Functional Nucleic Acid-Directed Assembly of Nanomaterials and Their Applications as Colorimetric and Fluorescent Sensors for Trace Contaminants in Water
Debapriya Mazumdar1, Juewen Liu2 and Yi Lu3, 1ANDalyze, Inc. Chicago, IL, USA, 2University of Waterloo, Waterloo, ON, CA, 3Center of Advanced Materials for the Purification of Water with Systems, Department of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Largely due to the high extinction coefficients and distance-dependent optical properties, metallic nanoparticles and quantum dots have been shown to be very attractive in DNA-related colorimetric and fluorescent assays, respectively. We have used DNAzymes (DNA molecules with catalytic activities) for directed assembly of gold nanoparticles and its application as colorimetric sensors for metal ions such as lead. This methodology has been expanded to an even broader range of molecules by using aptamers (nucleic acid-based binding molecules obtained through systematic evolution of ligands by exponential enrichment (SELEX)). We have developed a general sensor design method that is simple to design, easy to operate and gives fast color change with minimal materials consumption. To demonstrate the generality, sensors for adenosine and cocaine have been designed, both of which can produce color changes in seconds and at room temperature. Since no special feature on the aptamers is required, the design should be able to be used for any molecule of choice. Finally, to make the operation even easier to use and less venerable to errors, we have demonstrated dipsticks tests for a broad range of contaminants in water. These and other recent advances in this area will be summarized.
Keywords
Metallic nanoparticles; quantum dots; DNA-related colorimetric and fluorescent assays; DNAzymes; gold nanoparticles; colorimetric sensors; dipstick sensors; sensors for metals; sensors for cocaine
5.1 Detection of trace contaminants in water
Trace contaminants in our water resources are serious issues, as they come from both natural sources and human activities. The United States Environmental Protection Agency (US EPA) has set limits for about 90 contaminants in drinking water, which span an enormous range, from metal ions, radionuclides, volatile organics, synthetic organics, disinfectants, and their by-products, to viruses, bacteria, and other microbes. As highlighted in recent reviews, new classes of contaminants are added to the list every year [1]. These contaminants pose numerous health risks and their detection and quantification are essential to assess the risks, design removal strategies, and evaluate the effectiveness of the removal technology. The low quantities and high interference are the major obstacles associated with the detection and quantification.
To detect and quantify different contaminants, a number of analytical techniques are used. For example, inductively coupled plasma-mass spectrometry (ICP-MS) is generally used for the detection of inorganic metal species, liquid chromatography (LC)/MS is widely used for detection of organics, and matrix-assisted laser desorption/ionization (MALDI)-MS is increasingly being used for the detection of microorganisms [2]. These types of techniques are very sensitive and can detect a number of contaminants simultaneously; however they require expensive instrumentation and skilled operators, making on-site, real time detection difficult. In order to fulfill this demand, a number of materials that recognize a certain class of contaminants or a particular molecule have been developed into sensors that are often easy to use, inexpensive, and portable. In spite of this, there exists a need to obtain a general technology that incorporates both target recognition and signal generation for the rational design of sensors that can detect a wide range of contaminants. Recent development of functional nucleic acids and their application in directed assembly of nanomaterials such as gold nanoparticles and quantum dots have met the needs.
5.2 Functional nucleic acids for molecular recognition
Nucleic acids have recently emerged as an important platform for selective molecular recognition, one major requirement for sensors. Long considered as passive molecules for the storage of genetic information, RNA and DNA molecules with catalytic function similar to protein enzymes were discovered in the early 1980s and 1990s, respectively [3–5]. These enzymes are called ribozymes (catalytic RNA) and deoxyribozymes or DNAzymes (catalytic DNA). The nucleic acid enzymes usually require a metal ion co-factor to perform their catalytic function and can be tailored to be specific for a particular metal ion. In addition, nucleic acids that bind to a target molecule with high specificity and affinity (analogous to protein antibodies) have also been obtained, and these are called aptamers [6–9]. Nucleic acid enzymes and aptamers have also been fused to form a new class of allosteric enzymes called aptazymes [10,11]. Collectively, the nucleic acid enzymes, aptamers, and aptazymes are termed functional nucleic acids.
