4
Miscellaneous Pigments
This chapter lists pigments that, either for reasons of different chemical structure or for lack of knowledge thereof, are not included in Chapters 2 or 3 of this book.
4.1 Triarylcarbonium Pigments
As a class, these pigments share the triarylcarbonium structure. At least two of the aryl moieties carry amino groups, which act as electron-donating substituents.
The compounds are therefore basic and thus capable of combining with acids to form insoluble salts, which is the form in which they can be applied as pigments.
Triarylcarbonium compounds can be described by several mesomeric structures, either in quinoid form with an ammonium ion or by a benzenoid structure with a carbonium ion:

For reasons of molecular symmetry, only the carbonium structure is used in this book to describe triarylcarbonium pigments.
Two types of insoluble triarylcarbonium compounds are used industrially as pigments. Both are salts of these basic dyes. So-called Alkali Blue type triarylcarbonium pigments are inner salts of sulfonic acids, while the second group consists of salts of complex inorganic anions of heteropolyacids.
For a long time, triarylcarbonium compounds were used exclusively as textile dyes [1]. Wool was coloured using acidic dyes, that is, compounds that contained several sulfonic acid functions, while silk was dyed with basic products. Cotton was prepared by ‘mordanting’ the fibre with amine/tartar emetic (potassium antimonyl tartrate), the dye was fixed in the form of an insoluble compound. ‘Lakes’ were prepared by precipitating aqueous solutions of basic dyes with tannine/tartar emetic onto a mineral carrier (aluminium oxide, barium sulfate, calcium sulfate). Although the resulting ‘pigments’ provided brilliant shades, they offered insufficient lightfastness and are thus no longer important.
4.1.1 Inner Salts of Sulfonic Acids (Alkali Blue Types)
These compounds are triphenylmethane pigments. Strictly speaking, they should be referred to as tri(aminophenyl)methane derivatives. The parent structure is known as reddish violet parafuchsin (119) or its anhydro base pararosaniline (119a). The parent compound may bear between one and three methyl substituents (fuchsin, new fuchsin).

Scheme 4.1 Molecular structures of parafuchsin and pararosaniline.
The entire group of these compounds dates back to the very beginnings of organic dye chemistry. In 1858, E. Verguin in France oxidized a material that he named ‘aniline’ but which was in fact a mixture of aniline, o-toluidine, and p-toluidine. He performed the reaction in nitrobenzene in the presence of tin(IV) chloride or iron(III) chloride to produce bluish red fuchsine (120). The process has been industrially exploited since 1859. The central carbon atom is furnished by the CH3 group of p-toluidine, which is initially oxidized to its aldehyde.

Shortly afterwards, a route to acidic triphenylmethane compounds, referred to as Alkali Blue types, was developed.
In 1860, Girard and de Laire heated fuchsine with aniline and found triphenylfuchsine, known as Lyon Blue. Nicholson, in an attempt to make this structure water soluble, in 1862 introduced free sulfonic acid groups into the molecule through sulfonation.
The thus-prepared product, however, being the inner salt of a sulfonic acid, turned out to be entirely insoluble in water. The compound only became a suitable textile dye after it had been converted into its sodium salt, that is, through application in a slightly alkaline medium (= Alkali Blue).
The principle of forming the important inner salts of sulfonated triphenylmethane derivatives had thus been discovered. All of the commercially important products within this group are derived from phenylated rosaniline corresponding to structure 121 below.
4.1.1.1 Chemistry, Manufacture
Alkali Blue type pigments are based on the following general structure:

Manipulation of the number of phenyl groups R1 to R3 on the rosaniline nucleus and their substitution pattern has proven a useful tool in producing various commercially important derivatives. Currently, compounds with two (R1, R2 =C6H4CH3/C6H5; R3 = H) and especially three phenyl and/or toluene moieties (R1, R2, R3 = C6H5/C6H4CH3) are technically important. The CH3 groups are primarily located at the meta-position relative to the secondary amino group.
Notably, all triarylcarbonium pigments are described by structures that are no more than a useful approximation of reality. These products in fact represent mixtures of various compounds that are obtained through an intricate reaction pattern, the respective written structure being the main component.
The substituted triphenylmethyl (trityl) system 121 may basically be synthesized by two different routes, both of which are in use.
Route A involves preparation of triaminotriarylmethane derivatives and their subsequent reaction with aromatic amines in the presence of acidic catalysts.
Route B proceeds via trihalogentriarylcarbonium compounds, which are reacted with aromatic amines.
The most important starting materials for process A are 4,4′,4″-triaminotriphenylmethane, pararosaniline (119a), and parafuchsin (119). Aniline and formaldehyde are treated at 170 °C to form, apart from some formaldehyde-aniline intermediates, 1,3,5-triphenylhexahydrotriazine as the main component. Subsequent treatment with an acidic catalyst, for instance with hydrochloric acid, in excess aniline as a solvent initially affords 4,4′-diaminodiphenylmethane, which is finally oxidized to yield parafuchsin (119). Iron(III)chloride and nitrobenzene, which in the past were employed as oxidants, are no longer used. The reaction is now performed by air oxidation in the presence of vanadium pentoxide as a catalyst:

Other intermediate products are [2]:

Pararosaniline, after being formed from parafuchsin with sodium hydroxide, is fused at 175 °C with aniline and/or m-toluidine, for instance in the presence of catalytic amounts of benzoic acid, and thus arylated:

The aniline or toluidine residue in the melt is removed by vacuum distillation at 150 °C. The melt is allowed to cool to room temperature and then broken.
Various degrees of arylation may be achieved, depending on the fusion time and on the amount of benzoic acid used. Longer fusion times afford more greenish Alkali Blue types, corresponding to a higher yield of phenylated triaryl product, while the amounts of the more reddish diaryl and monoaryl pararosanilines decrease with the time. Careful reaction control makes it possible to convert the materials into the corresponding water insoluble monosulfonates through sulfonation with concentrated sulfuric acid:

The crude pigment provides only limited tinctorial strength. This necessitates the following aftertreatment: dissolution as a sodium salt, precipitation with mineral acid, possibly in the presence of surface active agents, followed by drying or flushing to transfer the material from the aqueous to the oily phase (mineral oil, linseed oil).
Flushing involves kneading the aqueous pigment presscake with varnish until a complete exchange has taken place between the two liquid phases. The aqueous phase is then separated and residual water removed by vacuum distillation at elevated temperature. Flushing affords a product that can be incorporated directly into a printing ink (Section 1.6.5.8).
Route A is generally unacceptable, due to a poor parafuchsin yield (in the past 35%, now approx. 60%). The arylation step, on the other hand, proceeds with good yield. The formation of a comparatively large amount of by-products makes it necessary to purify the product extensively before it is processed further. Pathway B starts from p-chlorobenzotrichloride and chlorobenzene, which undergo a Friedel–Crafts reaction, forming the tetrachloroaluminum complex of trichlorotriphenylmethyl chloride (122). Compound 122 reacts with aromatic amines only if it is first condensed with at least one meta- or para-substituted aniline. Unsubstituted aniline, deactivating the aluminium complex, does not afford acylation products. Meta- or para-substituted anilines, however, produce arylated pararosanilines in almost theoretical yield.

Subsequent treatment with alkali provides a free base solution in excess amine, which can be separated from the aqueous/alkaline aluminate layer. The thus-prepared product is precipitated with acid, possibly converted into the free base with aqueous alkaline solution, and isolated. The dried base or its salt is then sulfonated with concentrated sulfuric acid to form the monosulfonic acid 121.
Route B of this process may be substantially improved in terms of yield and product quality (purity) of the resulting triarylaminoarylcarbonium pigment. To this end, the solution of the free dye base is treated with an excess of aqueous sulfuric acid (20–40%) in a solvent such as chlorobenzene or an aromatic amine. This method produces the sulfate of the basic dye, which is insoluble in this medium, together with the soluble sulfates of the primary aromatic amines, which can therefore easily be separated. The isolated sulfate of the basic dye is then washed and in dry or wet condition monosulfonated with 85–100% sulfuric acid. Based on the dye base sulfate, this step affords 96–98% yield, compared to only 83–89% achieved by the previously described method. The entire synthesis, including the intermediate isolation of the triarylaminoarylmethane sulfate, may also be performed by continuous process [3].
Route B has the added advantage over route A of making it possible to perform a stepwise reaction of trichlorophenylmethyl-tetrachloroaluminate 122 (i.e. the complex compound formed from tri(chlorophenyl)methyl chloride and AlCl3) with various amines. It is thus possible to systematically synthesize triphenylmethane pigments with two or three differently substituted arylamino groups.
4.1.1.2 Properties and Application
As a result of the nonuniform reaction process, all commercial products within the Alkali Blue series represent mixtures of various products. The respective structure listed in the Colour Index only reflects the main component of a differently arylated mixture. Moreover, the aromatic moieties not only represent differently substituted compounds but also mixtures of various degrees of sulfonation.
Special-purpose triphenylmethane pigments are frequently even ‘tailor-made’, that is, designed to suit the individual needs of different areas of application.
Alkali Blue types cover a wide range of shades from the reddish blue to the violet portion of the spectrum. The shade becomes greener as the number of phenyl groups in the molecule increases. Both the tinctorial strength and the solubility of the pigment are controlled by manipulating the degree of sulfonation: one sulfonic acid function per molecule affords an optimum in tinctorial strength, while a higher degree of sulfonation adversely affects the strength but simultaneously improves the solubility of the pigment in water. This explains why compounds with two to three sulfonic acid groups (such as ‘Water Blue’ for paper mass colouration) are only infrequently used as pigments (by forming an insoluble aluminium lake on adding alum). Products featuring four to five sulfonic acid groups (such as ‘Ink Blue’ for inks) are completely unsuitable for use as pigments.
4.1.1.3 Commercially Available Alkali Blue Pigments
The list of significant Alkali Blue type triarylcarbonium pigments includes compounds derived from diarylated and triarylated rosanilines. Table 4.1 shows the pigments that are listed in the Colour Index.
Table 4.1 Alkali Blue type triaryl carbonium pigments.

|
||||||
| C.I. Name | C.I. Constitution No. | R | R′ | R1 | R2 | R3 |
| P.B.18 | 42 770:1 | C6H5NH | H | H | H | Ha |
| P.B.19 | 42 750 | NH2 | CH3 | H | H | H |
| P.B.56 | 42 800 |

