O.31 considerably affects the shrinkage of polyolefins in injection moulding (Section 1.8.3.2). 1/3 SD specimens equal step 6 on the Blue Scale for lightfastness. Good heat fastness made the pigment a suitable candidate especially for polypropylene spin dyeing, mainly to produce shades of brown and beige. P.O.31 was also used in textile printing, most of its textile fastnesses are very good.
2.9.4.3.2 Pigment Red 144
P.R.144 is a medium to slightly bluish red pigment, which probably reigns supreme within its class. It is broad in scope and is mainly used to colour plastics, including spin dyeing products. The commercially available types of the acicular pigment differ considerably in terms of particle size, consequently demonstrating a range of colouristic properties. This is especially true for the tinctorial strength. P.R.144 is less stable to various organic solvents than other dihydrazone condensation pigments of its class, such as P.R.166.
P.R.144 is almost completely fast to migration in plasticized PVC. One of the most tinctorially strong dihydrazone condensation pigments, only about 0.7% P.R.144 is needed to produce a 1/3 SD PVC sample containing 5% TiO2. Comparative values are listed for other pigments within the same class and also in other classes. P.R.144 is very fast to light. Its weatherfastness in rigid PVC is less satisfactory, it fails to meet the standards of long-term exposure.
Within this group of pigments, P.R.144, incorporated in PE, ranks second only to P.R.221 regarding tinctorial strength. Some 0.13% pigment is required to produce 1/3 SD specimens containing 1% TiO2. In HDPE, P.R.144 withstands temperatures up to 300 °C at 1/3 to 1/25 SD, both with and without TiO2. It considerably affects the shrinkage of the plastic at processing temperatures between 220 and 280 °C, which should be taken into consideration if the pigment is to be used in injection moulding. P.R.144 has excellent lightfastness: 1/3 SD colourations in HDPE containing 1% TiO2 equal step 7–8 on the Blue Scale, while transparent samples even reach step 8.
P.R.144 is also suited to use in other plastics, such as polystyrene, polyurethane, elastomers or cast resins, including those made from unsaturated polyester.
Good heat stability and lightfastness make P.R.144 a suitable candidate especially for polypropylene spin dyeing. It is equally important for polyacrylonitrile spin dyeing, but does not satisfy the demands for use in canvasses. The more important textile fastnesses are very good, but the pigment is not completely stable to dry and wet crocking. In this respect, it scores as high as step 4 on the 5 step Grey Scale.
The printing ink industry uses P.R.144, like other pigments of its class, primarily in high quality products. The commercial types exhibit specific surface areas between approximately 50 and 90 m2 g−1, which makes for more or less transparent prints. Numerous fastnesses that are important for packaging purposes (Section 1.6.2), such as resistance to soap, butter, cheese, paraffin, acid and alkali, are perfect. P.R.144 is fast to clear lacquer coatings and sterilization, which makes it suitable for metal deco printing. The pigment has very good lightfastness; letterpress proof prints equal step 6 to step 7 on the Blue Scale, depending on the pigment area concentration. In this respect, P.R.144 (at low pigment area concentrations such as 1/25 SD) is somewhat inferior to P.R.166.
Throughout the paint industry, P.R.144 functions as a pigment in general industrial coatings, automotive finishes and architectural paints.
2.9.4.3.3 Pigment Red 166
P.R.166 affords clean yellowish shades of red. It is broad in scope and in this respect resembles the somewhat bluer dihydrazone condensation pigment P.R.144. Its main area of application, however, is in plastics and in spin dyeing.
In the plastics sector, P.R.166 is used primarily to colour PVC and polyolefins. The pigment is almost completely fast to bleeding in plasticized PVC. Similarly coloured pigments of other classes perform poorer in terms of migration and lightfastness and also regarding heat stability. These pigments are considered suitable alternatives to P.R.166 only where the application requirements are less stringent.
P.R.166 exhibits medium-to-good tinctorial strength compared to other pigments covering the same range of shades. Between 0.9% and 1.2% pigment is needed, for instance, to produce 1/3 SD PVC samples containing 5% TiO2. The exact amount depends on the grade. Commercial types of this pigment provide medium to high hiding power. The lightfastness and weatherfastness of P.R.166 in PVC are very good. However, its weatherfastness is often not sufficient to meet the standards of long-term outdoor exposure.