As a major component of sensors, nucleic acids possess many advantages. First, DNA/RNA targeting essentially any molecule of choice can be obtained through combinatorial selections [6–8,12], providing a unique opportunity to construct a general sensing platform for a broad range of analytes. This process is described in detail in the next section. Second, nucleic acids, particularly DNA, are very stable and can be denatured and renatured many times without losing their binding abilities, allowing a long shelf life. Third, nucleic acids have predictable base pairing interactions, which have been proven to be very useful for rational sensor design; such rational designs are difficult when making protein or organic molecule based sensors. Finally, DNA with a broad range of chemical modifications can be chemically synthesized with relatively low cost. These properties make DNA/RNA ideal candidates to create sensors. The examples discussed in this chapter mainly focus on DNA as the sensing molecule because DNA is much more stable than RNA and also less expensive, thus making it a more desirable candidate. It should however be noted that a large number of RNA aptamers and ribozymes are known and have been utilized to construct sensors. The stability of these nucleic acids can be further improved by chemical modifications and using nucleic acid analogs.
5.2.1 In vitro selection of functional nucleic acids that are selective for a broad range of target analytes
Although a number of naturally occurring ribozymes have been discovered in nature [3,4], DNAzymes and aptamers are obtained by a combinatorial biology technique called in vitro selection or systematic evolution of ligands by exponential enrichment (SELEX) [6–8,12]. This technique can be used to obtain nucleic acid sequences that recognize a target contaminant with sensitivity and specificity. Figure 5.1A is a schematic representation of the selection process. A large pool of nucleic acid sequences represented by the colored objects (approximately 1014–1016 different sequences) is incubated with a target of interest in each round of selection. The “winner sequences,” which bind to the target analyte (in the case of aptamer selection) or catalyze a reaction in the presence of the target (in the case of nucleic acid enzyme selection), are separated by various techniques such as gel electrophoresis, column separation, and capillary electrophoresis. These “winners” are amplified using polymerase chain reactions (PCR) and used for the next round of selection. During each round of selection, the stringency can be increased by decreasing the interaction time between the target and the nucleic acid, or by decreasing the concentration of the target. Iterative rounds of selection are continued until the pool is sufficiently enriched with sequences of desired sensitivity and specificity (represented by blue cubes in Fig. 5.1A). This technique is particularly powerful as it provides a method for improving the specificity for a given target by incorporating rounds of negative selection, wherein the pool is incubated with potentially competing targets and the sequences that interact with these are removed from the pool [13]. Finally, the winner molecules that are isolated are identified by sequencing, and after some further biochemical and/or spectroscopic characterization they are used for different applications, particularly sensing [12,14–20], therapeutics [21–24], materials science, and nanotechnology [25–27].

5.2.2 Analytes or contaminants recognized selectively by functional nucleic acids
In vitro selection has been used to obtain a number of metal specific DNAzymes, such DNAzymes that are dependent on Pb2+ [28,29], Zn2+ [30], Co2+ [13], Cu2+ [31], UO22+ [32], Hg2+ [33], As5+ [33], some of which have been converted into fluorescent and colorimetric sensors as described in the following sections. A number of these metal ions, notably Pb2+, Hg2+, As5+, are heavy metalions that are particularly toxic; UO22+ is a radionuclide. The maximum contamination level of these metal ions in drinking water is strictly regulated by the US EPA and few sensors can detect metal ions below those levels, and selectivity of those sensors should also be improved in order to be practically applicable. Therefore the utility of DNAzymes as toxic metal sensors is of great importance. The predicted secondary structures of a few DNAzymes are shown in Fig. 5.1B–E. The strands in green represent the enzyme and the strands in black are the nucleic acid substrate. All the DNAzymes are RNA cleaving enzymes that catalyze the cleavage of a single ribo-linkage (represented by the arrow) embedded in the DNA substrate. Some of the fastest DNAzymes have a catalytic efficiency (kcat/Km) of 109 M−1 min−1 [34], rivaling that of protein enzymes and thus they are ideal for fast sensing.