|
H | CH3 | CH3 | H |
| P.B.61 | 42 765:1 | C6H5NH | H | H | H | H |
| a The constitution which is listed in the Colour Index erroneously contains another SO3H group in the R2 and R3 carrying ring. In fact, however, P.B.18 and P.B.61 are chemically identical. | ||||||
This chapter discusses relevant fastness properties and aspects of pigment performance as well as various facets of pigment application. The entire class is treated comprehensively instead of focussing on individual types. In Europe, Alkali Blue pigments are also referred to as Reflex Blue pigments, a trade name created by the local manufacturer.
The main area of application for these products is in printing inks, especially in offset and letterpress application and to a lesser extent also in aqueous flexographic inks. The pigments have less of an impact on the office articles sector. The printing ink industry employs triarylcarbonium pigments less as independent self colours but rather as shading pigments to tone black inks. Used at low concentrations, these pigments correct the brown touch of carbon black and provide a colouristically neutral, particularly deep black.
Alkali Blue types cover the reddish blue portion of the spectrum. Even the very greenish specimens are still considerably redder than α- or ɛ-Copper Phthalocyanine Blue. Alkali Blue pigments are uncommonly strong. At standardized conditions concerning printed film thickness, 1/3 SD letterpress proof prints are prepared from inks containing only approx. 3.5% pigment. More than twice as much pigment is needed to prepare corresponding α-Copper Phthalocyanine Blue prints.
As a consequence of their polarity, triarylcarbonium pigments show a considerable tendency to agglomerate. Compared to other organic pigments, they are very difficult to disperse. Normal powder pigments are therefore rarely used in actual practice – the pigments are supplied predominantly in predispersed form as flushed pastes (Section 1.6.5.8). Pigments may be flushed into very different types of binders, which makes it possible to optimize the products for specific printing purposes, such as heat set inks. The fact that a flushed paste consists of predispersed pigment makes it possible to add any amount of paste, as needed, to a ready-made dispersion of carbon black.
The rheological and colouristic differences between flushed pastes are partly attributed to the pigment concentration. Notably, however, tinctorial strength is a minor concern in a shading pigment: these products are selected primarily for their shading behaviour. This depends not only on the type of carbon black, for instance its colour cast, but it is also a function of the shade of Alkali Blue. Black prints made from inks containing Alkali Blue tend to bronze, a feature that equally contributes to the shading behaviour of the product. This effect, which is difficult to assess and measure, results from various factors [4]. Studies have indicated that, depending on the pigment concentration, practically all blue pigments bronze to a certain extent. The minimum extent of bronzing is given by the optical constants of the pigment. Stronger bronzing is attributed to floating of the blue pigment or to the penetration of a considerable amount of binder into the surface of the substrate, consequently increasing the pigment concentration on the surface of the dry printed film, a phenomenon that makes the reflection of light at the surface sensitive to the wavelength of the incident light.
Commercial flushed pastes commonly contain about 40% pigment; however, these products are standardized not in terms of pigment concentration but regarding their tinctorial strength. The ratio between carbon black and Alkali Blue pigment in a toned product may range between approximately 2 : 1 and 4 : 1, depending on the nature of the components and on the desired effect.
For several years, specialized Alkali Blue pigments, referred to as easily dispersible, have been available in powder form. Already during synthesis, these pigments are prepared with suitable resins or other agents to reduce the problem of pigment agglomeration during drying and milling. In addition, thus prepared pigments also have the advantage of facilitating the deagglomeration of agglomerated units within a printing ink. These types, however, do not disperse as readily as the easily dispersible members of other classes of organic pigments, for instance of the diarylide yellow pigment series.
Powder grades are commonly dispersed using agitated ball mills, often directly in combination with the carbon black. Theoretically, one should expect pigments that are used in offset and letterpress inks to dissolve in their medium or to more or less recrystallize. Such effects are normally expected in view of the considerable thermal demands placed upon pigments, especially carbon black, by modern dispersion technology. In practice, however, no colouristic evidence of dissolution or recrystallization has been found. The primary components in the respective oil-based binders, that is, offset and letterpress printing inks, are largely nonaromatic mineral oils as well as linseed oil and similar solvents. Alkali Blue pigments, tested for solvent fastness in pigment powders at standardized conditions (ambient temperature) (Section 1.6.2) prove to be completely resistant to these solvents, but not to alcohols and ketones. Insufficient fastness to alcohols is one of the main factors in precluding Alkali Blue from use in nitrocellulose-based printing inks. The printing ink often loses tinctorial strength and gloss, an effect that, due to pigment recrystallization, cannot be controlled.
Although Alkali Blue pigments are considerably faster to aromatic hydrocarbons than to alcohols and ketones, they are by no means entirely fast to these solvents. Alkali Blue is not applied in toluene publication gravure printing inks. Such black systems are typically shaded with Milori Blue (Iron[III]hexacyanoferrate), although sometimes in combination with Alkali Blue. Milori Blue exhibits a shading effect that is different from that of Alkali Blue types. At the concentrations needed to afford a satisfactory shading effect in carbon black, Milori Blue, incorporated in toluene-based publication gravure printing inks, unlike Alkali Blue, does not adversely affect the rheological properties of the black ink.
Prints containing Alkali Blue are not fast to the standard DIN 16524 solvent mixture, but they are fast to acid, paraffin, butter and other materials. Tested in accordance with normative testing standards (Section 1.6.2.2), the prints unexpectedly also show fastness to alkali. Notably, however, at higher alkali concentrations the tinctorial strength of the system declines and the shade becomes duller. This is because the pigment reacts with alkali.
Alkali Blue pigments are used to a considerable extent in aqueous flexographic inks, despite a slightly alkaline pH value. The resulting printing inks, however, may show a viscosity increase. Such inks are prepared from powder grades, because flushed pastes contain binders that are mostly incompatible with aqueous printing inks. Special-purpose pigment preparations have also recently been introduced to the market for this application.
Alkali Blue pigments may safely be exposed to 140–160 °C for 30 min. They are not fast to clear lacquer coatings and to sterilization. The pigment is therefore normally excluded from use in metal deco printing, although there are exceptions. Alkali Blue is employed, for instance, for blue cream containers that are not exposed to direct sunlight. Pigment blends of Copper Phthalocyanine Blue and Pigment Red 57:1 are frequently utilized as suitable shading pigments for black metal deco printing inks or for other black printing inks that are fast to overlacquering.
In terms of lightfastness, Alkali Blue performs moderately. 1/1 SD letterpress proof prints equal step 3 on the Blue Scale, while 1/3 to 1/25 SD formulations equal only step 2. However, as shading components their lightfastness is excellent and satisfies the requirements for all types of applications. This is because a large portion of the incident light is absorbed by carbon black.
Alkali Blue pigments are used in large volume to colour office articles, especially ribbons for typewriters and computers, as well as blue copy-paper. Other areas of application, such as the plastics industry, do not employ Alkali Blue pigments because of their lack of fastness.
4.1.2 Dye Salts with Complex Anions
This class of pigments consists of salts of the anions of complex inorganic acids with dye cations, primarily with triarylcarbonium cations.
As early as 1913, BASF research succeeded in precipitating cationic (‘basic’) dyes with a heteropolyacid. The specific acid used in this case was phosphotungstic acid (which at the time was still applied on aluminium oxide hydrate as a carrier). The new products were patented. Using these and other heteropolyacids proved a useful tool in improving the lightfastness of the dye salts, and the importance of the pigments was enhanced even further as it became apparent that eliminating the mineral carrier led to a drastic increase in the tinctorial strength. As a consequence of these advances in pigment technology, complex salts became very important in the time between the First and the Second World War. Other heteropolyacids, especially phosphomolybdic acid and finally also a combined phosphotungstomolybdic acid, likewise stimulated considerable interest.
The shortage of tungsten and molybdenum during the 1930s in Germany necessitated the use of copper ferrocyanide as anion. Some of these salts have maintained their commercial position to the present time. In addition, silicomolybdates are also used to produce this type of pigments.
4.1.2.1 Chemistry, Manufacture and Crystal Structures
The list of dye cations used to prepare these complex salts includes primarily two types of triarylcarbonium compounds, namely:
- triphenylmethane or diphenylnaphthylmethane derivatives with the chemical structure 123;
- phenylxanthene derivatives with structure 124.
The most important group in this series consists of compounds with the structure 123 (A− = acid anion):

with R = methyl or ethyl and Ar = phenyl, 4-dimethylaminophenyl, or 4-ethylaminonaphthyl.
It is the substitution pattern that defines whether a product provides violet, blue or green shades.
Phenylxanthene compounds with the structure 124:

with X = hydrogen or methyl and Y = hydrogen or ethyl primarily afford bluish red (‘pink’) colours.
Another member of this group is a yellowish green pigment, structurally derived from a benzothiazolium system (125):

Compound 125, which is supplied in the form of a complex salt with a heteropolyacid, is used in combination with Pigment Green 1.
Heading the list of possible acid anions, A–, are complex phosphoric acids, particularly those with Mo3O102− or W3O102− ligands. Formally, these acids are derived from phosphoric acid H3PO4 by replacing all oxygen atoms by molybdate and/or tungstate groups. The resulting heteropolyacids may be written as follows:
The same structures may also be represented as:
Alternatively, it is possible to replace the phosphorus atom by silicon, which affords products amongst which silicomolybdic acids H4[Si(Mo3O10)4]·aq and H4H4[Si(Mo2O7)6]·aq are commercially of some significance. The second kind of formula emphasizes the fact that only three (or four) out of seven (or eight) hydrogen atoms provide possible substitution sites.
The dye salts are therefore represented as follows (D = dye group):
However, notably, the stoichiometric aspect is idealized. The ratios between the different components can vary widely and are in actual fact controlled by the pH value and the precipitation temperature.
Reducing agents, such as zinc dust or sodium dithionite, convert heteropolyacids into deeply coloured blue compounds consisting of heteropolyacids with four hydrogen atoms to be substituted. This makes it possible to precipitate more dye with the same heteropolyacid to produce an insoluble pigment.
Copper ferrocyanide complex salts, which are occasionally used, are derived from copper(I)hexacyano-iron(II)-acid, HCu3[Fe(CN)6]. Three equivalents of copper are required for each unit of ferrocyanide, which furnishes the following general pigment structure:
4.1.2.1.1 Dye Synthesis
This section outlines the different synthetic routes to the most significant cationic dyes that these pigments are derived from.
Basically, compounds with a central C atom, which are considered electrophilic reaction centres, are treated with a nucleophilic aromatic compound. Subsequent oxidation affords the desired carbonium compound:

4.1.2.1.2 Triphenylmethane and Diphenylnaphthylmethane Derivatives
Methyl Violet consists of the basic structure of Pigment Violet 3 (126).
It is prepared by air oxidation of dimethylaniline in the presence of phenol and copper salts as well as sodium chloride. The reaction product consists of tetra- to hexamethylated pararosanilines (see 119, Scheme 4.1).

In contrast to Methyl Violet, Crystal Violet (127) is a uniform compound. This is the dye which constitutes Pigment Violet 39.

The compound is obtained from ‘Michler's ketone’ (128) and dimethylaniline in the presence of POCl3. Michler's ketone in turn is synthesized from N,N′-dimethylaniline (DMA) and phosgene with zinc chloride:

Another route proceeds via bis(dimethylaminophenyl)methane, which is synthesized from dimethylaniline and formaldehyde. The thus prepared intermediate may be oxidized to form the hydrol and then reacted again with dimethylaniline to afford the leuco base, which is finally oxidized to yield 127:

A blue-shift is observed if the phenyl ring Ar in structure 123 is replaced by a naphthyl ring. The product is known as ‘Victoria Blue’ (129):

The product may be obtained from tetraethyldiaminebenzophenone and N-ethylnaphthylamine with phosphorus oxychloride or with phosphorus trichloride; the route parallels the synthesis of Crystal Violet. The resulting colourant is the basic dye for Pigment Blue 1.
Substituting the Ar moiety in structure 123 by a phenyl group without alkylamino groups affords green shades. A typical product is ‘Brilliant Green’ (130), which is the basis for Pigment Green 1. With the chemical substituent N(C2H5)2, this compound is homologous to Malachite Green with N(CH3)2:

Compound 130 is manufactured by condensing one equivalent of benzaldehyde with two equivalents of diethylaniline in the presence of zinc chloride or hydrochloric acid. Proceeding via the carbinole, the reaction ultimately affords the leuco base, which is oxidized with agents such as PbO2 to afford the dye.
4.1.2.1.3 Phenylxanthene Derivatives

As a group, these typically pink compounds are derived from the xanthene structure (131):

Condensation of a m-dialkylaminophenol with one equivalent of benzaldehyde in the presence of sulfuric acid or zinc chloride, followed by oxidation (for instance with FeCl3), provides the bis(dialkylamino)-phenylxanthenium skeleton, which is the parent compound for dyes with structure 124 (see above):
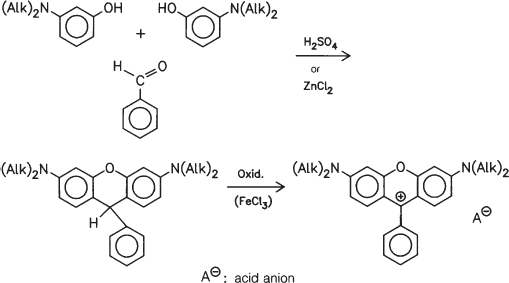
The most significant parent structure for pigments of this group is obtained by a slightly modified route by simply using phthalic anhydride instead of aldehyde. Reaction with m-diethylaminophenol at 180 °C in the presence of sulfuric acid or zinc chloride and subsequent oxidation with iron(III)chloride thus affords a dye known as ‘Rhodamine B’ (132), the basis of Pigment Violet 1:
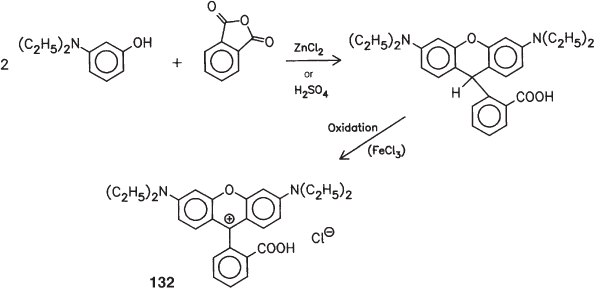
The ethyl ester of (132) is the dye component of Pigment Violet 2.
Starting from 3-ethylamino-4-methylphenol, it is possible to synthesize the cation (133) by using phthalic anhydride, as described above, and form the ethyl ester. The thus prepared product is the dye cation of Pigment Red 81.

4.1.2.1.4 Benzothiazolium derivatives
The benzothiazolium component with structure 125 is obtained through so-called primuline fusion: p-toluidine is heated with sulfur to 200–280 °C. Subsequent distillation affords not only the ‘primuline base’:

but also a product referred to as dehydrothio-p-toluidine, that is, 2-(4-aminophenyl)-5-methylbenzothiazole (134). Permethylation of the nitrogen atoms with agents such as methanol and hydrochloric acid and simultaneous quarternization affords the thiazolium salt 135.
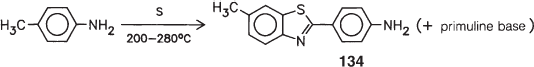
Compound 134 should first be removed from the primuline base by distillation:

4.1.2.1.5 Manufacture of the Heteropolyacids and Pigments
Mixing aqueous disodium hydrogenphosphate solutions with sodium molybdate and/or sodium tungstate and subsequently acidifying the mixture with mineral acids yields the desired heteropolyacids. While phosphotungstic acid is used only to a minor extent, phosphomolybdic acid and phosphotungstomolybdic acid reign supreme in the industrial manufacture of pigments of this class. Adding molybdate to phosphotungstic acid confers much higher brilliance and improved lightfastness on the resulting pigment complexes.
The pigments are obtained by preparing an aqueous solution of disodium hydrogenphosphate and adding a sodium molybdate solution (containing molybdenum trioxide and aqueous sodium hydroxide). Acidified with hydrochloric acid or preferably with sulfuric acid, the reaction mixture is then added to an aqueous solution of the cationic dye at 65 °C.
The product is after-treated at refluxing temperature, possibly in the presence of a surfactant. The pigment is then isolated by filtration, washed, dried and milled. Instead of drying and milling, it is also useful to flush the aqueous pigment presscake.
The corresponding copper ferrocyanide salts of basic dyes are obtained by treating potassium ferrocyanide, K4[Fe(CN)6], with sodium sulfite. Dissolved together, these two constituents are transferred to a solution of the cationic dye. A copper sulfate solution is finally added at 70 °C.
4.1.2.1.6 Crystal Structures
Crystal structures of dye salts with complex anions have not been determined yet.
4.1.2.2 Properties
As a class, these pigments are characterized by uncommonly clean and brilliant shades. They provide several shades, particularly red and violet ones, that can be duplicated by other organic pigments, but without their brilliance and cleanness. These pigments fail to satisfy more stringent fastness requirements. They are not stable to polar solvents, such as alcohols, ketones and ethylene glycol. Moreover, they also decompose if exposed to alkali.
For economic reasons these complex pigments are declining in use, despite their excellent colouristic properties. Many attempts have been made to replace more expensive types, such as those based on phosphotungstomolybdic acid, with less costly ones. As a consequence of these efforts, the properties of the pigments have improved greatly in recent decades, especially in terms of tinctorial strength and lightfastness. Complex pigments typically demonstrate average lightfastness. This property is a function both of the type of heteropolyacid and of the depth of shade. Copper ferrocyanide types, for instance, score an average of 2 steps lower on the Blue Scale than phosphomolybdic acid, phosphotungstomolybdic acid and silicomolybdic acid types.
4.1.2.3 Application
These complex pigments are utilized almost exclusively in printing inks, especially in packaging and special printing inks. Of the large number of pigments falling within this class, only the most significant are surveyed here. The exact chemical compositions of the individual species, especially the respective dye/heteropolyacid ratio, remain to be published.
4.1.2.4 Commercially Available Dye Salts with Complex Anions
4.1.2.4.1 General
The pigments are commonly referred to not only by their Colour Index name but also by an added abbreviation for the respective heteropolyacid. For instance:
- PM (or PMA), phosphomolybdic acid,
- PT (or PTA), phosphotungstic acid,
- PTM (or PTMA) for phosphotungstomolybdic acid,
- SM (or SMA) for silicomolybdic acid,
- CF, copper ferrocyanide (copper(I)-hexacyano-iron(II)-acid).
Table 4.2 lists the predominant species among these pigments.
Table 4.2 Commercial products of cationic triphenylmethane and diphenylnaphthylmethane dyes with inorganic heteropolyacids. For the abbreviations of the anions see text. PM = phosphomolybdate, PT = phosphotungstate, PTM = phosphotungstomolybdate, SM = silicomolybdate, CF = copper ferrocyanide.