P.R.166 shows excellent heat stability in polyolefins, for instance in HDPE. 1/3 SD samples containing 1% TiO2, for instance, are fast up to 300 °C. This high heat stability in HDPE is compromised by the fact that P.R.166 causes considerable distortion in this medium, irrespective of the processing temperature. Transparent HDPE samples equal step 8 on the Blue Scale for lightfastness, while corresponding polypropylene colourations match step 7. 1/3 SD colourations containing 1% TiO2 reach step 6–7 and step 7, respectively. High lightfastness makes P.R.166 a suitable candidate for polypropylene spin dyeing. Its application in polyacrylonitrile spin dyeing is restricted by the fact that it fails to satisfy the high lightfastness and durability standards of materials such as canvasses. P.R.166 exhibits perfect or almost perfect textile fastnesses. It is also used in polystyrene, rubber and other elastomers. P.R.166 is encountered in polyurethane that is intended for use in coatings, and applied in cast resins made from unsaturated polyester or aminoplast resins.
P.R.166 is recommended in the paint field for use in high grade industrial paints, for original automotive finishes, and for automobile refinishes, as well as for architectural paints and emulsion paints. Incorporated in baking enamels, deep shades (1:1 TiO2) and white reductions (1:20 TiO2) exhibit good weatherability. However, P.R.166 is not quite as weatherfast in these media as the somewhat yellower and considerably cleaner Anthanthrone pigment P.R.168 (Section 3.7.4.2). Dispersed in an alkyd-melamine resin that is baked at 120 °C, the pigment is almost completely fast to overcoating.
Like other members of its class, P.R.166 is used throughout the printing ink industry for high grade prints, especially for packaging purposes. It basically possesses all-round suitability for various printing techniques. P.R.166 is fast to clear lacquer coatings and to sterilization, which qualifies it particularly for use in metal deco printing. It provides very lightfast prints: 1/1 to 1/3 SD letterpress proof prints equal step 7 on the Blue Scale. P.R.166 is thus faster to light than the somewhat bluer dihydrazone condensation pigment P.R.144. P.R.166 shows excellent fastness to agents such as alkali, acids, soap, fats, paraffin and others, which are frequently encountered in packaging printing inks. The pigment is also used in textile printing.
2.9.4.3.4 Pigment Red 214
P.R.214 affords a medium to bluish shade of red. It exhibits average stability to organic solvents, an aspect in which it resembles other members of its class. P.R.214 is very lightfast and fast to overcoating. Its weatherfastness, however, does not quite reach the standards of other types of pigments within its class: it is somewhat inferior, for instance, to that of P.R.242.
The paint industry uses P.R.214 primarily for various types of industrial finishes. The pigment demonstrates very good tinctorial strength in plastics. Some 0.13% pigment is required, for instance, to produce 1/3 SD HDPE colouration containing 1% TiO2. P.R.214 produces a shade which, although cleaner, is very similar to that of P.R.144, a member of the same class of pigments. The two pigments also show similar tinctorial strength. The fact that P.R.214 considerably affects the shrinkage of the plastic makes it less important in injection moulding. It should be emphasized that P.R.214 possesses excellent heat stability. 1/3 to 1/25 SD HDPE samples containing 1% TiO2, as well as transparent specimens of the same depth of shade, are fast to temperatures up to 300 °C. P.R.214 is also used in PP spin dyeing. It shows good textile fastness. A pigment with high tinctorial strength in plasticized PVC, P.R.214 is not completely fast to bleeding in this medium. The pigment is also recommended for use in polystyrene and in several engineering plastics.
Like other representatives of its class, P.R.214 lends itself particularly to use in high grade printing inks that are targeted for purposes such as posters, packaging gravure printing inks for PVC films, and metal deco printing. The prints exhibit good fastness properties, they are stable to soap, alkali and acids and withstand temperatures up to 200 °C. They are also fast to clear lacquer coatings and to calendering.
2.9.4.3.5 Pigment Red 220
P.R.220 affords a yellowish red shade, which is somewhat bluer than that of P.R.166 and slightly yellower than that of P.R.144. P.R.220 is considerably weaker, however, than the latter: 0.31% pigment is required to produce 1/3 SD HDPE samples containing 1% TiO2. Although P.R.220 affects the shrinkage of injection-moulded HDPE at processing temperatures of 220 °C, it does this to an extent that is acceptable for most purposes. The effect is negligible at 260 °C. 1/3 SD specimens are fast to temperatures up to 300 °C and equal step 7 on the Blue Scale for lightfastness. P.R.220 is thus a suitable candidate for polypropylene spin dyeing and is also used in polyacrylonitrile spin dyeing.
2.9.4.3.6 Pigment Red 221
P.R.221 is a slightly bluish red pigment. Its main field of application is also in plastics, especially in PVC and in PUR. P.R.221 is sufficiently fast to migration in plasticized PVC to satisfy the requirements for most applications. The pigment demonstrates very high tinctorial strength in plasticized PVC; its full shade and related shades are the strongest in its class. Only 0.58% pigment is needed to produce a 1/3 SD sample containing 5% TiO2. The tinctorial strength of lighter shades corresponds to that of other dihydrazone condensation pigments in the red range. On the other hand, P.R.221 is less lightfast than many other types. The pigment does not exhibit plate-out in PVC.