The list of aptamers obtained by in vitro selection is even longer, and more importantly the analytes recognized are far more diverse and include small molecules, antibiotics, proteins, nucleotides, and even viruses and bacteria cells and other microbes. As the nucleic acid equivalent of antibodies, this flexibility in the choice of targets for which aptamers can be obtained is a competitive advantage over antibodies for sensing applications [35,36]. Antibodies, on the other hand, cannot be raised against molecules too small to generate enough binding repertoires (e.g., metal ions not associated with any chelators), or compounds or proteins with poor immunogenic properties or with high toxicity. An online searchable aptamer database that contains detailed information of aptamer sequences for different analytes has been constructed by Ellington and coworkers [37]. Although a large majority of research in the field of aptamers is devoted to aptamers for therapeutic applications [21–24], their utility in nanotechnology and sensing has been widely explored [12,14–20].
Table 5.1 is a partial list of literature-reported functional nucleic acid targets that are considered contaminants in water or are being investigated as contaminants. The sensing of many pharmaceutical compounds, hormones, and receptors that are considered as emerging water contaminants benefit from the research into aptamers for therapeutic applications. Also, as can be seen in Table 5.1, aptamers are being developed for binding specifically bacterial cells, viral spores, and toxins that can be used as biological warfare agents.
Table 5.1
Partial List of Literature-Reported Functional Nucleic Acids that Target Water Contaminants or Water Contaminant Candidates
| Contaminant Type | Examples and References |
| Metal ions | Pb2+ [28,29], Cu2+ [31], UO22+ [32], Hg2+ [33,38,39], As5+ [33], Zn2+ [30] |
| Radionuclides | UO22+ [32] |
| Toxins | Ricin [40], Abrin toxin [41], Microcystin [42] |
| Antibiotics | Vasopressin [43], Streptomycin [44], Tetracycline [45], Viomycin [46], Chloramphenicol [47] |
| Endocrine disrupting compounds and hormones | 17β -estradiol [48], Thyroxine hormone [49,50] |
| Protein | HA1 proteins of H5N1 influenza virus [51] |
| Other small organic molecules | Cocaine [15], Cholic acid [52], (R)-thalidomide [53], Ethanolamine [54] |
| Cells and bacteria | Anthrax spores [55], Campylobacter jejuni [56] |
It is quite clear that functional nucleic acids provide a unique recognition platform for a large range of different contaminants that are already known and are emerging every year.
5.3 Functional nucleic acid-directed assembly of nanomaterials for sensing contaminants
Since natural nucleic acids do not possess functional groups that can generate absorption in the visible region or fluorescence, external signaling labels need to be applied to convert them into sensors. To achieve this goal, many organic fluorophores and inorganic nanoparticles, which are summarized in the next few sections, have been employed.
5.3.1 Fluorescent sensors
Fluorescent sensors provide an opportunity for on-site and real time sensing as recent advances have led to development of hand-held fluorimeters that can be used as a stand-alone device or connected to a laptop computer. Also, fluorescent sensors have the advantage of high sensitivity [57–60].
5.3.1.1 Sensing metal ions using DNAzyme based fluorescent sensors
Many metal specific DNAzymes have been successfully converted into fluorescent sensors using a catalytic beacon technology [61]. The general design is illustrated in Fig. 5.2A. The catalytic beacon is engineered to place a fluorophore (green sphere) on one substrate arm and a quencher (brown sphere) on the enzyme arm. When the sensor is assembled, the fluorescence emission is quenched due to the proximity of the quencher to the fluorophore, brought about by DNA hybridization. A second quencher on the other arm of the substrate helps to reduce background fluorescence arising from non-hybridized substrate. In the presence of contaminant metal ion, the cleavage reaction causes the cleaved substrate fragment containing the fluorophore to be released into solution, thus enhancing the fluorescence strongly. The 17E DNAzyme was converted into a Pb2+ sensor with a detection limit of 10 nM [29], which is lower than the US EPA threshold for Pb2+ in water, set at 75 nM. Additionally, this sensor is over 80-fold more specific for Pb2+ over highly competing metal ions Co2+ and Zn2+ and over 1,000-fold more specific over other divalent metal ions including Mg2+ and Ca2+ that are found in water. Figure 5.2B is the fluorescent image of a microwell plate where the 17E DNAzyme is incubated with varying concentrations of Pb2+ and other competing metal ions. Concentration dependent enhanced fluorescence is only seen for Pb2+. This technique has been tested on real-world water samples, such as from Lake Michigan, spiked with Pb2+.