|
|||||
| C.I. Name | C.I. Constitution Number | A− | Ar | R | Common name of the corresponding dye |
| P.V.3 | 42 535:2 | PTM PM |
CH3 | Methyl Violet (Basic Violet 1) | |
| P.V.3.1 | 42 534:4 | SM | CH3 | Methyl Violet (Basic Violet 1) | |
| P.V.4 | 42 510:2 |

|
CH3 | Methyl Violet(Basic Violet 1) | |
| P.V.27 | 42 535:3 | CF | 
|
CH3 | Methyl Violet (Basic Violet 1) |
| P.V.39 | 42 555:2 | PM PTM |
CH3 | Crystal Violet | |
| P.B.1 | 42 595:2 | PTM PM |

|
C2H5 | Victoria Pure Blue B (Basic Blue 26) |
| P.B.2 | 44 045:2 | PTM PM |

|
CH3 | Victoria Blue 4R |
| P.B.9 | 42 025:1 | PTM PM |
 |
CH3 | — |
| P.B.10 | 44 040:2 | PTM PM PT |
|
CH3 | — |
| P.B.14 | 42 600:1 | PTM PM |
CH3 | Ethyl Violet | |
| P.B.62 | 44 084 | CF | 
|
C2H5 | Victoria Blue R |
| P.Gr.1 | 42 040:1 | PTM PM |
C2H5 | Diamond Green G | |
| P.Gr.4 | 42 000:2 | PTM PM |
CH3 | Malachite Green | |
| P.Gr.45 | — | CF | — | — | — |
4.1.2.4.2 Triphenyl and Diphenylnaphthyl Derivatives
4.1.2.4.2.1 Pigment Violet 3
P.V.3 provides a very bluish violet shade, which is considerably bluer than that of its counterparts P.V.1 and P.V.2. In addition, P.V.3 exhibits higher fastness properties than P.V.27. This is particularly true with respect to the lightfastness: 1/3 SD P.V.3 letterpress proof prints score approximately 1 1/2 steps higher on the Blue Scale than comparative P.V.27 prints. Similarly, P.V.3 is used primarily in printing inks, especially in flexographic and packaging gravure printing inks. The list of suitable media also includes oil-based vehicle systems. Possible processing problems are referred to under P.V.2. US manufacturers also supply flushed pastes in which P.V.3 is dispersed in systems such as offset and letterpress vehicles or in mineral oil.
4.1.2.4.2.2 Pigment Violet 3:1
Pigment Violet 3:1 (C.I. 42535:4, CAS 68647-35-8) is a silicomolybdic acid derivative of P.V.3. It has a bright blue violet colour with outstanding brilliant hue, purity and high tinting strength. P.V.3:1 is used in water based printing inks as well as in polyamide, nitrocellulose and oil inks.
4.1.2.4.2.3 Pigment Violet 27
P.V.27 is found on the product line of several European manufacturers. The pigment affords a very bluish, clean violet shade, similar to that of P.V.39. P.V.27, however, is tinctorially stronger and can therefore be used more economically than P.V.39. It is, on the other hand, less fast than P.V.39, and the difference is occasionally considerable. This is partly attributed to the complex acid component. P.V.27, for instance, is less resistant to a large number of organic solvents than P.V.39, the difference being at least 1 step on the 5 step scale (Section 1.6.2.1). Similar observations have been recorded for the fastness of prints. They showed much less stability than prints containing the considerably redder P.V.1 and 2 types. Prints containing P.V.1 and 2, for instance, are entirely fast to water, while similar P.V.27 samples distinctly colour moist filter paper (Section 1.6.2.2). Moreover, P.V.27 specimens, in contrast to P.V.1 prints, are not acid proof. P.V.27 is very fast to aliphatic and aromatic hydrocarbons. The pigment is used to shade and tone black inks. Insufficient fastness properties preclude P.V.27 from being used in packaging gravure and flexographic printing inks based on NC (Nitrocellulose), but the pigment is used in aqueous flexographic inks. Copper ferrocyanide as a complex acid in oil-based systems, such as for letterpress or offset printing inks, catalytically accelerates the drying process. This drying effect largely restricts the use of P.V.27, since it is responsible for phenomena such as film formation on the ink surface, thickening of the ink and yellowing of the print.
4.1.2.4.2.4 Pigment Violet 39
Types containing phosphotungstomolybdic acid and types based on phosphomolybdic acid are commercially available. The tinctorially somewhat stronger PM version is a more economical alternative. Both types afford the same shade, a very clean, bluish violet, which cannot be duplicated by other types of pigments. Compared to Dioxazine Violet, which is the standard pigment in this range of shades, P.V.39 exhibits a still considerably bluer and noticeably cleaner shade. The pigment darkens upon exposure to light. 1/3 SD letterpress proof prints equal step 3–4 on the Blue Scale for lightfastness.
P.V.39 is primarily applied in special printing inks. In addition, it is also used as a shading pigment in other colours. Possible disadvantages to pigment processing in different binders are listed under P.V.2. Incorporated in NC printing inks, P.V.39 does not change colour upon storage.
4.1.2.4.2.5 Pigment Blue 1
P.B.1, which is supplied by several manufacturers, affords clean reddish shades of blue. Although its shade is also accessible by using chemically different pigments, for instance by combining α- or ɛ-Phthalocyanine Blue and Dioxazine Violet, these shades are not as brilliant. P.B.1 is available in the form of PM, PT, PTM and SM types. The PM and SM varieties are tinctorially stronger, but offer less clean shades than PT and PTM types. The higher the PT content in the pigment, the cleaner the shade, an advantage somewhat compromised by the fact that the tinctorial strength decreases in the same order. P.B.1 is largely as fast as other types of pigments within the same class. The products, for instance, are very unstable to polar solvents such as alcohols, ketones and esters. P.B.1 is comparatively lightfast. 1/3 SD letterpress proof prints equal approximately step 4 on the Blue Scale, although the pigment darkens distinctly.
Its colouristic properties make P.B.1 a suitable and widely used colourant for special printing inks. The pigment may be incorporated without problems in oily binders, that is, in letterpress and offset inks. It is equally often used in publication gravure printing inks based on toluene and in packaging printing inks based on NC. Problems that may arise as P.B.1 is incorporated in these vehicles are mentioned under P.V.2. In addition, P.B.1 lends colour to paper, wallpaper, typewriter ribbons and other media, although it is often difficult to disperse.
4.1.2.4.2.6 Pigment Blue 2
At present, P.B.2 is only produced in the USA, where both PM and PT types are available. They are greener than grades of P.B.1. P.B.2 has much less impact on the market than P.B.1.
4.1.2.4.2.7 Pigment Blue 9
P.B.9 is similarly restricted to the North American market, which supplies both PM and PTM types. The PM varieties are stronger than PTM types but furnish shades that are not as clean.
P.B.9 matches the standard cyan for three and four colour printing (Section 1.8.1.1). The pigment is continually losing significance as it is being displaced by the similarly shaded β-modification of Copper Phthalocyanine Blue. The latter offers several application and economic advantages. P.B.9 continues to be used in mass coloured paper, textile printing and in coloured pencils.
4.1.2.4.2.8 Pigment Blue 10
P.B.10 is only utilized locally in East Asia and has no commercial significance.
4.1.2.4.2.9 Pigment Blue 14
Pigment Blue 14, like P.B.2 and 9, is produced exclusively in the USA, where it has only gained limited commercial importance. Both PM and PTM types are available. They provide very reddish shades of blue.
4.1.2.4.2.10 Pigment Blue 62
In terms of shade, P.B.62 parallels the PTM and PM types of P.B.1. P.B.62 exhibits high tinctorial strength. Its shade is not as clean as those of similar pigments, but it may be applied more economically. P.B.62 is less lightfast: 1/3 SD letterpress proof prints do not quite reach step 3 on the Blue Scale, and the samples darken upon exposure to light. The same applies to stability to organic solvents: compared to the two P.B.1 types, P.B.62 is somewhat less fast to several solvents. P.B.62 is primarily used as a shading pigment for publication gravure printing inks, as well as for aqueous flexographic inks. It is not recommended for NC-based printing inks. Incorporated in oily inks for applications such as offset printing inks, P.B.62 tends to catalytically accelerate the drying process, which restricts its suitability for these media. The pigment is also used to colour office articles.
4.1.2.4.2.11 Pigment Green 1
P.Gr.1 is less brilliant than other triarylcarbonium pigments. Its shade may also be obtained by combining pigments of other chemical classes, such as copper phthalocyanine blue pigments or Copper Phthalocyanine Green types with monohydrazone yellow pigments. The resulting shades are even more brilliant than those of P.Gr.1. P.Gr.1 has an advantage over other pigments in providing higher tinctorial strength, which makes it an attractive product despite its lack of brilliance.
In terms of lightfastness, P.Gr.1 parallels the blue types. Irradiated 1/3 SD letterpress proof prints equal step 3 on the Blue Scale, although the shade darkens considerably. P.Gr.1 is recommended for use in oily binders, publication gravure printing inks and NC-based printing inks. Possible problems are referred to under P.V.2.
4.1.2.4.2.12 Pigment Green 4
P.Gr.4 is manufactured in the USA, but has only attracted very limited attention. Both PM and PTM types are available. P.Gr.4 provides an intermediate shade between those of P.Gr.1 and 2. The pigment performs much like other green pigments of its class.
4.1.2.4.2.13 Pigment Green 45
Both the chemical constitution of this basic dye and the complex acid of P.Gr.45 remain to be published. So far, the pigment is described as a triphenylmethane compound forming a complex with copper hexacyanoferrate. P.Gr.45 affords yellowish shades of green, which are within the range of shades provided by P.Gr.36, the brominated copper phthalocyanine pigment. The shade produced by P.Gr.45 may be matched by combining pigments of other classes. Suitable blends are provided by greenish P.Y.74 type monohydrazone yellow pigments with the β-modification of Copper Phthalocyanine Blue. The resulting shade equals P.Gr.45 shades in terms of brilliance. P.Gr.45 is appreciated for its high tinctorial strength. High gloss and transparency add to the value of the pigment. It is used primarily in offset and aqueous printing inks, but also in solvent-based printing inks, such as those based on NC.
4.1.2.4.3 Phenylxanthene Derivates
The structures are given in Table 4.3.
Table 4.3 Cationic phenylxanthene dyes with heteropoly acids. PM = phosphomolybdic acid, PT = phosphotungstic acid, PTM = phosphotungstomolybdic acid, SM = silicomolybdic acid, CF = copper ferrocyanide.
 |
||||||
| C.I. Name | C.I. Constitution Number | A− | R | X | Y | Common name of the corresponding dye |
| P.R.80 | 45 160 | PTM | H | CH3 | C2H5 | Rhodamine 6G (Basic Red 1) |
| P.R.81 | 45 160:1 | PTM | H | CH3 | C2H5 | Rhodamine 6G (Basic Red 1) |
| P.R.81:1 | 45 160:3 | STM | H | CH3 | C2H5 | Rhodamine 6G |
| P.R.81:2 | 45 161:1 | SM | H | CH3 | CH3 | Basic Red 1:1 |
| P.R.81:3 | 45 161:2 | PM | H | CH3 | CH3 | Basic Red 1:1 |
| P.R.81:4 | 45 161:5 | PTM | H | CH3 | CH3 | Basic Red 1:1 |
| P.R.81:5 | 45 161:4 | SM | H | CH3 | C2H5 | Rhodamine 6G |
| P.R.81:6 | 45 161:7 | PM | H | CH3 | C2H5 | Rhodamine 6G |
| P.R.169 | 45 160:2 | CF | H | CH3 | C2H5 | Rhodamine 6G |
| PV.1 | 45 170:2 | PTM | C2H5 | H | H | Rhodamine B (Basic Violet 10) |
| P.V.1:1 | — | PM | C2H5 | H | H | Rhodamine B |
| P.V.1:2 | 45 170:4 | M | C2H5 | H | H | Rhodamine B |
| P.V.2 | 45 175:1 | PTM | C2H5 | H | C2H5 | Rhodamine 3B ethylester |
4.1.2.4.3.1 Pigment Red 81/81:1/81:2/81:3/81:4
P.R.81 affords a very clean bluish red shade, which matches the purple-red on the DIN 16508 colour scale for letterpress application and also on the DIN 16509 offset scale. The shade is not accessible by using other pigments (Section 1.8.1.1). Pigments, which are closely related by shade, such as P.R.147, provide shades which are not even remotely as clean. The standard magenta on the European Colour Scale for letterpress (DIN 16538) and offset printing (DIN 16539) is somewhat yellower. For such printing inks, certain high solvent fastness properties are required to safely overlacquer the prints. These conditions cannot be met with P.R.81. The pigment in particular lacks fastness to polar solvents, such as alcohols, ketones and esters, as well as to the DIN 16524 solvent mixture. On the other hand, P.R.81 prints are very fast to aliphatic and aromatic hydrocarbons, paraffin, butter and many other fats, although they are not entirely stable to sterilization. Like other members of its class, P.R.81 affords prints that are not fast to alkali and soap but largely fast to acid.
Tinctorially, P.R.81 is a strong pigment, which, as with other representatives, is especially true for the PTM types. The pigment commonly darkens noticeably as it is exposed to light. 1/3 SD letterpress proof prints equal step 4 on the Blue Scale for lightfastness. The lightfastness of P.R.81 systems may be adversely affected if the surface of the pigment particles is attacked by polar solvents, such as esters, ketones, low molecular weight alcohols or glycol ether. Alkali has a similar effect.
P.R.81 is used especially in three and four colour printing and lends itself to various printing processes; therefore, pigments of this type are referred to as ‘Process Red’ in the USA. Used as a colourant for NC-based printing inks, SM types of P.R.81 may present problems as they are dispersed with steel balls or stored in steel containers as well as at elevated temperature. Catalytic decomposition of the binder and damage to the pigment may induce a colour shift and increase the viscosity.
4.1.2.4.3.2 Pigment Red 169
P.R.169 parallels the P.R.81 types in terms of shade and fastness properties. Thus, P.R.169 types are equally suited to letterpress and offset printing inks, where they provide the standard purple red for three and four colour printing in accordance with the so-called DIN scale (Section 1.8.1.1). Moreover, P.R.169 is frequently used for toluene-based publication gravure printing inks, where it is sometimes applied in combination with P.R.57:1. Several grades, however, are not as fast to toluene as comparable P.R.81 varieties. One of the major fields of application for P.R.169 is in aqueous flexographic printing inks.
There are various systems in which the salt character of these pigments adversely affects the ease of processing in an ink or even the finished print. Most of these problems are attributed to the copper ferrocyanide content, which is used as a complex acid in P.R.169 and comparable types of other basic dyes. In offset inks and other oxidatively drying printing inks, catalytic acceleration of the drying process may occur, resulting in a thickening of the ink, film formation on the surface of the ink and yellowing of the prints. Incorporation in polyamide vehicles has the disadvantage that P.R.169 catalytically decomposes the resin and thus causes the prints to stick and develop an odour. An unsuitable or shifting pH value in an aqueous ink may thicken the vehicle and affect the colouristic properties of the product. Highly acidic binder components may chemically react with the pigment or with the dye component and thus adversely affect the colouristic properties, an effect that is often accompanied by considerable flocculation. Deficiencies also tend to appear in NC-based printing inks. These are a consequence of dye nitrosation through formation of nitrous gases. As a result, gas develops, the shade demonstrates an often considerable shift, and the printing ink loses some of its strength. Pigment manufacturers supply their products with detailed information on how to obviate these difficulties.
4.1.2.4.3.3 Pigment Violet 1
P.V.1 is broad in scope; it is closely related to P.V.2, both in terms of colouristics and fastness properties. Although the shades are very similar, P.V.1 usually provides not only higher tinctorial strength and greater brilliance but also higher cleanness of shade. In terms of lightfastness, P.V.1 scores about 1/2 step on the Blue Scale less than P.V.2 types. Moreover, P.V.1 prints are also less fast to organic solvents and to agents such as soap and butter than prints made from P.V.2. The areas of application are the same as for the other violet type.
4.1.2.4.3.4 Pigment Violet 2
One of the predominant features of P.V.2 varieties is the fact that they are comparatively lightfast. At standardized conditions regarding printed film thickness and substrate, 1/3 SD letterpress proof prints equal step 5 on the Blue Scale. Like other representatives of this class of pigments, P.V.2 loses much of its lightfastness through exposure to polar solvents, such as low molecular weight alcohols, esters and ketones. Alkali has a similar effect. Moreover, such solvents (and also alkali) enhance the tendency of the pigment to migrate and reduce its fastness to overlacquering. PTM types are noticeably less fast to polar solvents than pigments based on other complex acids. P.V.2 is acid fast in accordance with standard test conditions (Section 1.6.2.2).
The pigment provides a very bluish shade of red, referred to as violet. It furnishes a very clean shade. P.V.2 systems show a brilliance that is not reproduced by using chemically different pigments. Tinctorially, P.V.2 is an especially strong product.
The printing ink industry utilizes P.V.2 particularly for special printing inks and as a shading pigment. It is much too blue to be used in process colours. As is the case with other PTM types and with types containing phosphomolybdic acid or silicomolybdic acid, there is some difficulty in dispersing P.V.2 in certain binders. Both the processing of the ink and the printed products may be affected. Inks containing polyamide, for instance, if printed onto polyolefin films may exhibit dye migration through the film. This phenomenon is attributed to the presence of small amounts of dye within the pigmented system that have not been converted into salts. NC-based printing inks containing P.V.2 are affected through contact with steel. This is likely to occur, for instance, as printing inks are prepared using steel balls or as they are transported in steel containers. Consequences include catalytic degradation of nitrocellulose and damage to the pigment, visibly resulting in increased viscosity and colouristic changes involving transparency, tinctorial strength and shade. Elevated temperature has a similar effect or may even enhance the action of steel. Pigments decompose in strongly basic aqueous systems and suffer colouristic and rheological changes. Interaction between pigment and polar solvents also frequently affects the shade and the rheology of the system, thickening the material or flocculating the pigment. Finally, the different components of a blend of this type of pigment, incorporated in a liquid printing ink, may interact with one another, not only forming a sediment on the bottom of the container but also affecting the colouristic properties of the ink. Pigment manufacturers provide their products with information on how to meet these problems.
4.1.2.4.4 Benzothiazolium Derivatives
4.1.2.4.4.1 Pigment Green 2
P.Gr.2 is a mixture of P.Gr.1 (see Table 4.2) and

