Nanotechnology for Contaminated Subsurface Remediation
Possibilities and Challenges
Denis M. O’Carroll, Department of Civil and Environmental Engineering, The University of Western Ontario, London, Ontario, Canada
Groundwater represents a significant source of potable and industrial process water throughout the world. With population growth the availability of this precise resource is becoming increasingly scarce. Historically, the subsurface was thought to act as a natural filter of wastes injected into the ground. The potential for these wastes to persist in the subsurface for decades, potentially contaminating drinking water sources was ignored. Not only do toxic compounds have significant detrimental impacts on the environment and human health, there are also economic and social costs associated with contaminated groundwater. Due to increased demands on groundwater resources and historical contamination there is a need to remediate contaminated groundwater to meet current and future demands. At many hazardous sites, however, current remediation technologies routinely defy attempts at satisfactory restoration. As a result new, innovative remediation technologies are required.
Nanomaterials are receiving widespread interest in a variety of fields due to their unique, beneficial chemical, physical, and mechanical properties. They have recently been proposed to address a number of environmental problems including the remediation of the contaminated subsurface. A wide variety of nanoparticles, such as metallic (e.g., zero valent iron or bimetallic nanoparticles) and carbon based nanoparticles (e.g., C60 nanoparticles) have been investigated to assess their potential for contaminated site remediation. Studies suggest that nanoparticles have the ability to convert or sequester a wide variety of subsurface contaminants (e.g., chlorinated solvents and heavy metals). In addition they are more reactive than similar, larger sized, reactive materials. The majority of these studies have, however, been conducted at the batch scale. Considerable work is necessary prior to the application of nanotechnology for contaminated site remediation. One problem, for example, is the delivery of reactive nanometals to the contaminated source zone where they will react. This chapter will summarize the use of nanoparticles for contaminated site remediation and highlight some of the challenges that remain unresolved.
Keywords
Groundwater; potable water; industrial process water; underground injection; contaminated groundwater; groundwater remediation
28.1 Introduction
Groundwater is an important source of water throughout the world and as such, its protection is paramount. It is estimated that groundwater sources provide between 95 and 100 percent of potable water supplies in some developing countries (e.g., Oman and Cuba) whereas most regions of the world obtain 30–50 percent of their potable water supplies from groundwater sources [1,2]. Specifically, 70–90 percent of drinking water supplies come from groundwater sources in Central America and 70 percent from groundwater sources in China [2,3]. In arid regions such as Northern China where groundwater sources represents 87 percent of the total water consumed in 27 cities, this resource is vital [4]. Even in Canada, a country with abundant surface water resources, 30 percent of the population rely on groundwater for domestic use [5]. In the United States approximately one-quarter of consumed fresh water comes from groundwater sources [6]. Despite its importance throughout the developed and developing world, contamination of drinking water resources by natural and anthropogenic substances is common. For example it is estimated that 90 percent of groundwater in Chinese cities is contaminated to some extent and 61 percent of tested wells in Nicaragua were contaminated with pesticides above United States Environmental Protection Agency (US EPA) drinking water standards [2,3]. This contamination causes serious health and environmental problems. Furthermore, brownfield sites, abandoned properties with soil and groundwater contamination, severely limit redevelopment and reinvigoration of many urban cores. Contamination of water resources, therefore, has significant health, ecological, and economic societal impacts.