Following this, a number of other ions, UO22+ [32], Hg2+ [39], and Cu2+ [62], were converted into sensitive and selective fluorescent sensors. The performance of the UO22+ sensor is particularly impressive as the detection limit is reported to be 45 pM, which is not only lower than the EPA threshold (130 nM), but remarkably it is also lower than the detection limit for the widely used instrumental technique, ICP. Furthermore, the sensor has also been used to detect UO22+ extracted from soil of a nuclear waste site, as these sites are dangerous sources for contamination of groundwater and surface water with radionuclides.
A number of other designs for the position of the fluorophore–quencher have also been investigated and resulted in improvements in sensor designs [63]. In addition, new DNAzymes with fluorescently modified nucleotides were isolated by Li and coworkers [64,65] and utilized for metal sensing [66].
5.3.1.2 Sensing organic and biological molecules using aptamer-based fluorescent sensors
A general approach for converting aptamers into fluorescent sensors using the change in secondary structure of the aptamer upon target binding was reported by Li and coworkers (Fig. 5.2C). This method has been termed “structure switching signaling aptamers”[16]. A tripartite assembly is made between a fluorophore (green sphere) labeled DNA, a quencher (brown sphere) labeled DNA, and another linker DNA strand that contains the aptamer sequence and hybridizes to both the other strands, bringing the fluorophore and quencher into close proximity, which leads to fluorescence quenching. Introduction of the target causes the aptamer to wrap around the target causing the disruption of base pairing interactions between the quencher containing DNA and the linker, thereby leading to its dissociation and subsequent fluorescence enhancement. A number of alternates to this design have been reviewed [58] and aptamer based fluorescent sensors have been demonstrated for organic molecules, proteins, and cells.
5.3.2 Colorimetric sensors
Although fluorescent sensors are a very sensitive method for contaminant detection, they still require an instrument and the organic fluorophores can photo bleach relatively quickly. Colorimetric sensors eliminate the need for instruments and the following two sections discuss the use of metallic nanoparticles for the development of these sensors.
5.3.2.1 Sensing metal ions using DNAzyme/gold nanoparticle-based colorimetric sensors
Gold nanoparticles (AuNPs) make an attractive candidate for colorimetric labels as they have a very strong extinction coefficient (108 Σ cm−1 for 13 nm AuNPs, which is about three orders of magnitude higher than the best organic chromophores) and they display distance dependent optical properties. Dispersed nanoparticles are red in color, whereas aggregated nanoparticles are blue/purple in color. A quantitative analysis can be carried out by measuring the absorbance of the samples, for which portable colorimeters are also available. In 1996, Mirkin and coworkers utilized the DNA induced assembly of AuNPs to make a colorimetric sensor for nucleic acids [67]. Lu and coworkers expanded the scope of sensing to analytes beyond nucleic acids, by combining AuNPs with DNAzymes [68–71]. The sensing method is depicted using the Pb2+ dependent 17E DNAzyme as a representative example (Fig. 5.3A). AuNPs functionalized with short thiol modified DNA are assembled on the arms of the extended substrate, which is in turn hybridized to the enzyme. Since each AuNP is functionalized with many DNA strands, blue aggregates are formed. In the presence of Pb2+, the enzyme catalyzed cleavage of the substrate will disassemble the aggregate producing red color. The color can be spotted on a thin layer chromatography (TLC) plate and one such representative test is shown in the inset of Fig. 5.3A. Red color of increasing intensity is seen with Pb2+, whereas the sensors containing other metals are blue. The reaction is fast with color change occurring within 10 minutes under optimized conditions and the detection limit is approximately 100 nM.
A unique feature of this sensor is that the dynamic range of the sensor can be tuned by careful mutation of the DNA sequence, which is very useful for making sensor systems that can change colors at different threshold levels that match the maximum contaminant levels (MCLs) defined by EPA or CDC. The dynamic range of the Pb2+ sensor was shifted from 10 to 100 ∝M Pb2+ using this strategy [68].