Scheme 4.2 Molecular structure of P.Y.18, which is part of P.Gr.2 (PTM = phosphotungstomolybdic acid).
P.Gr.2 provides yellowish shades of green that are considerably more yellowish than those furnished by P.Gr.1. P.Gr.2, like P.Gr.1, lacks brilliance, a deficiency that within this class of pigments is unique to these two species. The shade produced by P.Gr.2 is also obtained with blends consisting of other organic pigments, such as phthalocyanine green pigments and monohydrazone yellow pigments or diarylide yellow pigments. A prominent characteristic of P.Gr.2 is its high tinctorial strength. In recent years, P.Gr.2 has lost most of its commercial importance and continues to be used only in the USA. Both PTM and PM types are available. The pigment parallels other representatives of its series in terms of fastness properties. It shows good lightfastness, but darkens slightly upon exposure.
4.1.3 Aluminium Pigment Lakes
This class contains the aluminium salts of some carboxylic acids or sulfonic acids of triphenylcarbonium dyes.
4.1.3.1 Pigment Red 172
P.R.172, C.I.45430:1 is the aluminium salt of tetraiodofluorescein (136):

Scheme 4.3 Molecular structure of P.R.172.
Fluorescein (137) is prepared by the same route as Pigment Red 90 (Section 4.3.8). The compound is iodized with iodine and potassium iodate in an acidic medium. The iodic acid reoxidizes the resulting hydrogen iodide back to iodine:
Aluminium chloride is used to convert tetraiodofluorescein, also known as erythrosine, into the P.R.172 lake.
Provided it meets certain purity criteria, the pigment may be used in pharmaceuticals and cosmetics, but less in foodstuff. It is registered as E 127 throughout the EC and as FD&C Red No.3 in the USA. The bluish red pigment P.R.172 provides little tinctorial strength. The pigment generally shows poor application and fastness properties, including fastness to organic solvents, alkali and acids, as well as heat stability and lightfastness. Consequently, there are limits to its technical applicability.
4.1.3.2 Pigment Blue 24:x, Pigment Blue 24:1 and Pigment Blue 78
The parent structure is a trisulfonated triphenylmethane dye (138):

Scheme 4.4 Molecular structure of P.B.24:x, P.B.24:1 and P.B.78.
It is obtained by condensing benzaldehyde-o-sulfonic acid with 2 equivalents of N-ethylbenzylaniline, followed by sulfonation, oxidation and conversion into the ammonium salt. The dissolved dye is then immersed in a dispersion of aluminium hydroxide and converted into the corresponding salt using aluminium chloride or barium chloride solution:
Both types of lakes are available.
4.1.3.3 Pigment Blue 24:x
P.B.24:x, the Al salt of 138, a greenish blue compound, is registered in the USA as FD&C Blue 1 for use in foodstuffs, pharmaceuticals and cosmetics, provided certain purity standards are met. The pigment possesses poor lightfastness. It largely parallels P.B.24:1, also in terms of tinctorial strength and stability to organic solvents.
4.1.3.4 Pigment Blue 24:1
P.B.24:1, 42 090:1, the Ba-salt of 138, provides brilliant greenish shades of blue. Although a pigment with very good tinctorial strength, P.B.24:1 has largely been displaced by Copper Phthalocyanine Blue. In the past, P.B.24:1 used to be applied in large volume as a process colour blue for three and four colour printing. The pigment lacks fastness to acid, alkali and soap, and its lightfastness is poor. P.B.24:1 is found in inexpensive letterpress printing inks as well as in office articles such as coloured pencils and less costly water colours.
4.1.3.5 Pigment Blue 78
P.B.78 is listed under Constitution Number 42 090:2. The structure is identical with the aluminium salt of 138. The pigment is used for food, pharmceutical and cosmetic products and toys.
4.2 Metal Complex Pigments
This section describes metal complexes of azo and azomethine compounds. Copper phthalocyanine pigments are decribed in Section 3.1.
Metal complex pigments have attracted attention [5, 6] for their commercially interesting properties. Water-soluble complexes, especially those of the azo series, have long maintained an important position as colourants for textiles. Besides a few complex metal salts of hydrazone pigments, it is primarily azomethine metal complexes that have gained commercial significance.
Apart from alizarin ‘lake’, which today is formulated as an aluminium/calcium complex [7] (Section 3.7.2), the oldest known metal complex pigment is an iron complex. In 1885, O. Hoffmann reported on the iron complex of 1-nitroso-2-naphthol, which under the name of Pigmentgrün B (Pigment Green 8, 10006) was first industrially exploited in 1921 by BASF. Largely displaced by copper phthalocyanine green pigments, this product has lost most of its commercial importance.

Scheme 4.5 Molecular structure of P.Gr.8.
In 1946, Du Pont described a nickel/azo complex pigment, which was introduced in 1947 as Green Gold (Pigment Green 10, 12775, see Section 4.2.4.1.2.2). For a long time, this pigment was the most lightfast and weatherfast product within the greenish yellow range of the azo series.
The promotion of metal complex pigments in the azo and azomethine series with improved fastness properties stalled until the early 1970s, as chemically novel structures were developed.
This group of metal complex pigments not only exhibits good fastness properties, but has proven economically advantageous enough to maintain limited but solid ground within the ranges of organic pigments.
4.2.1 Chemistry, Synthesis and Crystal Structures
The commercially most interesting metal complex pigments within the azo series are those obtained from aromatic o,o′-dihydroxyazo compounds, while important products within the azomethine series are nickel or copper complexes of aromatic o,o′-dihydroxyazomethine compounds.

The aromatic moieties are, possibly, substituted benzene or naphthalene rings. In azomethine pigments, only one form of metal complex is possible. This is in contrast to azo metal complexes, which may assume either structure 139 or 140:

The nitrogen atom that is connected to the least nucleophilic aromatic moiety is always the one to serve as a ligand.
The commercially interesting metal complex pigments usually contain the coordinative tetravalent Cu2+ or Ni2+ ions, or less commonly Co2+ ions. The copper and nickel complexes are mostly planar molecules.
It is important for metal complex compounds to be free from solubilizing groups in order to provide the necessary pigment characteristics.
4.2.1.1 Azo Metal Complexes
Azo metal complexes may also be seen as ‘hydrazone metal complexes’, since some of them are synthesized from hydrazone pigments (see below). However, the difference between the azo form and the hydrazone form vanishes, when the proton is removed during complexation with the metal. Therefore, in this book the name ‘azo metal complexes’ is retained.
The above-mentioned Pigment Green 10 is the nickel complex of the hydrazone pigment obtained from p-chloroaniline and 2,4-dihydroxyquinoline (140):

Scheme 4.6 Synthesis of P.Gr.10.
Its synthesis follows the usual pathway of coupling diazotized 4-chloroaniline onto 2,4-dihydroxyquinoline and subsequently treating the product with a nickel(II) salt.
The fact that protons are released through complexation makes it possible sometimes to enhance the reaction and to improve the yield by adding a base (such as sodium acetate).
An interesting product in the range of azo metal complexes is P.Y.150, which is the 1 : 1 nickel complex of azo barbituric acid (142) [8]:

Scheme 4.7 Molecular structure of P.Y.150.
This molecule is believed to assume the structure of a 1 : 1 sandwich complex [9].
A reaction known as diazo group transfer produces diazo barbituric acid from barbituric acid and p-toluenesulfonyl azide. Additional barbituric acid affords azo barbituric acid [10]. Subsequent complexation with a nickel(II) salt yields a greenish yellow pigment.
4.2.1.1.1 Other Complexation Methods
Apart from the reaction of the o,o′-dihydroxyazo compound with a metal salt, two more complexation techniques are worth mentioning, although these are primarily used to synthesize azo metal complex dyes.
Demethylating while copperizing is achieved only under severe conditions, that is, at temperatures in excess of 100 °C, frequently under pressure. Suitable starting materials are o-hydroxy-o′-methoxyazo compounds.
During the oxidative copperizing process, an o-hydroxyazo compound reacts with a copper(II) salt in the presence of hydrogen peroxide. Both methods broaden the scope of metal complexation reactions by extending the selection of suitable diazo components [11, 12].
4.2.1.2 Azomethine Metal Complexes
Copper, nickel and cobalt complexes reign supreme amongst industrially interesting products within this class. The complexes are manufactured by condensing aromatic o-hydroxyaldehydes with aromatic o-hydroxyamines, frequently o-aminophenols, in an aqueous medium or in organic solvents at 60 to 120 °C. The intermediate products, usually orange to red azomethines, are either separated or immediately reacted further by adding a Cu, Ni or Co(II) salt. The conversion is carried out at similarly elevated temperature, although the prime concern is the presence of a solvent to afford the largely insoluble azomethine metal complexes. As above, the presence of a base may sometimes be useful.