P.R.221 is not recommended for use in polyolefins, since it also causes considerable distortion. It has, however, gained recognition as a pigment for high grade printing inks, especially for metal deco printing.
2.9.4.3.7 Pigment Red 242
P.R.242 affords a yellowish red shade, referred to as scarlet. It exhibits good-to-excellent resistance to organic solvents, such as alcohols, esters, ketones and aliphatic hydrocarbons. The pigment is more soluble in aromatic hydrocarbons. P.R.242, like other members of its class, is fast to alkali and acid.
P.R.242 is used throughout the plastics industry as a colourant for PVC as well as for polyolefins, polystyrene and other engineering plastics. It exhibits average tinctorial strength: 0.2% pigment is required to formulate 1/3 SD HDPE samples containing 1% TiO2. P.R.242 is heat stable up to 300 °C. 1/25 SD specimens containing 1% TiO2, as well as transparent samples at 1/3 to 1/25 SD, are stable to temperatures up to 280 °C. The pigment has a considerable effect on the shrinkage of the plastic. It is of interest to PP spin dyeing, for which a special-purpose pigment preparation is available.
In plasticized PVC, P.R.242 shows good fastness to bleeding and provides average tinctorial strength. 1/3 SD colourations containing 5% TiO2 require 1.1% pigment. Good heat stability makes P.R.242 a suitable candidate for use in polystyrene, ABS, PMMA and polyester.
P.R.242 is an equally valuable product for paints, especially for various types of industrial paints. It is also recommended for use in automotive finishes. Both lightfastness and weatherfastness are excellent, but do not quite reach the levels of the appreciably yellower anthanthrone P.R.168. P.R.242 is fast to overcoating and heat stable above 180 °C. It is also employed in emulsion paints based on synthetic resin.
In the printing ink field P.R.242 is utilized to colour high grade systems, such as printing inks for PVC films or metal deco printing. P.R.242 exhibits good fastness properties in application. It shows some bleeding in styrene monomer, which makes the pigment unsuitable for decorative printing for laminated plastic sheets based on styrene/polyester.
2.9.4.3.8 Pigment Red 248
P.R.248 was introduced to the market a few years ago, but marketing has already been discontinued. It was used to colour plastics, especially polyolefins and polystyrene. It provides bluish shades of red.
1/3 SD HDPE samples containing 1% TiO2 are heat stable up to 290 °C. The distortion problem in injection-moulded HDPE gains significance as the processing temperature increases. While at 220 °C the material is distorted to a still acceptable degree, considerable shrinkage is observed at 250 °C and higher temperatures. P.R.248 is one of the types in its class that provides high tinctorial strength. Its strength is comparable to that of P.R.166: 1/3 SD systems containing 1% TiO2 are formulated at 0.16% pigment. 1/3 SD HDPE samples equal step 7 on the Blue Scale for lightfastness. P.R.248 was not recommended for polypropylene spin dyeing.
P.R.248 exhibits excellent bleed resistance in plasticized PVC. Transparent specimens equal step 8 on the Blue Scale for lightfastness, while 1/3 SD samples match step 7. The pigment does not perform as well in terms of weatherfastness, for instance in PVC plastisols for coil coating. P.R.248 was also recommended for use in elastomers, polyurethane and unsaturated polyester.
2.9.4.3.9 Pigment Red 262
P.R.262 is a comparatively recent product that affords bluish shades of red. P.R.262 is recommended for polypropylene spin dyeing, but possesses all-round suitability for other applications, as long as the requirements do not exceed the fastness properties of this class of pigments.
2.9.4.3.10 Pigment Brown 23
P.Br.23 confers reddish shades of brown on its application media. In numerous areas, this pigment is in direct competition with the colouristically similar, somewhat redder and more transparent P.Br.25.
In terms of stability to organic solvents, P.Br.23 performs like other red pigments within its class. It is thus somewhat inferior to the yellow products. Regarding fastness to various ketones, esters and alcohols, as well as to dioctyl phthalate and dibutyl phthalate, P.Br.23 equals step 3–4 and step 4, respectively, on the 5 step scale. P.Br.23 is broad in scope, but its main field of application is in plastics.
Incorporated in plasticized PVC, P.Br.23 exhibits average to good tinctorial strength: 0.75% pigment is required to produce 1/3 SD samples containing 5% TiO2. The pigment is not entirely bleed resistant (step 4), but exhibits excellent lightfastness and weatherfastness. In rigid PVC, for instance, P.Br.23 is fast to long-term exposure. It is primarily used to colour window frames and similar articles.