5.3.2.2 Sensing organic and biological molecules using aptamer/gold nanoparticle-based colorimetric sensors
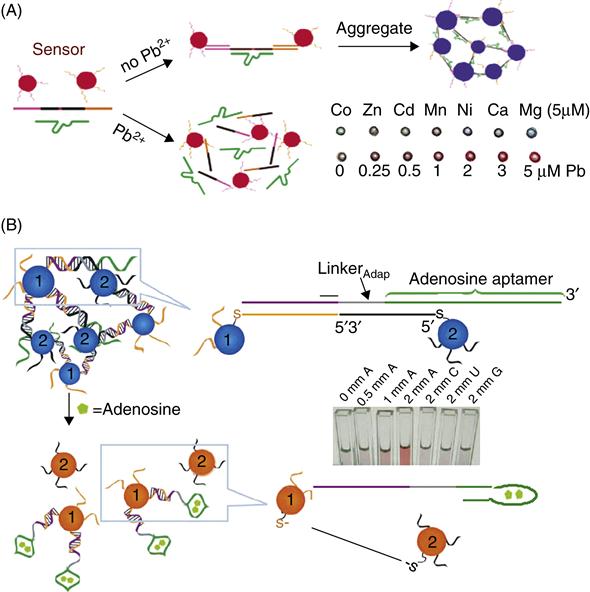
To detect contaminants beyond metal ions, aptamers have been used instead for directed assembly of nanomaterials such as gold nanoparticles [19]. Figure 5.3B is an illustration of the detection scheme using an adenosine (A) aptamer. Two types of oligonucleotide functionalized AuNPs (particles 1 and 2) are assembled on a linker DNA consisting of the adenosine aptamer. The addition of adenosine induces structure switching, leading to disassembly of the blue aggregate, producing a red color characteristic of dispersed AuNPs, which is not seen in the presence of control analytes (C), cytidine (C), uridine (U), and guanine (G) (inset of Fig. 5.3B). The generality of this method has been demonstrated by making a cocaine sensor in the same manner. Sensors that respond to multiple chemical stimuli have also been constructed by combining two aptamers in the same system [72] such that the sensor responds either in the presence of both analytes just one of the analytes.
Recently, a new method has been reported for colorimetric sensing utilizing AuNPs, called the label free method [73]. Here the AuNPs do not need to be functionalized with thiol modified DNA, and thus there can be significant reduction in sensing costs as the DNA utilized will not require the chemical (thiol) modification required for attaching DNA to AuNPs. This method is based on the principle that single stranded DNA (ssDNA) can adsorb on AuNPs more effectively and therefore protect them from salt induced aggregation to a greater extent as compared to double stranded DNA or structured DNA, such as quadruplex DNA. Aptamer-based sensing has been demonstrated for some analytes by utilizing the change in the DNA secondary structure upon target binding [74–76].
5.4 Simultaneous multiplexed detection using quantum dots and gold nanoparticles
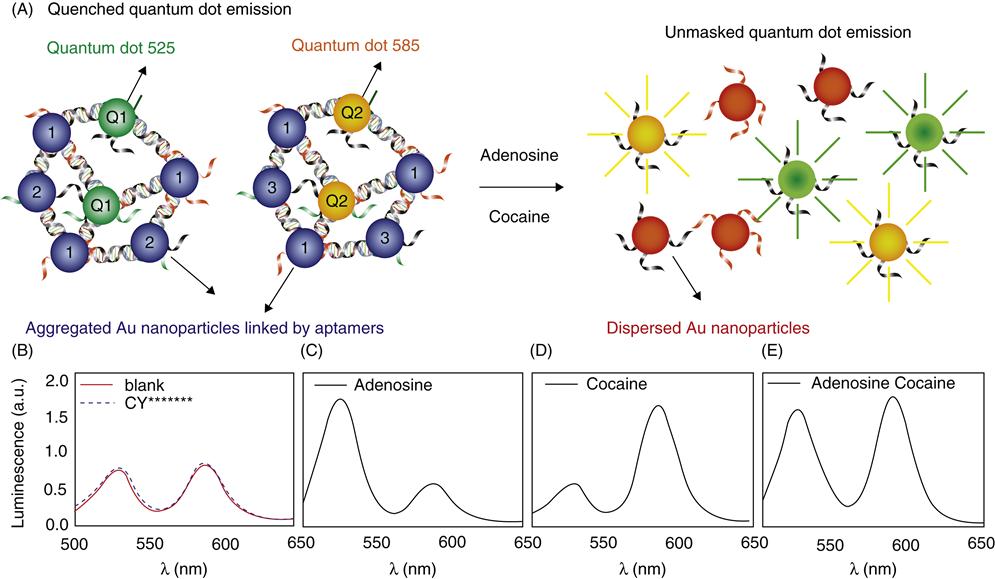
In order to probe complex chemical environments, it may be desirable to construct materials that are responsive to multiple chemicals in the same system, producing a unique signal for each chemical. Semiconductor quantum dots (QDs) are ideal as the emission wavelength of QDs can be tuned by varying their size, shape, and chemical composition, while keeping the excitation wavelength the same, thus making it possible to have QDs with different emission wavelengths to encode for different analytes. Multiplexed detection of four toxins was previously achieved using QDs linked to antibodies [77]. Liu et al. combined QDs and AuNPs with aptamer technology to demonstrate one-pot simultaneous detection of two analytes, adenosine, and cocaine (Fig. 5.4A) [78]. The detection scheme is based on disassembly of particles as described in Section 5.3.2. For adenosine detection, in addition to AuNPs 1 and 2, QD1 (emission at 525 nm), which is functionalized with the same DNA as AuNP 2, was also incorporated to make aggregates using the linker containing adenosine