There is another structure which, although it does not represent a typical azomethine compound, is synthesized from nickel complexes of the anilide of diimino butyric acid (143).

The synthesis involves nitrosating the corresponding substituted acetoacetic anilide with sodium nitrite in acetic acid and subsequently, by adding hydroxylamine to the same reaction vessel, converting the resultant compound into the oxime. Finally, complexation is achieved by means of a Ni(II) salt.

Yet another structural principle is represented by metal complex pigments based on isoindolinones. Condensation of amino-iminoisoindolinones (iminophthalimide) with 2-aminobenzimidazole in a high boiling solvent affords an azomethine (144). This compound reacts with salts of divalent metals, such as Co, Cu, Ni, to yield yellow azomethine metal complex pigments [13, 14]:
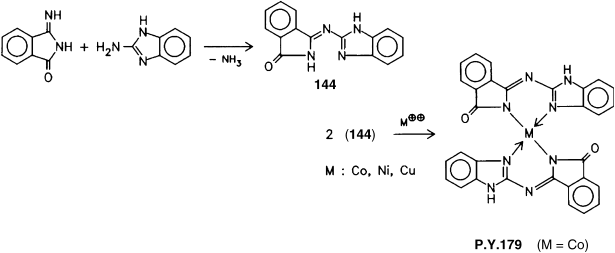
Scheme 4.8 Synthesis of P.Y.179.
The cobalt complex is commercially available as P.Y.179, C.I. 48 125.
Pigment Yellow 177, C.I. 48 120 is synthesized by using 2-cyanomethylene-benzimidazole instead of 2-aminobenzimidazole to react with iminophthalimide, followed by complexation with Co2+.

Scheme 4.9 Molecular structure of P.Y.177.
For more information about cobalt complexes, refer to Reference [15].
Crystal structures have been determined for P.Y.177 [13], P.O.59 (α- and β-phases) [16], P.O.65 [17], and for pyridine solvates of P.Y.129 [18, 19].
In P.O.65 the Ni2+ ion is coordinated in a square-planar geometry by the pigment dianion (Figure 4.1). The molecules form pairs that are held together by van der Waals and Coulomb interactions (Figure 4.2). The pairs arrange in a herringbone packing (Figure 4.3).

Figure 4.1 Molecular structure of the nickel complex P.O.65.

Figure 4.2 Pairs of molecules in P.O.65. View perpendicular to the molecular planes. Intramolecular distances (Å): Ni–N = 1.85, Ni–O = 1.83. Intermolecular distances between the two molecules (Å):N⋯O = 3.20 + 3.26, Ni⋯Ni = 3.29, Ni⋯N = 3.39 + 3.46.

Figure 4.3 Molecular packing in P.O.65. To guide the eye, the molecular centres of the five pairs are connected by dotted lines.
4.2.2 Properties
Commercially available azo and azomethine metal complex pigments cover the spectral range from considerably greenish to reddish yellow and yellowish orange. Compared to their parent structures (the corresponding azo and azomethine compounds), azomethine metal complexes frequently exhibit a distinctly duller shade. Formation of the metal complex often shifts the colour of an originally yellow material in the greenish yellow direction.
There are several advantages to complexation. It imparts better weatherfastness and lightfastness, as well as enhanced solvent resistance and migration fastness compared with the metal free compound.
A number of the commercially available members of this class are characterized by high transparency. Their tinctorial strength, however, is not always satisfactory. Both lightfastness and weatherfastness are generally very good, sometimes excellent. While some grades are fast to overcoating under application conditions, which include temperatures up to 160 °C, other products bleed at less than 120 °C. Likewise, the pigments vary considerably in terms of heat stability.
4.2.3 Application
Metal complex pigments are mainly used in paints. The products are fast enough to be applied especially in industrial finishes. Some representatives, particularly azomethine copper complex pigments, are very weatherfast, which makes them suitable candidates for automotive finishes. High transparency in combination with good weatherfastness is an asset for use in metallic finishes. It is not uncommon for metal complexes to lose much of their brilliance in white reductions. Some are also recommended for use in architectural paints, especially for emulsion paints.
In addition, metal complex pigments are also used in printing inks as well as in other areas of application.
4.2.4 Commercially Available Metal Complex Pigments
4.2.4.1 General
A small number of azo metal complexes and a large number of azomethine metal complexes are commercially available (Table 4.4).
Table 4.4 Examples of azo and azomethine metal complex pigments.
| C.I. Name | C.I. Constitution Number | Structure | Shade | Reference |
| Azo series | ||||
| P.Gr.8 | 10 006 | 
|
Green | |
| P.Gr.10 | 12 775 |  |
Very greenish yellow | |
| P.Y.150 | 12 764 |  |
Greenish yellow | [8, 20] |
| P.Br.2 | 12 071 |  |
Brown | |
| Azomethine series | ||||
| P.Y.117 | 48 043 |  |
Greenish yellow | [20, 21] |
| P.Y.129 | 48 042 |  |
Very greenish yellow | |
| P.Y.153 | 48 54548 |  |
Greenish yellow | [20, 22, 23] |
| R2, R4, R5 = H | ||||
| P.O.59 | 5455 | structure see P.Y. 153 R2 = OCH3, R4, R5 = H |
orange | |
| — | — | structure see P.Y. 153 R2, R4 = CH3, R5 = H |
red | |
| P.Y.177 | 48 120 |  |
Dull yellow | |
| P.Y.179 | 48 125 |  |
Reddish yellow | |
| P.O.65 | 48 053 |  |
Dull reddish orange | |
| P.O.68 | 486 150 |  |
Orange | [24] |
| P.R.257 | 562 700 | 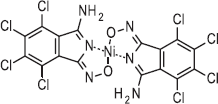 |
Red–violet | [24] |
| P.R.271 | 487 100 |  |
Red |
4.2.4.2 Pigment Green 8
P.Gr.8, also referred to as Pigment Green B, provides dull yellowish shades of green or, respectively, greenish shades of yellow up to the olive hues. Over the last few decades, P.Gr.8 has largely given way to the cleaner phthalocyanine green pigments.
P.Gr.8 exhibits good fastness to organic solvents, but performs poorly towards esters, such as ethyl acetate, ketones, such as methyl ethyl ketone, and ethyl glycol (ethylene glycol monoethylether). In contrast to possessing excellent fastness to alkali and lime, P.Gr.8 is not stable to acids. The pigment exhibits good lightfastness, but it is not as lightfast as its copper phthalocyanine counterparts. 1/3 SD P.Gr.8 emulsion paint samples, for instance, equal step 7 on the Blue Scale for lightfastness, while more reduced specimens up to 1:200 equal step 6–7. Corresponding phthalocyanine green pigments match step 8 on the Blue Scale.
With its moderate price, P.Gr.8 is used in emulsion paints and also in concrete mass colouration. Like P.Gr.7, it is one of the few organic pigments suitable for a large variety of concrete formulations. P.Gr.8 in general is not lightfast and weatherfast enough to be used in outdoor application. Its dull shade is of no consequence to indoors application, the shade is cleaner than that of Chrome Oxide Green. As always in this type of application, preliminary testing is recommended.
An important application of P.Gr.8 is in the colouration of rubber. The pigment, however, is not suitable for use in blends that contain large amounts of basic fillers. It is somewhat sensitive to cold vulcanization. The coloured articles usually perform well in general application but are not entirely fast to aromatic hydrocarbons and to some fats, and they are sensitive to acid and sulfur dioxide. P.Gr.8 also colours some plastics, especially LDPE and polystyrene. Heat stable up to 220 °C, P.Gr.8 grades equal step 2–3 on the Blue Scale for lightfastness. Other areas of application include wallpaper and artist's colours.
4.2.4.3 Pigment Green 10
P.Gr.10, a nickel azo complex known since 1947, affords a dull, very greenish yellow shade that, as in the Colour Index, may also be considered a very yellowish green (in white reduction and in full shade, respectively). It is a comparatively weak product and is used primarily in high grade industrial paints, especially as a shading pigment for blue and green colours. The commercial types are highly transparent, which makes them suitable candidates for metallic, transparent and hammer tone finishes. P.Gr.10, however, fails to satisfy the requirements for use in automotive finishes. The colourations remain very lightfast even in highly reduced white reductions: reductions up to 1:200 SD equal step 8 on the Blue Scale for lightfastness. The weatherfastness level is equally very good. In this respect, P.Gr.10 performs similarly to P.Y.117, an azomethine copper complex pigment.
Incorporated in finishes, P.Gr.8 is heat stable up to about 180 °C. It is not entirely fast to overcoating. Most systems containing P.Gr.8 will fail to maintain their colour value if they are exposed to acids: there is a shift towards yellower hues, a phenomenon attributed to demetallation of the pigment.
4.2.4.4 Pigment Yellow 117
The marketing of P.Y.117, an azomethine copper complex, has recently been discontinued. It provides very greenish shades of yellow that resemble those of P.Y.129. Combination with TiO2, for instance at a ratio of 1 : 50, considerably reduces the cleanness of the system, resulting in very dull olive green shades. As P.Y.117 exhibits high transparency, it was recommended for use in metallic finishes, especially throughout the automotive industry. It was also recommended for olive shades in automotive finishes. Blends with copper phthalocyanine blue and green pigments were equally recommended for automotive finishes.
Compared to other pigments covering the greenish yellow range of the spectrum, P.Y.117 exhibits good tinctorial strength. It shows good stability to organic solvents and under standardized conditions exhibits complete fastness to mineral spirits. Its fastness to toluene, alcohol or esters, such as ethyl acetate, equals step 4 on the 5 step scale; and the pigment equals step 3 in terms of stability to ketones, such as methyl ethyl ketone, or ethyl glycol.
P.Y.117 demonstrates good fastness to alkali and acids. It is completely fast to overcoating up to 160 °C and heat stable up to 180 °C. For a system consisting of P.Y.117 incorporated in a silicone resin paint, the manufacturer listed a temperature stability of up to 300 °C for 1 h exposure.
P.Y.117 is very lightfast and weatherfast. 1/3 to 1/25 SD formulations in an alkyd-melamine resin equal step 8 on the Blue Scale for lightfastness, while 1:200 SD samples still reach step 7. The weatherfastness of the pigment in this range of standard depths and in this binder system equals approximately that of P.Y.153, but is not quite as high as that of P.Y.129. In addition, P.Y.117 was also recommended for use in emulsion paints.
P.Y.117, like P.Y.129, undergoes recomplexation if it is incorporated in plasticized PVC in conjunction with tin stabilizers, such as dibutyltin thioglycolate. The colour shifts towards reddish shades, and the pigment begins to exhibit an appreciable tendency to migrate in the polymer. If suitable stabilizers are added, P.Y.117 is very weatherfast in PVC and shows high tinctorial strength. Some 0.65% pigment is required to produce 1/3 SD samples containing 5% TiO2.
4.2.4.5 Pigment Yellow 129
P.Y.129 exhibits a clean, very greenish yellow shade. Its only field of application is in paints, especially in automotive and industrial finishes. High transparency makes it a useful pigment to produce interesting metallic finishes. Much of the cleanness is lost as white pigment, such as TiO2, is added, resulting in olive shades. The coatings are very lightfast and weatherfast. Up to 140 °C, P.Y.129 is fast to overcoating. Reduced 1 : 50 with TiO2, the systems are heat stable up to 150 °C. P.Y.129 is also recommended for use in architectural paints.
P.Y.129 is not employed in plastics. The type of metal in the stabilizer defines how much of a colour change is observed as the chelated metal in the pigment molecule is exchanged. Tin stabilizers produce red complexes, which, like corresponding lead or zinc chelates, respond very poorly to light and weather (see also Section 1.6.7).
4.2.4.6 Pigment Yellow 150
This pigment, an azo/nickel complex, affords dull, medium shades of yellow. The pigment is recommended for use in paints and printing inks. P.Y.150 produces very lightfast paints: samples up to 1/25 SD equal step 8 on the Blue Scale. The weatherfastness of these specimens, however, deteriorates as more white pigment, such as TiO2, is added. Systems up to 1/3 SD are heat stable up to 180 °C. P.Y.150 is used as a colourant for general industrial and architectural paints wherever the durability requirements are not too high. It exhibits good resistance to organic solvents but is not entirely fast to overcoating.
P.Y.150 is also very lightfast in printing inks. The prints, however, are neither acid nor alkali resistant. Moreover, they are somewhat sensitive to several organic solvents, including the DIN 16524/1 standard solvent mixture, and soap. The systems are heat stable up to 140 °C. Prints made from P.Y.150 are almost completely fast to sterilization. A pigment with high tinctorial strength, P.Y.150 is particularly suitable for use in decoration printing inks for laminates.
A new special-purpose type of P.Y.150 was introduced to the market a few years ago. It is recommended for use in spin dyeing polypropylene and polyamide fibres. In this type of application, the pigment exhibits good heat stability and also good lightfastness and weatherfastness. Under common processing conditions in injection moudling, P.Y.150 is likely to react with the zinc sulfide that is often found in polyamide, a tendency that precludes its use in polyamide injection moulding.
4.2.4.7 Pigment Yellow 153
P.Y.153 is a nickel complex that was introduced to the market in the late 1960s. It produces slightly dull reddish shades of yellow. Although not fast to acids, the pigment may safely be exposed to alkali. It is fast to mineral spirits and alcohols, but only moderately so to aromatic solvents, such as xylene, and to esters, such as ethyl acetate.
Medium to light shades of this relatively weak pigment are used to colour high grade industrial paints, especially automotive solid shades, as well as metallic finishes. P.Y.153 withstands exposure to up to 160 °C for 30 min. Incorporated in an alkyd-melamine resin, full shades (10%) and 1/3 SD samples equal step 8 on the Blue Scale for lightfastness, while 1/25 SD specimens match step 7–8. In terms of lightfastness, P.Y.153 performs approximately like P.Y.138, a quinophthalone pigment. P.Y.153 is fast to overcoating up to 120 °C, but bleeds at higher temperatures.
High durability makes P.Y.153 a preferred product in emulsion paints, especially for exterior house paints. It is a relatively important pigment for these purposes.
4.2.4.8 Pigment Yellow 177
P.Y.177 is no longer listed as a commercial product. It was a special-purpose pigment for polypropylene and polyamide spin dyeing. As a colourant for these media, P.Y.177 has the added advantage of enhancing the stability of the fibres.
Baking enamels containing P.Y.177 are very sensitive to overcoating. Their shade depends largely on the depth of shade. Full shades are typically reddish brown, similar to iron oxide systems, while white reductions are greenish yellow.
4.2.4.9 Pigment Yellow 179
The production of P.Y.179, an isoindolinone/cobalt complex pigment, has been discontinued. It was recommended for use in paints, especially in automotive finishes. The pigment produces a reddish yellow shade. High lightfastness and excellent weatherfastness are an asset in pastel colours. Besides, good transparency made P.Y.179 a suitable product for metallic finishes. Yet, it is not quite as weatherfast as the equally reddish yellow P.Y.24, a flavanthrone pigment.
4.2.4.10 Pigment Orange 59
This pigment has been withdrawn from the market.
P.O.59 is a nickel complex that affords very dull yellowish shades of orange. The pigment was used to match deep to medium shades in the yellow and orange part of the spectrum for high grade industrial finishes, as well as for architectural paints.
P.O.59 exhibits good lightfastness and weatherfastness. Incorporated in a baking enamel based on alkyd-melamine resin, for instance, 10% full shades equal step 8 on the Blue Scale for lightfastness, while 1/3 to 1/25 SD samples correspond to step 7, and 1:200 SD specimens (with TiO2) match step 6 on the Blue Scale. The systems rapidly decrease in weatherfastness as more TiO2 is added. Up to 180 °C, the pigment is heat stable and fast to overcoating, as long as the baking temperature does not exceed 140 °C. P.O.59 is not alkali and acid resistant; pigment demetallation fades or lightens the shade.
4.2.4.11 Pigment Orange 65
P.O.65, was withdrawn from the market a few years after the production started. It is an azomethine/nickel complex pigment and produces dull, reddish shades of orange, but very brilliant shades of copper in metallic finishes. P.O.65 was therefore considered a speciality product for such purposes. The pigment is not entirely fast to overcoating. Its weatherfastness in metallic finishes is very good and satisfies the requirements for use in original automotive finishes. This is despite the fact that it fails to reach the durability standards of the reddish P.R.168, a dibromanthanthrone pigment.
4.2.4.12 Pigment Orange 68
P.O.68 lends itself for use in industrial paints, including automotive finishes, as well as for plastics. It provides dull, reddish shades of orange. Two varieties are commercially available: a version with a coarse particle size and a type with a fine particle size.
The pigment with a fine particle size provides good transparency at high tinctorial strength. It is recommended primarily for use in metallic finishes to produce shades of copper, gold and brown. Transparent P.O.68 is not completely fast to overcoating. This type exhibits good lightfastness and durability, but tends to darken. This is especially true for deep solid shades and colour intense metallic finishes.
The variety with a coarse particle size is a pigment with equally high tinctorial strength and provides good hiding power. It is bluer and somewhat duller than the version with a fine particle size. The opaque type, like the transparent variety, is not completely fast to overcoating. Lightfastness and durability equal those of the pigment with a fine particle size. Opaque P.O.68 is used in combination with quinacridone pigments, magenta or violet pigments to produce dull shades of red and maroon.
P.O.68 is also used in plastics for its high heat stability. 1/3 SD colourations in HDPE (high density polyethylene) containing 1% TiO2 withstand temperatures up to 200 °C. The shade is a dull orange. Some 0.15% pigment is required to formulate 1/3 SD samples containing 1% TiO2. Such specimens match step 7 on the Blue Scale for lightfastness. In some plastics (polycarbonate), which are processed at high temperature, the pigment withstands as much as 320 °C. This makes P.O.68 one of the most heat stable organic pigments known. The list of recommendations also includes polyamide colouration.
4.2.4.13 Pigment Red 257
P.R.257, a heterocyclic nickel complex, covers the range of reddish violet shades; its hue is distinctly yellower than that of the P.R.88 type thioindigo pigments and of the β-modification of P.V.19, an unsubstituted quinacridone pigment. In paints, P.R.257 is tinctorially weaker than these pigments. In combination with Molybdate Orange or opaque organic red pigments it is also less intense. The commercial grade demonstrates good hiding power, good rheology and good resistance to flocculation. Both lightfastness and weatherfastness are equally good and, both in full shades and in white reductions, comparable to the performance of P.R.88. In baking enamels, P.R.257 is fast to overcoating. Its full shade and similarly deep shades are used in original automotive and industrial finishes.
4.2.4.14 Pigment Red 271
This isoindoline nickel complex pigment is mainly recommended for the colouration of metallics and effect coatings, especially for water based systems. P.R.271 affords yellowish to medium red shades, providing a bright flop in metallics. Alkyd/melamine resin systems may safely be overcoated and withstand exposure of 140 °C for 30 min. The flow properties of P.R.271 in these systems and in polyestercellulose acetobutyrate base coat systems are good, but there is a certain tendency to flocculate.
4.3 Pigments with Known Chemical Structure Which Cannot be Assigned to Other Chapters
These products are classified according to their shade.
4.3.1 Pigment Yellow 101
P.Y.101, C.I.48 052, has the chemical structure of a disazomethine compound (145):