P.Br.23 shows excellent heat stability in polyolefins. 1/3 SD samples containing 1% TiO2, as well as transparent colourations at 1/3 SD in HDPE, are stable to exposure to 300 °C for 5 min. In injection moulding, P.Br.23 considerably affects the shrinkage of the plastic at 220 °C, an effect that diminishes with increasing temperature (Section 1.8.3.2).
P.Br.23, like other members of this series of pigments, is a suitable colourant for elastomers. However, due to the low fastness of rubber itself, P.Br.23 is only used in high grade elastomers. The pigment is also recommended for use in polystyrene. Transparent polystyrene samples are fast to exposure to 280 °C for 5 min, while light shades containing TiO2 (0.01% pigment + 0.5% TiO2) are only stable up to 240 °C. In terms of lightfastness, these samples equal step 7 and step 5, respectively, on the Blue Scale. Good fastness properties make P.Br.23 a suitable candidate for polyacrylonitrile spin dyeing, although its lightfastness and weatherfastness do not satisfy the stringent requirements for use in applications such as canvasses.
The paint industry utilizes P.Br.23 particularly in industrial paints. As a result of its lack of solvent fastness, P.Br.23 is not completely fast to overcoating if it is used in baking enamels at a curing temperature of 120 °C. It is, however, very fast to light and weather. In deep shades (1:1 TiO2), P.Br.23 equals step 8 on the Blue Scale for lightfastness, while light shades (1:20 TiO2) match step 7–8. To assess their weatherfastness, baking enamels containing P.Br.23 were subjected to two years of outdoor exposure. The colourations were found to equal step 4–5 and step 3–4 on the Grey Scale, respectively (Section 1.6.6). In the automotive industry, P.Br.23 is recommended for use in original automotive and repair finishes. The list of applications also includes emulsion paints and high grade architectural paints. Moreover, the pigment is also found in various types of wood stains and other special-purpose media.
P.Br.23 possesses all-round suitability for all printing applications. Its shade and its fastness properties make it particularly suitable for printing imitation wood, for instance on PVC. The prints show good fastness to clear lacquer coatings. Sterilization causes somewhat of a yellow shift. P.Br.23 prints demonstrate good lightfastness: under standardized conditions, 1/1 to 1/25 SD letterpress proof prints equal step 6–7 and step 6 on the Blue Scale, respectively, depending on the substrate.
2.9.4.3.11 Pigment Brown 41
This dihydrazone condensation pigment affords very yellowish shades of brown, covering the range of the displaced benzimidazolone pigment P.Br.32.
Incorporated in PVC, P.Br.41 provides medium tinctorial strength and good hiding power. The pigment is not completely fast to bleeding in plasticized PVC. In polyolefins, P.Br.41 also exhibits medium tinctorial strength: 0.21% pigment is required to formulate 1/3 SD HDPE samples containing 1% TiO2. Such specimens are stable up to 300 °C. P.Br.41 shows excellent weather fastness in rigid PVC and in impact-resistant PVC, which is also true for reductions with TiO2. The pigment even satisfies the requirements of long-term exposure. It is used, for instance, in window frames made of impact-resistant PVC.
Incorporated in paints, P.Br.41 exhibits equally high hiding power, which makes it a less attractive product for use in metallic finishes. Its tinctorial strength in these media is comparatively poor. However, P.Br.41 is completely fast to overcoating. The commercial grade shows considerable flocculation in various paint systems.
2.9.4.3.12 Pigment Brown 42
P.Br.42 provides yellowish shades of brown, which are on the yellowish side of P.Br.41. The pigment is particularly recommended for use in rigid PVC, in which it exhibits medium tinctorial strength: 0.85% pigment is required to produce 1/3 SD samples containing 5% TiO2. P.Br.42 is very lightfast and weatherfast, a quality that makes it suitable for long-term exposure. Incorporated in plasticized PVC, P.Br.42, like other brown pigments within its class, is not completely bleed resistant (step 3–4).
1/3 SD P.Br.42 samples in HDPE containing 1% TiO2 are stable up to 280 °C for 5 min, which indicates very good heat stability. Some 0.22% pigment is needed to formulate such 1/3 SD samples.
P.Br.42 affords somewhat dull, yellowish shades of brown in industrial finishes, such as alkyd melamine resin systems. The coating shows a certain opacity; the pigment, however, is recommended to be used in metallics and as opaque pigment as well.