aptamer. In the assembled state, the emission of the QD was quenched because of energy transfer to nearby AuNPs [79–83]. The addition of adenosine disassembles the nanoparticles, giving increased emission intensity at 525 nm. Similarly, cocaine sensors were also prepared with QD2 (emission at 585 nm). When the sensors are mixed, different QDs can be excited at the same wavelength, as shown in Fig. 5.4B: two emission peaks at 525 and 585 nm were observed (solid line), corresponding to the adenosine and cocaine sensors, respectively. Addition of cytidine and uridine (dashed line) did not change the emission intensity of either peak. The addition of adenosine alone increased only the 525 nm peak (Fig. 5.4C); the addition of cocaine alone only increased the 585 nm peak (Fig. 5.4D); and the addition of both analytes resulted in enhancements in both peaks (Fig. 5.4E).
5.5 Sensors on solid supports
5.5.1 Dipsticks
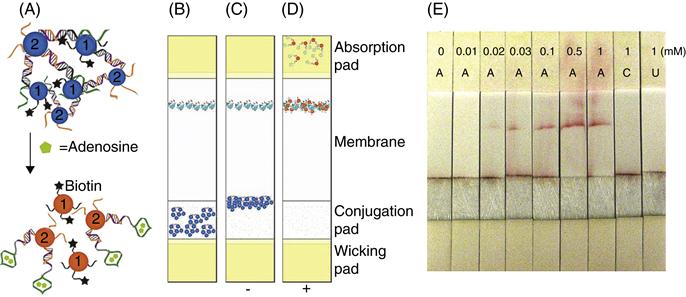
Although the colorimetric sensors provide a method of qualitative or semi-quantitative sensing without the requirement of an instrument, they still need precise transfer of solutions in small (usually microliter) quantities, which can be difficult for people without scientific training. In order to alleviate this problem, the aptamer–nanoparticle-based colorimetric tests can be converted into user-friendly “dipstick” tests using lateral flow devices that provide the reagents in a dry or nearly dry state immobilized on a pad. Several antibody-based dipstick tests are known, the home pregnancy test being one of the most common uses of this technology. The detection of DNA using lateral flow devices has also been demonstrated [84]. By utilizing the lateral flow devices for aptamer-based detection, the range of analytes that can be detected using this simple platform has been expanded [85]. A lateral flow device is constructed using four overlapping pads (wicking pad, conjugation pad, membrane, and absorption pad) placed on a plastic backing. The adenosine sensor consisted of the same components as described in Section 5.3.2, except in this case approximately 50 percent of DNA on particles 1 contained a biotin moiety, denoted by a black star (Fig. 5.5A). AuNP aggregates are dried on the conjugation pad of the devise and streptavidin is applied on the membrane (Fig. 5.5B). When the device is dipped into a solution without adenosine, the rehydrated aggregates migrate to the bottom of the membrane where they stop because of their large micrometer size (Fig. 5.5C). In the presence of adenosine, the dispersed nanoparticles [16,19] can migrate along the membrane and be captured by streptavidin to form a red line (Fig. 5.5D). Representative test results are depicted in Fig. 5.5E, where more intense red lines are seen with increasing concentrations of adenosine, but not with cytidine or uridine. These devices are also more sensitive than solution-based tests owing to the integration of binding, separation, and detection on a simple test-paper-like platform with no background interference.