Scheme 4.10 Molecular structure of P.Y.101.
Known since 1899, this product was initially patented as a fluorescent dye and was used later as a pigment for the mass colouration of viscose.
It is obtained by condensing 2 moles of 2-hydroxy-1-naphthaldehyde with one mole of hydrazine:

P.Y.101 produces uncommonly brilliant greenish yellow shades. It is referred to as a fluorescent pigment. In this respect, P.Y.101 is unique among the currently available organic pigments. All other industrial fluorescent pigments, which are not discussed in this book, represent fluorescent dyes that are dissolved in suitable media (resins). Some of these provide good migration resistance through chemical interaction between dye and resin. P.Y.101, on the other hand, is a crystalline pigment, although it shows somewhat less fluorescence than resin-based dyes. The optical effect of P.Y.101 and other fluorescent colourants is a consequence of selective light absorption and simultaneous luminescence, initiated by high-energy radiation, that is, UV radiation and/or light with a short wavelength.
The molecule adopts a trans conformation with two intramolecular hydrogen bonds. In the crystal it is fully planar. Figures 4.4 and 4.5 show the molecular structure and the crystal structure, respectively, of P.Y.101 [25, 26].

Figure 4.4 Molecular structure of P.Y.101 in the solid state.

Figure 4.5 Crystal structure of P.Y.101 showing four molecules.
Experimental investigations and quantum mechanical calculations (time dependent density functional theory) reveal that the OH group is necessary for the fluorescent behaviour. Derivatives without an OH group do not show fluorescence, because the excited state (ππ* state) converts through an in-plane CNN bending vibration into an nπ* state, from where a transition to the ground state (fluorescence) is optically forbidden; the system returns to the ground state by a radiationless decay. Lattice-energy calculations show that the corresponding CNN bending is facile in the crystal. In P.Y.101 (and other derivatives containing an OH group in the β-position of the naphthalene ring) the hydrogen bond between the OH group and the nitrogen atom lowers the energy of the n-orbital (lone pair at the N atom). Consequently, the nπ* state is much higher in energy, and thus this state cannot be reached after a ππ* excitation, and the system has to return to the ground state by fluorescence [26].
P.Y.101 is neither fast to acids nor bases and is not entirely fast to important organic solvents, either. It is moderately lightfast.
The commercial product is highly transparent and is considered a special-purpose product, particularly for printing inks. The colour intensity reaches an optimum when applied over light, if possible white, substrates. P.Y.101 is frequently applied in combination with nonhiding fillers, such as barium sulfate, but particularly with other yellow, green or red pigments. Even minor amounts of P.Y.101 noticeably increase the brilliance of prints containing nonfluorescent pigments.
Other areas of application are in office articles and in the arts field, where P.Y.101 lends colour to pencils, chalks, watercolours and other articles. It is also used for fluorescent markers.
4.3.2 Pigment Yellow 148
The chemical structure of P.Y.148, a greenish yellow pyrenyle triazine, is listed in the Colour Index under C.I. Constitution Number 50600:

Scheme 4.11 Molecular structure of P.Y.148.
This pigment is a special-purpose product for the spin dyeing of polyamide.
4.3.3 Pigment Yellow 182
P.Y.182 is a hydrazone pigment of the following structure [27]:

Scheme 4.12 Molecular structure of P.Y.182.
The corresponding coupling component 147 is synthesized by treating s-trichlorotriazine with N-acetoacetyl-2-methoxyaniline in an alkaline medium. Upon addition of sulfuric acid at room temperature the resulting compound 146 releases the acetyl group and hydrolyses to afford 147. The yield is more than 90% [28, 29]:

P.Y.182 was available for some years, but has been withdrawn. P.Y.182 provides somewhat reddish shades of yellow and is tinctorially strong. It is sensitive to various organic solvents, especially to ketones such as methyl ethyl ketone and cyclohexanone, as well as to aromatic solvents such as toluene or xylene. In this respect, the pigment equals step 2 on the 5-step scale (Section 1.6.2.1). P.Y.182 is targeted for the paint and the plastics industry.
The paint industry utilizes P.Y.182 especially in various types of industrial finishes wherever the weatherfastness requirements are not too stringent. A high TiO2 content adversely affects both the lightfastness and the weatherfastness of P.Y.182 systems. At typical baking temperatures (150 °C), such systems are not entirely fast to overcoating. The list of recommended applications for P.Y.182 includes emulsion paints, although a certain sensitivity of the pigment to alkaline agents prevents P.Y.182 containing paints from being applied onto alkaline substrates, such as fresh plaster or cement.
Used as a colourant for plastics, P.Y.182 exhibits medium to high tinctorial strength; 0.8% pigment is required to produce a 1/3 SD PVC sample containing 5% TiO2. There is some blooming at low pigment concentrations, and bleeding is observed over the entire concentration range. P.Y.182 is therefore only recommended for use in rigid PVC.
1/3 SD HDPE systems (1% TiO2) are thermally stable up to 250 °C, while 1/25 SD specimens withstand 280 °C. P.Y.182 does not affect the shrinkage of the plastic. Some 0.37% pigment is needed to formulate a 1/3 SD HDPE sample. Combining P.Y.182 with nickel stabilizers in polypropylene is to be avoided. The pigment dissolves in polystyrene as the temperature reaches 200 °C, a process that is accompanied by considerable colour change. P.Y.182 is also an unsuitable candidate for ABS.
4.3.4 Pigment Yellow 201
According to [30] P.Y.201 has the following structure:

Scheme 4.13 Molecular structure of P.Y.201.
The shown tautomeric form is the one that probably exists in the solid state. The pigment is a candidate for the use in packaging gravure and flexo printing inks. Its shade is a medium to somewhat reddish yellow and the tinctorial strength is rather high. The fastness to organic solvents like ethanol, ethyl acetate, methyl ethyl ketone or to the solvent mixture according to DIN 16524 (Section 1.6.2.1) is comparatively poor, which may lead to recrystallization in the ink during the dispersion process or to bleeding of the print. The pigment is therefore particularly recommended for water thinnable printing and UV inks.
Despite a specific surface area of 57 m2 g−1 the commercially available grade affords opaque prints. Prints of an ink with 7.5% P.Y.201 equal step 3–4 on the Blue Scale for lightfastness.
4.3.5 Quinolonoquinolone Pigments (P.Y.218, P.Y.220, P.Y.221)
The parent compound, quinolonoquinolone (QQ), is a yellow pigment with a high degree of lightfastness and a good tinctorial strength. The synthesis, which was developed by DuPont in 1964 [31], resembles the synthesis of quinacridones:

Halogen-substituted QQs are intense yellow pigments that tend to have a greenish yellow hue. The 2,8-dimethoxy derivative shows a distrinct reddish yellow shade. Many halogen-substituted QQs exist in at least two polymorphic forms, which often differ markedly in their stabiliy and their tinctorial properties.
Quinolonoquinolones have been studied as a possible alternative to Pigment Yellow 74. Some monohalogenated QQs were found to have comparable strength and shade with P.Y.74. The following three pigments could be converted into stable nanosized inkjet dispersions. They demonstrate outstanding lightfastness of prints, matching that of copper phthalocyanine and quinacridones. Dispersions of these QQs have very high colloidal stability and are not sensitive to co-solvents [32].
4.3.5.1 Pigment Yellow 218
The chemical structure of P.Y.218 is listed in the Colour Index under Constitution Number 561 805. It is a 3-fluoroquinolonoquinolone:

Scheme 4.14 Molecular structure of P.Y.218.
P.Y.218 has a bright yellow shade and is a high performance pigment for inkjet, plastics and paints. Lightfastness and thermostability are outstanding. It can also be used as a bright and strong yellow inkjet pigment.
4.3.5.2 Pigment Yellow 220
P.Y.220 is a 2-fluoroquinolonoquinolone, that is, an isomer of P.Y.218. In the Colour Index, the structure is listed under Constitution Number 561 806:

Scheme 4.15 Molecular structure of P.Y.220.
P.Y.220 has a bright and neutral yellow hue. It is a strong inkjet pigment with outstanding fastness properties.
4.3.5.3 Pigment Yellow 221
The chemical structure of P.Y.218 is listed in the Colour Index under C.I. Constitution Number 561 801:

Scheme 4.16 Molecular structure of P.Y.221.
P.Y.221 is a mixture of 80–90% 3-chloroquinolonoquinolone (C.I. 561802, R3 = Cl, R9 = H), quinolonoquinolone (C.I. 561800, R3 = R9 = H) and 3,9-dichloroquinolonoquinolone (C.I. 561812, R3 = R9 = Cl).
P.Y.221 is a yellow pigment that can be used for plastics and paints. Lightfastness and thermostability are outstanding. It can also be used as a bright and strong yellow inkjet pigment.
4.3.6 Pigment Orange 64
The chemical structure of P.O.64 is listed in the Colour Index under C.I. Constitution Number 12 760:
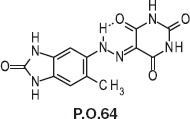
Scheme 4.17 Molecular structure of P.O.64.
P.O.64 is synthesized by diazotization of 5-amino-6-methylbenzimidazolone and subsequent coupling of the diazonium component onto barbituric acid. The pigment affords a yellowish orange shade.
Its main field of application is in plastics. 1/3 SD HDPE samples, both transparent and opaque, withstand exposure to 300 °C for 5 min, while 1/25 SD specimens are heat stable up to 250 °C. Temperatures in excess of this value shift the shade towards more yellowish hues. P.O.64 does not have a nucleating effect on its medium, that is, it does not affect the shrinkage of partially crystalline injection-moulded polymers.
P.O.64 shows good migration resistance in plasticized PVC and exhibits medium tinctorial strength. The pigment is also used in polystyrene and similar polymers and is also recommended as a colourant for rubber.
The printing ink industry employs P.O.64 in metal deco printing inks in view of the fact that the pigment is thermally stable up to 200 °C. The prints may safely be overcoated.
4.3.7 Pigment Orange 67
P.O.67 is a member of the pyrazolo-quinazolone pigment series. Its constitution is listed in the Colour Index under C.I. Constitution No. 12 915. The coupling component is a pyrazolo[5,1-b]quinazolone (Section 3.7.1.2). The diazonium component is 2-nitro-4-chloroaniline. Coupling affords the pigment, which was found to exhibit the hydrazone structure:

Scheme 4.18 Molecular structure of P.O.67.
The commercial grade is very opaque. Its main area of application is in paints, to which it lends full shades and relatively deep shades. P.O.67 is employed wherever use of Molybdate Orange pigments is to be avoided. P.O.67 affords brilliant yellowish shades of orange, which are close to the standard shade RAL 2004. The pigment is not sufficiently fast to important organic solvents. Incorporated in oven drying systems, the pigment is not entirely fast to overcoating at common baking temperatures (140 °C). Systems that are processed at low temperatures between 80 and 100 °C, however, may safely be overcoated. P.O.67 is particularly recommended for use in long-oil and medium-oil alkyd resin systems, especially for decorative paints and emulsion paints. In these media, P.O.67 exhibits very good lightfastness and weatherability. The pigment should not be used in epoxy resins.
P.O.67 is also recommended for printing inks, especially for flexo inks on NC base. Special pigment preparations for this application are on the market.
4.3.8 Pigment Red 90
P.R.90, C.I. 45380:1, is derived from the fluorescein structure (137). Fluorescein, a yellow dye with intensely green fluorescence, was discovered by A. v. Bayer in 1871. It is prepared by heating resorcin and phthalic anhydride with zinc chloride or concentrated sulfuric acid:

Brominating fluorescein in the presence of sodium chlorate affords a red tetrabromo derivative, whose sodium salt is known as eosine (Caro, 1871). Sodium chlorate reoxidizes the evolving hydrogen bromide to bromine, which can react again:

The bromination product is actually contaminated with a certain percentage of mono to tribromo derivatives. Although impurities, these side products apparently furnish the good tinctorial strength of P.R.90. Eosine itself is only a tinctorially weak pigment.
Treating the aqueous eosine solution with lead nitrate or lead acetate affords bluish red P.R.90, referred to as phloxine:

Scheme 4.19 Molecular structure of P.R.90.
Phloxine, the lead precipitate, was last manufactured in Japan and the USA, although its significance in these countries has now also rapidly declined as a result of its lead content. Worldwide the use of lead containing pigments is today very much restricted or even prohibited.
The pigment provides a brilliant medium red shade and high tinctorial strength. P.R.90 is used in inexpensive letterpress and offset inks, for which it was supplied in the form of flushed pastes. The prints lack fastness to organic solvents, especially to alcohols, esters and ketones, as well as to various chemicals such as alkali, acid and soap. Likewise, P.R.90 shows poor lightfastness and heat stability.
4.3.9 Pigment Red 252

Scheme 4.20 Molecular structure of P.R.252.
P.R.252 is a member of the pyrazoloquinazolone series, like P.O.67 (Section 2.7.4.2.7) and P.R.251 (Section 3.7.1.4.6). It has already been withdrawn from the market.
P.R.252 provides yellowish to medium red shades and is recommended particularly for use in architectural paints. The pigment shows very poor fastness to several organic solvents commonly used in paints, a deficiency that largely precludes it from being used in oven drying systems. Regarding lightfastness and weatherfastness, the only available type with coarse particle sizes performs somewhat better than the much more yellowish P.O.5.
4.3.10 Pigment Blue 63
P.B.63, 73 015:x, is an aluminium lake. It is derived from the indigo structure, which is sulfonated to afford indigo-5,5′-disulfonic acid. Reaction with aluminium trichloride yields the insoluble pigment:

Scheme 4.21 Molecular structure of P.B.63.
The aluminium lake is registered throughout the EC as E 132, in the USA as FD&C Blue 2 as a colourant for foodstuffs and pharmaceuticals, provided certain purity conditions are met. Its shade is a bluish red. The pigment is somewhat sensitive to chemicals and to overcoating as well as to light. It is a tinctorially weak product.
4.3.11 Pigment Brown 22
This reddish brown benzidine pigment is characterized by the following chemical structure:

Scheme 4.22 Molecular structure of P.Br.22.
P.Br.22, C.I. 10407, presents medium to slightly reddish shades. It is used for the spin dyeing especially of polyacrylonitrile and viscose.
Pigment preparations of P.Br.22 are supplied for these purposes. The pigment demonstrates good fastness properties: it is fast to perspiration, dry cleaning in perchloroethylene and dry heat. In terms of lightfastness, P.Br.22 systems equal step 5 to step 7 on the Blue Scale, depending on the depth of shade.
P.Br.22 is offered in the form of various pigment preparations, designed for industrial finishes, packaging gravure printing inks or wood stains. The pigment is not supplied as a powder.
4.3.12 Pigment Black 1
P.Bl.1, 50 440, referred to as Aniline Black, is a phenazine derivative:

Scheme 4.23 Molecular structure of P.Bl.1.
A technique of developing Aniline Black directly on the fibre was found by Lightfoot in the period between 1860 and 1863. In accordance with this process, the fibre is soaked with aniline, aniline hydrochloride and sodium chlorate in the presence of an oxidation catalyst (e.g. ammonium vanadate, potassium hexacyanoferrate(II)). The compound is ‘developed’ at 60–100 °C and then oxidized further with sodium chromate. Notably, however, Perkin had already synthesized a black compound that he called Aniline Black as early as 1856. He oxidized aniline (containing toluidine) with potassium dichromate and separated Aniline Violet from the resulting black mixture (Aniline Black).
Modern methods of manufacturing this the probably oldest representative amongst synthetic organic pigments involve dissolving aniline in strong sulfuric acid. Oxidation is achieved with sodium dichromate in the presence of a copper salt or one of the above-mentioned oxidation catalysts. Oxidation with sodium chlorate initially affords an indamine polymer (pernigraniline):