Notes
References for Chapter 2
- 1 For crystal structures of Naphthol AS pigments see, for example, Kobelt, D., Paulus, E.F., and Kunstmann, W. (1972) Acta Crystallogr., Sect. B, 28, 1319–1324; Kobelt, D., Paulus, E.F., and Kunstmann, W. (1974) Z. Kristallogr., 139, 15–32; Paulus, E.F. (1982) Z. Kristallogr., 160, 235–243; for Hansa Yellow pigments see, for example, Paulus, E.F., Rieper, W., and Wagner, D. (1983) Z. Kristallogr., 165, 137–149; for pyrazolone pigments see, for example, Whitaker, A. (1988) Acta Crystallogr., Sect. C, 44, 1587–1590.
- 2 For spectroscopic investigations in the solid state and in solution see, for example, Mustroph, H. (1987) Z. Chem., 27, 281–289 (review with 100 references); Antonov, L. and Stoyanov, S. (1995) Dyes Pigment., 28, 31–39 (UV-vis spectroscopy); Alarcon, S.H., Olivieri, A.C., Sanz, D., Claramunt, R.M., and Elguero, J. (2004) J. Mol. Struct., 705, 1–9 (NMR).
- 3 Paulus, E.F. unpublished results.
- 3a Program Mercury CSD. Cambridge Cristallographic Data Centre 2016.
- 4 See, for example, Barrow, M.J., Christie, R.M., and Monteith, J.E. (2002) Dyes Pigments, 55, 79–89 (diaryl pigments); van de Streek, J., Brüning, J., Ivashevskaya, S.N., Ermrich, M., Paulus, E.F., Bolte, M., and Schmidt, M.U. (2009) Acta Crystallogr., Sect. B, 65, 200–211 (benzimidazolone pigments); Ivashevskaya, S.N., van de Streek, J., Djanhan, J.E., Brüning, J., Alig, E., Bolte, M., Schmidt, M.U., Blaschka, P., Höffken, H.W., and Erk, P. (2009) Acta Crystallogr., Sect. B, 65, 212–222 (laked pigments).
- 5 Gilli, P., Bertolasi, V., Pretto, L., Antonov, L., and Gilli, G. (2005) J. Am. Chem. Soc., 127, 4943–4953; Schmidt, M.U., Brüning, J., Wirth, D., and Bolte, M. (2008) Acta Crystallogr., Sect C., 64, o474–o477.
- 6 Woroshzow, N.N. (1966) Grundlage der Synthese von Zwischenprodukten und Farbstoffen, 4th edn, vol. 6, Akademie-Verlag, Berlin.
- 7 Schweizer, H.R. (1964) Künstliche organische Farbstoffe und ihre Zwischenprodukte, Springer-Verlag, Berlin.
- 8 Winnacker, K. and Küchler, L. (1982) Chemische Technologie, 4th edn, Carl Hanser Verlag, Munich, pp. 143–310.
- 9 Zollinger, H. (1958) Chemie der Azofarbstoffe, Birkhäuser, Basel.
- 10 Zollinger, H. (2003) Color Chemistry, 3rd edn, VCH Verlagsgesellschaft, Weinheim; Zollinger, H. (1998) Diazo Chemistry I, VCH Verlagsgesellschaft, Weinheim.
- 11 Rochat, A.C. and Stocker, E. (1974) XII. Congress FATIPEC, Garmisch, 12–18 May 1974, Verlag Chemie, Weinheim, p. 371.
- 12 For example, Ronco, K. (1966) Ciba-Geigy DE-OS 1 644 119; Ronco, K., Müller, W. (1966) Ciba-Geigy DE-OS 1 644 127.
- 13 Jung, R., Kund, K., Nestler, B., Schmidt, M., Unverdorben, L., and Steiner, R.(Clariant) (2000) EP 1240253.
- 14 Topham, A. (1968) J. Appl. Chem., 18, 233–235.
- 15 Nakaten, H. (1961) Chimia, 15, 156–163; Patterson, D. (1967) Ber. Bunsenges. Phys. Chem., 71, 270–276.
- 16 Bayer (1977) EP-PS 1236; ICI (1978) EP-PS 3656.
- 17 Hoechst AG (1978) EP-PS 10219.
- 18 Ciba-Geigy (1975) DE-OS 2635536; Ciba-Geigy (1975) DE-OS 2635778.
- 19 Hoechst AG (1981) EP-PS 58296.
- 20 Meister Lucius & Brüning (1909) DRP 257488.
- 21 Paulus, E.F. (1984) Z. Kristallogr., 167, 65–72.
- 22 Whitaker, A. (1983) Z. Kristallogr., 163, 139–149.
- 23 Whitaker, A. (1987) Acta Crystallogr., Sect. C, 43, 2141–2144.
- 24 Paulus, E.F. unpublished results.
- 25 Christie, R.M., Hill, J.M., and Rosair, G. (2006) Dyes Pigments, 71, 194–198.