5.5.2 Incorporation of sensors into devices
Whereas the functional nucleic acids described earlier are excellent for on-site and real-time applications, most sensing formats are for one-time use. In some applications, long-term and unattended monitoring is required. Furthermore, despite the fact that most sensors have quite high sensitivity and low detection limit, it is desirable to improve the detection limit even further. The immobilization of the fluorescent DNAzyme based sensors on gold surface and into micro- or nanofluidic devices has made it possible to achieve the goals mentioned earlier. For example, the detection limit for the fluorescent Pb2+ sensor was improved from 10 to 1 nM by reducing background noise, improving the signal to noise ratio. This was accomplished by rinsing away unhybridized DNA substrate from the immobilized DNAzyme sensor prior to sensing [86,87]. Furthermore, these sensors were also incorporated into micro- or nanofluidic devices, which require low sample volumes, low amounts of detection reagents, and which provide a possibility of facile regeneration and enhance the real-time long-term monitoring capability [88,89].
5.6 Other sensing schemes utilizing electrochemistry and magnetic resonance imaging
It is important to mention that apart from colorimetric and fluorescent sensors, other sensing methodologies are also being explored. Functional nucleic acids have been labeled with redox active groups, such that target binding produces an electrochemical signal (reviewed by Willner and Zayats [20]). For example, in one particular realization of this technology, an aptamer immobilized on an electrode is labeled with a redox active group such as ferrocene or methylene blue. Structural change due to target binding can bring this label closer to the electrode leading to greater electron transfer and thus an electrochemical signal [90–93]. The advantages of these sensors are that electronic devices can be miniaturized easily and these sensors can often be regenerated.
Recently, aptamers have also been combined with magnetic particles (superparamagnetic iron oxide nanoparticles or SPIO), which are contrast agents, for the purpose of constructing magnetic resonance imaging (MRI) sensors [94,95]. This method utilizes the change in the T2 relaxation time of the medium when the aptamer assembled SPIO goes from an aggregated (low T2, darker MR image) to a dispersed state (high T2, brighter MR image) in the presence of the target. Although MR imaging may not be feasible for on-site monitoring, this method has the potential to be used for three-dimensional imaging in environmental monitoring, such as mapping the removal of contaminants during water purification using columns. Additionally, since MR imaging depends on a nuclear signal, it is less likely to have interference from turbid matrices that can mask fluorescent or colorimetric signals.
5.7 Conclusions and future perspective
Since the development of in vitro selection in the 1990s to obtain functional nucleic acids with desired recognition properties, a number of sensing applications have emerged very rapidly. A variety of materials and devices have been successfully developed based on functional nucleic acids for the detection of trace contaminants in water. This chapter highlighted some of the major strengths of this novel approach of combining nucleic acids with nanotechnology: (1) Nucleic acids provide a general class of molecules that can be selected to recognize a variety of different contaminants. (2) In vitro selection can be utilized to tailor the sensitivity and specificity of the nucleic acid for the contaminant, so as to obtain better sensors. (3) Functional nucleic acids can be readily labeled with fluorophores and inorganic nanoparticles to obtain sensors with tunable dynamic ranges. (4) The sensors can be assembled into dipstick tests and devices for ease of use, longer shelf life, and regeneration.
In spite of the generality of nucleic acid sensors, there still exist challenges in selecting nucleic acids for certain kinds of analytes, such as anions like perchlorate or nitrate, which are negatively charged and thus are repelled by the negatively charged backbone of nucleic acids. It is important to explore newer selection strategies to overcome these and further expand the repertoire of analytes recognized.
Finally, further development in this field would require development of sensor arrays containing nucleic acids that recognize many contaminants for simultaneous monitoring purposes, similar to the microarray technology commonly used for nucleic acid detection.
Acknowledgments
The authors would like to thank Dr Daryl P. Wernette for assistance with the figures. This material is based upon work supported by the US National Science Foundation through the Science and Technology Center of Advanced Materials for the Purification of Water with Systems (WaterCAMPWS, CTS-0120978), the Strategic Environmental Research and Development Program, the U.S. Department of Energy (DE-FG02–08ER64568), and the the U.S. National Institute of Health (ES016865).