This intermediate must be oxidized further to afford the azine pigment (Green, Willstätter, 1907–1909).
Aniline Black provides a deep, neutral shade of black. Extensive absorption and little scattering make for good hiding power. The commercial grades cover a comparatively wide range of particle size distributions. The types with fine particle sizes in particular provide characteristically dull, velvety effects in finishes and prints. Even types with fine particle sizes show only a very slight tendency to agglomerate, which makes them easy to disperse. The pigment is not an electrical conductor.
P.Bl.1 is used in various media. Incorporated in paints, full shades show excellent lightfastness and weatherfastness, qualities that deteriorate rapidly as more TiO2 is added. The pigment is tinctorially weaker than carbon blacks. Some types are not entirely fast to overcoating, a property that extends to acids and alkali, oxidants and reducing agents. The paint and printing ink industries utilize P.Bl.1 particularly where carbon blacks present processing problems or where a matt and velvety quality is required in a paint or print. P.Bl.1 is of interest in wood stains made from unsaturated polyester. In plastics, P.Bl.1 is used to advantage wherever carbon black cannot be used as a result of its inability to tolerate heat sealing.
4.4 Pigments with Hitherto Unpublished Chemical Structures
The fact that many of these pigments have not even been assigned to any particular class makes it more convenient to list them by their shade in the order of increasing Colour Index Numbers.
4.4.1 Pigment Yellow 99
P.Y.99, which is derived from the anthraquinone structure, is produced in Japan. It affords very reddish shades of yellow, even redder but at the same time distinctly duller than those of P.Y.83. It is recommended especially for use in textile printing but is also used in plastics. Although HDPE systems are heat stable up to 300 °C, they noticeably lack tinctorial strength. 1/3 SD HDPE samples containing 1% TiO2 are formulated at 0.53% pigment.
4.4.2 Pigment Yellow 187
The production of P.Y.187, a fluororubine pigment, has been discontinued. It was a special-purpose pigment for polyamide. Thermostable up to 320 °C in this plastic material, the pigment furnishes greenish yellow shades. 1/1 SD samples score only as high as step 4 on the Blue Scale for lightfastness.
Incorporated in plasticized PVC, P.Y.187 demonstrates moderate fastness to bleeding. At a processing temperature of 130 °C, the pigment affords a greenish shade of yellow, while an orange shade is observed at 160 °C.
4.4.3 Pigment Yellow 214
P.Y.214 is a dihydrazone pigment, of which the exact chemical structure has not yet been disclosed. It affords a very greenish yellow shade with high colour strength. The pigment is recommended for use throughout the plastics industry, in particular for the colouration of polyolefins, PVC, polystyrene (PS) and other engineering plastics as well as for the spin dyeing of polypropylene. High bleeding fastness in PVC, heat resistances of 260 °C (ABS), 280 °C (PE, polyethylene) and 300 °C (PS) and a general lightfastness of step 7 in full shade and step 6/6–7 in reduction makes P.Y.214 an interesting addition to the yellow range of plastics pigments. The pigment is suitable for fibre and thin wall applications as well as for low warping applications and cable sheathing.
4.4.4 Pigment Orange 83
Pigment Orange 83 is monohydrazone metal lake with hitherto unpublished chemical structure. It offers a bright reddish orange shade. The pigment can be used for industrial coatings, and in paints and plastics.
4.4.5 Pigment Red 204
The exact chemical constitution of P.R.204, a polycyclic compound, remains to be published. The pigment affords a dull, medium red full shade. In white reductions, the shade becomes much more bluish and comparatively cleaner. P.R.204 exhibits excellent fastness properties. Full shades (5%) in various paint systems equal step 7–8 on the Blue Scale for lightfastness, a value that is also reached by white reductions up to 1/25 SD. Moreover, P.R.204 also shows very good weatherfastness. The commercial grade demonstrated high hiding power and good rheology. Full shades and similar deep shades were of particular interest, as they were frequently combined with inorganic red pigments. The resulting blends were used to advantage in various types of industrial paints, including automotive finishes. P.R.204 provides good fastness to overcoating and shows a heat stability of 180 °C for 30 min. Higher baking temperatures cause a colour shift towards more bluish shades.
P.R.204 is also very lightfast in printing inks, although it was rarely encountered in these media. 1/1 to 1/25 SD letterpress proof prints, for instance, equal step 7 on the Blue Scale. The prints demonstrated very good fastness properties in application, despite the fact that they are not entirely fast to paraffin. In addition, they are not fast to sterilization.
At present the pigment is not available on the market.
4.4.6 Pigment Red 278
This is a bluish red monohydrazone pigment of which the chemical structure has not yet been disclosed. P.R.278 is recommended for use in printing inks.
The pigment is not commercially available.
4.4.7 Pigment Red 285
The chemical structure of this pigment has not yet been disclosed. The main area of application for the monohydrazone strontium lake pigment P.R.285 is in plastics, in which it produces very yellowish and transparent shades of red. The tinctorial strength is moderate, 0.33% is needed to achieve 1/3 SD in HDPE with 1% TiO2. Such colourations equal step 6–7 on the Blue Scale for lightfastness. P.R.285 exhibits excellent heat stability as it withstands temperatures of up to 290 °C in polyolefins and does not effect the shrinkage of partially crystalline polymers.
4.4.8 Pigment Red 293
Clariant introduced P.R.293 to the market in 2016 as a new magenta pigment for non-impact printing. P.R.293 is a hydrazone pigment of hitherto non-disclosed structure. The compound is chlorine-free and metal-free and as such has been developed to futural needs for environmentally favourable products. Having a shade close to that of P.R.57:1, P.R.293 exactly matches the hue required for a standard magenta (process magenta) used for four-colour printing.
In non-impact printing, P.R.293 closes the gap between the too-bluish P.R.122 and the too-reddish hydrazone pigments P.R.146, P.R.184 and P.R.269. Hence, P.R.293 has the advantage that – in contrast to the other hydrazone pigments – no mixing with P.R.122 is required to obtain the standard magenta shade.
In addition, P.R.293 shows a significantly higher color strength and an improved chroma, i.e. it is more brilliant when compared to P.R.57:1, P.R.146 or P.R.184. Consequently, P.R.293 caters for the largest accessible color space (color gamut expansion) when compared to other magenta pigments.
The toner grade of P.R.293 has a high transparency and a high specific surface of about 70 m2/g. Only 0.61% pigment is necessary to obtain a print with 1/1 standard depth. The solvent fastness properties are good, light fastness is moderate. The pigment is suitable for all common toner resins such as styrene-acrylic, polyester and epoxy resins. Due to its bluish shade and high chroma it helps to match offset quality.
P.R.293 is also suited for aqueous ink-jet applications, where it could replace the hitherto used mixtures of P.R.184 and P.R.122.
4.4.9 Pigment Violet 51
The exact chemical constitution of this laked monohydrazone pigment is still unpublished. The violet pigment has an excellent heat stability of over 310 °C. P.V.51 has been developed for the plastics industry, where it brings good value to the packaging and interior household durables segments. Tinctorially it is quite strong and similar in colour to quinacridone violet and can be used as a cost effective diluent, but its use is restricted to the interior due to its fastness properties. Pigment Violet 51 is significantly bluer than Pigment Violet 52 (Section 2.7.4.2.10) and is – other than P.V.52 – not in compliance with the FDA requirements for food contact under the conditions of use, (A) through (H) as described in Table 2 of 21 CFR Part 176.170(c). Pigment Violet 51 exhibits very low warpage characteristics in polyolefin moldings.
4.4.10 Pigment Black 20
The exact chemical structure of P.Bl.20, an anthraquinone pigment, has not been published. P.Bl.20 is a speciality product used in camouflage paint. The pigment satisfies certain specifications regarding infrared reflection. It is employed in paints. 1/3 SD equals step 6 on the Blue Scale for lightfastness. Thermally stable up to 200 °C, P.Bl.20 is not fast to overcoating, not even at low baking temperatures such as 120 °C. Although alkali proof, P.Bl.20 is not entirely fast to acid. The pigment is insufficiently fast to common organic solvents such as esters and aromatic hydrocarbons. In this respect, P.Bl.20 equals step 2 on the 5-step scale.
4.5 Organic/Inorganic Hybrid Pigments
The organic/inorganic hybrid pigments described in this chapter consist of layered silicates and organic colourants [33]. They mimic the ancient Maya Blue pigments.
In Mayan frescoes in Mesoamerica a brilliant blue colour is found on indoor murals as well as on buildings, sculptures and ceramics. Interestingly enough, despite long exposure to environmentally harsh humidity and the heat of Mesoamerica the colour hardly faded. Its stability defies exposure to acids, alkalis and chemical solvents and it resists natural biodegradation.
The blue paint consists primarily of indigo and the clay mineral palygorskite. This was already determined by X-ray powder diffraction in the 1950s [34]. However, neither component by itself would exhibit any of Maya Blue's characteristic properties. Attempts to duplicate the process by mixing the palygorskite clay and indigo produced the colour of Maya Blue but not its fastness properties. A heating process was found to be essential and by heating the clay/indigo mixture to about 125°C for five to six days the Maya Blue was formed [35].
The process behind the heating is that the layer structure of palygorskite contains channels enclosing three types of water molecules, two of them tightly bound into the structure and the third loosely held inside the channels. The third water molecule is readily removed through the heating process and the channels absorb the indigo molecules. The appropriate size of the indigo molecules results in them being locked within the channels which creates the Maya Blue. The results were supported by high resolution transmission electron microscopy (HRTEM), sychrotron X-ray powder diffraction [36], and molecular dynamics [37].
All industrial organic pigments are stabilized by their crystal lattice (Section 1.5.3). Pigments with good application properties show extremely dense and efficient molecular packings with high lattice energies, leading to a pronounced insolubility and a good light and weather fastness. When these pigment molecules are incorporated into the silicates, the molecular packing is broken up, and the molecules are “dissolved” in the silicate, forming a mixed crystal. Only molecules which contain hydrogen bond donors and acceptors (e.g. indigo) can form hydrogen bonds with the oxygen atoms and OH groups of the silicate framework, and with the remaining water molecules. The large non-polar aromatic groups (phenyl, naphthyl etc.) of the pigment molecules form only weak van der Waals interactions with the silicate. Hence, it strongly depends on the molecular constitution, if the silicate host can provide a better stabilisation of the molecules than the original lattice of the pigment itself. Correspondingly, the incorporation into a silicate makes only sense for those pigments, which as pure compounds have poor application properties. A good example is indigo, which is chemically less stable and fades upon weathering as a pure pigment, but can be stabilized by palygorskite. Similarly, dyes may be stabilized by silicates. A general disadvantage of the resulting hybrid pigments is their relatively low colour strength, caused by the “dilution” of the dye or pigment by the silicate.
In the 1990ies, several large producers of organic pigments undertook attempts to develop organic/inorganic hybrid pigments. To the best of the authors knowledge, none of their developments lead to a commercial product. In approximately 2005, the company Mayan Pigments, Inc. introduced a series of organic/inorganic hybrid pigments to the market. These pigments were based on developments at the University of Texas El Paso. Several inorganic/organic hybrid pigments were registered in the Colour Index. For most pigments, the exact chemical composition has not yet been disclosed. Presently, organic/inorganic hybrid pigments are produced on a small scale.
4.5.1 Pigment Yellow 222
This greenish-yellow pigment is based on quinoline, but the chemical structure has not been disclosed to the public. Properties and applications have not yet been published.
4.5.2 Pigment Yellow 223
This reddish-yellow needle-shaped pigment based on quinoline is claimed to exhibit a broad range of excellent chemical and physical properties in demanding environments and to be suitable for use in paints, coatings, cement/stucco, artist's colours, graphic arts, selected ink and thermoset applications. It is described to exhibit a very good lightfastness and migration fastness and resists solvents such as ethyl alcohol, ethyl acetate and xylene. The pigment requires no labelling for health hazards for art material customers.
4.5.3 Pigment Yellow 224
This greenish-yellow quinoline-based pigment, whose structure has not yet been published, is suitable for use in plastics such as polypropylene, polyethylene, polybutylene terephthalate, polycarbonate, PVC and nylon and also in engineering resins. The pigment is resistant against alkali and acids and insoluble in organic solvents such as ethyl acetate, ethyl alcohol or xylene. Lightfastness and migration stability are excellent.
4.5.4 Pigment Yellow 226
This yellow pigment of a dihydrazone type structure exhibits very good solvent fastness against ethyl acetate, ethyl alcohol and xylene and high stability against acids and alkali. Very good lightfastness and migration fastness allow applications in many types of plastics, as well as in paints and inks.
The pigment has been approved by the FDA for use in indirect food contact at levels not to exceed 1.0% by weight of the finished polymer. The finished articles are to contact food only under conditions of use B through G described in 21 CFR 178.3297 (Colorants for Polymers).
4.5.5 Pigment Orange 84
This needle-shaped monohydrazone type pigment of undisclosed chemical structure shows good solvent resistance to ethyl acetate, ethyl alcohol and xylene and very good migration fastness. P.O. 84 can be applied for use in plastics, paints and inks.
The pigment has been approved by the FDA for use in indirect food contact at levels not to exceed 1.0% by weight of the finished polymer. The finished articles are to contact food only under conditions of use B through G described in 21 CFR 178.3297 (Colorants for Polymers). Used in food packaging the pigment exhibits a strong potential for strengthening and reducing wall thickness in plastics. It shows very good thermal stability and does not induce warping.
4.5.6 Pigment Red 286
P.R.286 exhibits very good stability against acids and alkali and high solvent fastness for ethyl acetate and ethyl alcohol. The indigoid type pigment is suitable for use in plastics and engineering resins and specifically recommended for polyethylene, ABS, polybutylene terephthalate, polycarbonate, PVC and acrylics, and with limited use depending on application temperature and resin grade for, for example, polypropylene and polyester.
4.5.7 Pigment Red 287
This indigoid type pigment provides very good stability againsts acids and alkali and high solvent fastness for ethyl acetate and ethyl alcohol. It is suitable for use in paints, coatings, paper and paper board, cement/stucco, artist's colours, graphic arts, selected ink and thermoset applications.
The pigment requires no labelling for health hazards for art material customers.
4.5.8 Pigment Red 288
A dihydrazone type pigment of which the chemical structure has not yet been disclosed. P.R.288 exhibits very good stability against acids and alkali and high solvent fastness for ethyl alcohol, ethyl acetate and xylene. Lightfastness and migration fastness are also described as very good.
The pigment is FDA approved for use in indirect food contact and recommended for applications in plastics, paints and inks.
For use in indirect food contact the level should not exceed 1.0% by weight of polymers. Approved for use in all polymers the thickness should not exceed 0.05 mm. The finished articles are to contact food only under conditions of use A through H described in 21 CFR 178.3297 (Colorants for Polymers), food types: aqueous, acidic, low alcohol.
4.5.9 Pigment Violet 58
The violet and blue pigments were derived from the research and investigation into the ancient Maya Blue.
P.V.58 is an indigoid type pigment, the structure of which has not been disclosed. The application properties make P.V.58 suitable for use in plastics such as HDPE, ABS, polyethylene terephthalate, polystyrene and acrylics.
4.5.10 Pigment Blue 82
P.B.82 is synthesized by precipitating Indigotin (148) on clay.

P.B.82 combines the chroma of an organic colourant with selected properties of an inorganic pigment. It is claimed to provide good application properties regarding lightfastness, migration stability, resistance against acids and alkali and solvent resistance to ethyl alcohol, ethyl acetate and xylene.
The pigment is suitable preferably for use in plastics, but also in paints, coatings, cement/stucco, artist's colours, graphic arts and selected ink and thermoset.
P.B.82 does not require labelling for health hazards for art material customers.
4.5.11 Pigment Blue 84
This hybridized indigoid type pigment is derived from naturally occurring sources consisting of clay with natural plant and dye extracts. Fastness against acids and alkali and solvent fastness for ethyl acetate, ethyl alcohol and xylene are described as very good as well as the migration fastness. The lightfastness is not as good as that of Pigment Blue 82.
The pigment is suitable for use in plastics such as polyolefines and also in biodegradable polymers such as poly-3-hydroxybutyric acid, polylactic acid and polyhydroxy alkanoates.
References for Chapter 4
- 1 Hunger, K. (ed.) (2003) Industrial Dyes, Wiley-VCH Verlag GmbH, Weinheim, pp. 59–67.
- 2 Ringel, Kh. et al. (1982) Zh. Org. Chim., 18, 1018–1022.
- 3 Hoechst (1969) DE-AS 1919 724.
- 4 Schmelzer, H. (1976) XIII. Congr. FATIPEC, Juan les Pins 1976, Congress book, pp. 572–574.
- 5 Baumann, H. and Hensel, R. (1967) Fortschr. Chem. Forsch., 7, 4.
- 6 Stallmann, O. (1960) J. Chem. Educ., 37, 220–230.
- 7 Kiel, E.G. and Heertjes, P.M. (1963) J. Soc. Dyers Colour, 79, 21.
- 8 Bayer (1970) DE-OS 2 064 093.
- 9 Stephan, G. (1976) 6th International Colour Symposium, Freudenstadt.
- 10 Regitz, M. (1967) Angew. Chem., 79, 786–801.
- 11 Schindehütte, K.H. (1965) Houben-Weyl, Methoden der org. Chemie, vol. X/3, 4th edn, Stuttgart, p. 213.
- 12 Pfitzner, H. and Baumann, H. (1958) Angew. Chem., 70, 232.
- 13 L'Eplattenier, F.A., Frey, C., and Rihs, G. (1977) Helv. Chim. Acta, 60, 697–709.
- 14 Ciba-Geigy (1974) DE-OS 2 546 038.
- 15 Frey, C. and Lienhard, P. (1984) XVII. Congress FATIPEC Lugano, 1984, Congress Book, pp. 283–301.
- 16 Hädicke, E., Henning, G., and Mez, H.-C. (1973) European Crystallographic Meeting.
- 17 Blagus, A. and Kaitner, B. (2009) Acta Crystallogr., Sect. C, 65, m455.
- 18 Zhang, X.-L., Ren, C.-X., Chen, X.-M., and Ng, S.W. (2003) Acta Crystallogr., Sect. E, 59, m1176.
- 19 Liu, K., Liu, G., Cao, Z., and Niu, M. (2010) Acta Crystallogr., Sect. E, 66, m78.
- 20 Fetsko, J.M. (1983) NPIRI Raw Materials Data Handbook, vol. 4, Pigments. National Printing Ink Research Institute, Bethlehem, Penn. USA.
- 21 BASF (1966) DE-OS 1 544 404.
- 22 Sakai, H. et al. (1982) J. Jpn. Soc. Colour Mater., 55, 685.
- 23 BASF (1967) DE-OS 1 252 341; BASF (1966) DE-OS 1 569 666.
- 24 P.A. Lewis (ed) (1988). Pigment Handbook, vol. 1, John Wiley & Sons, Inc., New York, pp. 723, 725.
- 25 Guo, D., Li, J., Xie, J. et al. (2002) Chin. J. Inorg. Chem., 18, 1215–1220.
- 26 Dreuw, A., Plötner, J., Lorenz, L., Wachtveitl, J., Djanhan, J.E., Brüning, J., Metz, T., Bolte, M., and Schmidt, M.U. (2005) Angew. Chem., 117, 7961–7964.
- 27 Lewis, P.A. (ed.) (1988) Pigment Handbook, vol. 1, John Wiley & Sons, Inc., New York, p. 723.
- 28 Kaul, B.L. (1989) Soc. Plastics Eng., Huron (Ohio), 213–226.
- 29 Kaul, B.L. (1986) XVIII. Congress FATIPEC, Venedig, 1986, Vol. 3 73–93.
- 30 Winter, R., Zidan, I., Ott, U., and Sieber, A. (Clariant) (1998) DE 19710977 A1.
- 31 Aldridge, G.R., Jaffe, E.E., and Matrick, H. (DuPont) (1964). US 3334102.
- 32 Shakhonovich, A. (2009) NIP 25: International Conference on Digital Printing Technologies and Digital Fabrication 2009, Louisville, Kentucky, September 20, Society for Imaging Science Technology, pp. 276–278.
- 33 Polette, L., Ugarte, N., Yacamán, José, M., and Chianelli, R. (2000) Sci. Am., Discovering Archeology, July–August, p. 46.
- 34 Gettens, R. (1962) J. Am. Antiq., 27, 557–564.
- 35 Reyes-Valerio, C. (1993) De Bonampak al Templo Mayor: El azul maya en Mesoamérica, Mexico D.F., Siglo XXI editores, ISBN 968-23-1893-9.
- 36 Polette, L., Ugarte, N., and Chianelli, R. (2000) Presentation made at the Workshop on Synchrotron Radiation in Art and Archaeology, SSRL, 18 October 2000.
- 37 Fois, E., Gambo, A., and Tilocca, A. (2003) Microporous Mesoporous. Mater., 57, 263–272.