- 26 Whitaker, A. (1988) Acta Crystallogr., Sect. C, 44, 1767.
- 27 Hunger, K., Paulus, E.F., and Weber, D. (1982) Farbe + Lack, 88, 453–458.
- 28 Paulus, E.F., Rieper, W., and Wagner, D. (1983) Z. Kristallogr., 165, 137–149.
- 29 BASF (1976) DE-OS 2 616 981; BASF (1981) EP 37972; Hoechst (1988) DE-OS 3833226.
- 30 Schmidt, M.U., van de Streek, J., and Ivashevskaya, S.N. (2009) Chem. Eur. J., 15, 338–341.
- 31 Ivashevskaya, S.N., van de Streek, J., Djanhan, J.E., Brüning, J., Alig, E., Bolte, M., Schmidt, M.U., Blaschka, P., Höffken, H.W., and Erk, P. (2009) Acta Crystallogr., Sect. B, 65, 212–222.
- 32 US Food and Drug Administration (2000) Inventory of Effective Food Contact Substance (FCS) Notifications, FCN No. 25.
- 33 US Food and Drug Administration (2000) Inventory of Effective Food Contact Substance (FCS) Notifications, FCN No. 84.
- 34 Whitaker, A. (1983) J. Soc. Dyers Color., 99, 121–123.
- 35 Whitaker, A. (1983) Z. Kristallogr., 163, 19–30.
- 36 Whitaker, A. (1985) J. Soc. Dyers Color., 101, 21–24.
- 37 Whitaker, A. (1983) J. Soc. Dyers Color., 99, 157–159.
- 38 Whitaker, A. (1988) J. Soc. Dyers Color., 104, 225–226;Whitaker, A. (1988) Acta Crystallogr., Sect. C, 44, 1587–1590.
- 39 Whitaker, A. (1986) J. Soc. Dyers Color., 102, 136–137.
- 40 Whitaker, A. (1986) J. Soc. Dyers Color., 102, 109–110.
- 41 Whitaker, A. (1984) J. Soc. Dyers Color., 100, 123–124.
- 42 Schmidt, M.U., Acs, A., Jung, R., and Schui, F. (Clariant) (2002) US 6602342.
- 43 Griesheim-Elektron (1911) DRP 251479.
- 44 Hoechst AG (1953) DE 921404.
- 45 McKay, R.B. (1990) Farbe + Lack, 96, 336–339.
- 46 Herbst, W. and Hunger, K. (1978) Prog. Org. Coat., 6, 106–270.
- 47 Kozo, T. et al. (1963) Shikizai Kyokai-shi, 36, 16–21.
- 48 Herbst, W. and Merkle, K. (1976) DEFAZET Dtsch. Farben Z., 30, 486–490.
- 49 Schmidt, M.U. (1999) Colour Science '98, Volume 1: Dye and Pigment Chemistry, (ed. J. Griffiths), University of Leeds, Leeds, pp. 72–81.
- 50 Barrow, M.J., Christie, R.M., Lough, A.J., Monteith, J.E., and Standring, P.N. (2000) Dyes Pigments, 45, 153–160.
- 51 Barrow, M.J., Christie, R.M., and Monteith, J.E. (2002) Dyes Pigments, 55, 79–89.
- 52 Barrow, M.J., Christie, R.M., and Badcock, T.D. (2003) Dyes Pigments, 57, 99–106.
- 53 Schmidt, M.U., Dinnebier, R.E., and Kalkhof, H. (2007) J. Phys. Chem. B, 111, 9722–9732.
- 54 Christie, R.M. and Standring, P.N. (1989) Dyes Pigments, 11, 109–121.
- 55 Az, R., Dewald, B., and Schnaitmann, D. (Hoechst AG) (1991) Dyes Pigments, 15, 1–15.
- 56 Kawamura, T. (1987) Japanese Patent JP 62153353.
- 57 Tuck, B., Stirling, J.A., Farnocchi, C.J., and McKay, R.B. (Ciba SC) (1997) EP 0790282.
- 58 Osamu, J., Shuji, M., and Masami, S. (Toyo) (2002) JP 2002161214.
- 59 See, for example, Hari, S. and Ronco, K. (Ciba-Geigy) (1972) DE-OS 2 243 955; Ciba-Geigy (1973) DE-OS 2 410 240.
- 60 Ivashevskaya, S.N., van de Streek, J., Brüning, J., and Schmidt, M.U. unpublished results.
- 61 Rieper, W. and Baier, E. (Hoechst) (1991) EP 545 072.
- 62 Jung, R., Metz, H.J., Weber, J., Schmidt, M.U., Schupp, O., and Wacker, A. (2001) (Clariant) EP 1188800.
- 63 Griesheim-Elektron (1910) DRP 236856.
- 64 Grainger, C.T. and McConnell, J.F. (1969) Acta Crystallogr., Sect. B, 25, 1962–1970.
- 65 Whitaker, A. (1980) Z. Kristallogr., 152, 227–238; Whitaker, A. (1981) Z. Kristallogr., 156, 125–136.
- 66 Whitaker, A. (1978) Z. Kristallogr., 147, 99–112.
- 67 Whitaker, A. (1977) Z. Kristallogr., 145, 271–288.
- 68 Yatsenko, A.V., Paseshnichenko, K.A., Chernishev, V.V., and Schenk, H. (2001) Acta Crystallogr., Sect. E, 57, o1152–o1153.
- 69 Schmidt, M.U., Buchsbaum, C., Schnorr, J.M., Hofmann, D.W.M., and Ermrich, M. (2007) Z. Kristallogr., 222, 30–33.
- 70 Kobelt, D., Paulus, E.F., and Kunstmann, W. (1972) Acta Crystallogr., Sect. B, 28, 1319–1324; Kobelt, D., Paulus, E.F., and Kunstmann, W. (1974) Z. Kristallogr., 139, 15–32.
- 71 Whitaker, A. (1977) Z. Kristallogr., 146, 173–184.
- 72 Ribka, J. (Hoechst AG) (1969), DE 12287731; Ribka, J. (Hoechst AG) (1970), DE 2 043 482.
- 73 Schmidt, M.U., Hofmann, D.W.M., Buchsbaum, C., and Metz, H.J. (2006) Angew. Chemie, 118, 1335–1340; Angew. Chem., Int. Ed., 45, 1313–1317.
- 74 Warshamanage, R., Linden, A., Schmidt, M.U., and Bürgi, H.-B. (2014) Acta Crystallogr., Sect. B, 70, 283–295. Teteruk, J., Glinnemann, J., Gorelik, T.E., Linden, A., and Schmidt, M.U. (2014) Acta Crystallogr., Sect. B, 70, 296–305.
- 75 Chang, C.-H., Christie, R.M., and Rosair, G.M. (2003) Acta Crystallogr., Sect. C, 59, o556–o558.
- 76 Hundsdorf, T. and Rauschmann, W. (Clariant) (2008) EP 2170999.
- 77 Czajkowski, W. (1980) Dyes Pigments, 1, 17–25.
- 78 Czajkowski, W. and Jones, F. (1977) J. Soc. Dyers Color., 93, 313–317.
- 79 Hoechst (1982) EP 97 913.
- 80 Dainippon (1996) Jap. Patents 09194752-A, 09227791-A, 092411524, 09268259-A.
- 81 Schmidt, M.U. and Metz, H.J. (Clariant) (1999), EP 965616.
- 82 Schmidt, M.U. and Metz, H.J. (Clariant) (1999), EP 965617.
- 83 Schmidt, M.U. (Clariant) (1999), EP 1010732.
- 84 Gorelik, T., Schmidt, M.U., Brüning, J., Bekoe, S., and Kolb, U. (2009) Cryst. Growth Des., 9, 3898–3903.
- 85 Kennedy, A.R., Andrikopoulos, P.C., Arlin, J.-B., Armstrong, D.R., Duxbury, N., Graham, D.V., and Kirkhouse, J.B.A. (2009) Chem. Eur. J., 15, 9494–9504.
- 86 Stenger, J., Kwan, E.E., Eremin, K., Speakman, S., Kirby, D., Stewart, H., Huang, S.G., Kennedy, A.R., Newman, R., and Khandekar, N. (2010) e-Preservations Sci., 7, 147–157.
- 87 Czajkowski, W. (1987) Dyes Pigments, 8, 141–150.
- 88 See, for example, Kennedy, A.R., McNair, C., Smith, W.E., Chisholm, G., and Teat, S.J. (2000) Angew. Chem., 112, 652–654; Angew. Chem. Int. Ed., 39, 638–640.
- 89 Hoechst (1982) DE-OS 3 223 888.
- 90 Blank, U. et al. (1982) Winnacker-Küchler, Chemische Technologie, 4th edn, vol. 6, Carl Hanser Verlag, Munich, pp. 143–310.
- 91 Woroshzow, N.N. (1966) Grundlage der Synthese von Zwischenprodukten und Farbstoffen, 4th edn, Akademie-Verlag, Berlin.
- 92 Harris, R.K., Jonsen, P., Packer, K.J., and Campbell, C.D. (1987) J. Chem. Soc., Perkin Trans. II, 1383–1387.
- 93 Bekoe, S.L., Hammer, S.M., and Schmidt, M.U. (2012) Angew. Chem., 124, 4814–4818; Angew. Chem. Int. Ed., 51, 4735–4738.
- 94 Lebensmittelzusatz-Zulassungsverordnung, 30 December 81, EG-Nr. E 180i DFG-Farbstoffkommission (Dyestuff Commission); Ringbuch – Farbstoffe für Lebensmittel, Colours for Foods (1988), LB-Rot 2; Kosmetikverordnung vom 21. Dezember 77/Anl. 3, Teil A; DFG-Farbstoffkommission (Dyestuff Commission); Ringbuch – Kosmetische Färbemittel, Colours for Cosmetics (1991), C-Rot 12.
- 95 Hoechst (1988) EP 320 774; Hoechst (1987) DE-OS 3 742 815.
- 96 US Food and Drug Administration (2000) Inventory of Effective Food Contact Substance (FCS) Notifications, FCN No. 27.
- 97 US Food and Drug Administration (2000) Inventory of Effective Food Contact Substance (FCS) Notifications, FCN No. 23.
- 98 US Food and Drug Administration (2002) Inventory of Effective Food Contact Substance (FCS) Notifications, FCN No. 274.
- 99 FDA (2000) Food Contact Substances Notification, FCS No. 27, 02 May 2000 (Engelhard Corp.).
- 100 FDA (2000) Food Contact Substances Notification, FCS No. 26, 04 August 2000; Bindra, A. (2006) US Pat 7041421 (Engelhard Corp).
- 101 FDA (2000) Food Contact Substances Notification, FCS No. 23, 04 August 2000; Bindra, A. (2006) US Pat. 7041421 (Engelhard Corp).
- 102 FDA (2002) Food Contact Substances Notification, FCS No. 274, 14 November 2002 (BASF Corporation).
- 103 Dietz, E. and Fuchs, O. (1973) Farbe + Lack, 79, 1058–1063.
- 104 Schunck, R.P. and Hunger, K. (1988) Pigment Handbook, vol. I (ed. P.A. Lewis), John Wiley & Sons, Inc., New York, pp. 523–533.
- 105 Paulus, E.F. and Hunger, K. (1980) Farbe + Lack, 86, 116–120.
- 106 Hunger, K., Paulus, E.F., and Weber, D. (1982) Farbe + Lack, 88, 453–458.
- 107 Paulus, E.F. (1982) Z. Kristallogr., 160, 235–243.
- 108 van de Streek, J., Brüning, J., Ivashevskaya, S.N., Ermrich, M., Paulus, E.F., Bolte, M., and Schmidt, M.U. (2009) Acta Crystallogr., Sect. B, 65, 200–211.
- 109 Schmidt, M.U. (Hoechst AG), unpublished results.
- 110 Schmidt, M.U., Brühne, S., Wolf, A.K., Rech, A., Brüning, J., Alig, E., Fink, L., Buchsbaum, C., Glinnemann, J., van de Streek, J., Gozzo, F., Brunelli, M., Stowasser, F., Gorelik, T., Mugnaioli, E., and Kolb, U. (2009) Acta Crystallogr., Sect. B, 65, 189–199.
- 111 Schmid, M. (1955) DEFAZET Dtsch. Farben Z., 9, 252–255.
- 112 Gaertner, H. (1963) J. Oil Colour Chem. Assoc., 46, 13–46.
- 113 Ruf, K., Gülec, B., Reisinger, M., Surber, W. (Ciba-Geigy) (1991) EP498 469.
- 114 Mory, R., Stöcklin, E., and Schmid, M. (Ciba) (1956) DE 1026750.
- 115 NPIRI (1983) Raw Materials Data Handbook, vol. 4, Pigments, National Printing Ink Research Institute, Bethlehem, Penn. USA.
- 116 Ciba (1957) DE-PS 1 150 165; Ciba (1964) DE-PS 1 544 453.
- 117 Dainichi Seika (1972) DE-OS 2 312 421.
- 118 Ciba (1957) DE-PS 1 150 165.
- 119 Ciba (1966) DE-OS 1 644 117.
- 120 Lewis, P.A. (ed.) (1988) Pigments Handbook, vol. 1, John Wiley & Sons, Inc., New York, p. 724.
- 121 Kaul, B.L. (1987) J. Col. Chem., 12, 349–354; Kaul, B.L. (1989) Soc. Plastics Eng., 213–226.
- 122 Kaul, B.L. (1993) Rev. Prog. Color., 23, 19–35; Ciba-Geigy (1974) DE-PS 2 500 005.
- 123 Keller, E. (1999) SCHAKAL 99, Kristallographisches Institut der Universität Freiburg, Freiburg, Germany.