28.2 Sources of groundwater contamination and remediation costs
From the industrial revolution until the 1970s, vast amounts of hazardous waste and spent chemicals were disposed directly to the subsurface with the belief that the subsurface acted as a natural filter of disposed chemicals. In the United States it was not until an abnormally high number of birth defects and miscarriages were found in Love Canal, New York, that the public became aware of the impacts of improper contaminant disposal practices. Many of the chemicals historically disposed of to the subsurface are particularly persistent in the subsurface, frequently contaminating water sources for decades or centuries. As an example of the magnitude of the problem, Canada is home to an estimated thirty thousand brownfield sites, many of which exhibit contamination of soil and groundwater by hazardous industrial chemicals. The restoration of these brownfield sites has been identified as critical to the health and overall sustainability of Canada, by providing significant economic, environmental, and social benefits [7]. It is estimated that for every dollar spent on restoration and redevelopment, this investment yields a fourfold return in terms of economic benefit to the Canadian economy (direct and indirect), providing up to seven billion dollars annually in expected net national public benefit [8]. To put this benefit in context, it represents approximately 0.6 percent of Canada’s gross domestic product [9]. A 1997 US National Research Council report suggests that there are between three hundred thousand and four hundred thousand contaminated sites in the United States requiring cleanup [10]. Furthermore it is estimated that there are over thirty thousand dense nonaqueous phase liquid (DNAPL) contaminated sites in the United States including twenty thousand sites at existing or former dry cleaner installations [11]. These DNAPL sites represent a subset of the larger number of brownfield sites in the United States and are often considered the most persistent and problematic sites. In 1996 approximately $9 billion were spent on environmental remediation [10]. To restore brownfield sites the US EPA Superfund Program in 2006 alone obligated $540 million for the construction and active remediation of contaminated sites where no other responsible parties could pay for remedial activities. Of this amount 45 percent went to only 14 sites, or an average of $17 million per year for each of these 14 sites. If remedial activities from the private sector were also included in this figure the total spent on remediation in the United States would increase considerably, as indicated by the 1996 figure.
Anthropogenic groundwater contaminants were often generated in various industrial processes prior to their disposal to the subsurface. For example chemical solvents were commonly used to clean cutting tools, entraining small heavy metal fragments from the cutting process and generating significant quantities of waste liquids. A survey of 91 US Department of Energy (DOE) wastes sites found that 20 percent of their sites were contaminated with this complex waste mixture [12]. Other industrial and commercial uses of common groundwater contaminants include the use of polychlorinated biphenyls (PCB) in electrical transformers, heavy metals as paint additives or in metal plating and smelting operations, pesticides for agriculture, and chlorinated solvents in dry cleaning installations [10]. All of these operations have caused subsurface contamination due to their improper disposal of hazardous chemicals. Common disposal practices included accidental releases (e.g., due to leaky underground storage tanks or compromised landfill liners) and intentional releases (e.g., underground storage systems designed to slowly leach liquids into the subsurface, subsurface injection wells, or land application of contaminants) [10,11,13]. These contaminants can cause serious health problems and even death. For example, PCBs, and the chlorinated solvent and DNAPL, trichloroethylene (TCE), are carcinogens [14,15]; pesticides have been found to cause birth defects [16], and lead can impair organ development in fetuses [17]. Unfortunately groundwater contamination of drinking water sources is widespread in the developing and developed world due to these poor disposal practices, causing significant health problems. As a result the development of innovative remediation technologies is an active area of research.
28.3 Remediation alternatives
Substantial advances in our understanding of the phenomena governing subsurface remediation have been made and a number of innovative remediation technologies have been developed, such as steam and brine flooding or density modified displacement [18–20]. A 2005 US National Research Council report grouped remediation technologies of DNAPL and chemical explosive contaminated sites into two classifications, extraction or transformation [11]. Many of these remediation alternatives could be used for a variety of other classes of subsurface contaminants. Extraction refers to the removal of the contaminant from the subsurface for subsequent aboveground treatment and disposal. Transformation refers to the conversion of hazardous subsurface contaminants to more benign daughter products or forms. Examples of extraction technologies include excavation and pump and treat, and examples of transformation technologies include chemical oxidation and enhanced bioremediation. This report concluded that, for the reviewed technologies, contaminant mass removal did not significantly reduce toxicity or mass flux from the contaminant source zone. Furthermore, the report found that no large-scale DNAPL contaminated site has been cleaned up such that the aquifer water meets drinking water standards. This report did not include the potential for nanotechnology-based remediation alternatives in its assessment. Based on this report and other similar studies, existing technologies for the remediation of more persistent contaminants (i.e., chlorinated solvents and heavy metals) are rarely able to clean up contaminated sites such that the water source meets drinking water objectives at the completion of remedial activities. The problem relates to the inability of existing remedial technologies to remove enough contaminant mass in the subsurface to significantly reduce dissolved aqueous phase concentrations. The decision to remediate contaminated sites is, therefore, still a source of considerable debate despite over two decades of active research and development [11,21,22]. The development of new and innovative remediation technologies is, therefore, crucial to achieve clean up goals at contaminated sites.
28.4 Contaminated site remediation via reactive nanomaterials
Nanoparticles are receiving widespread interest in a variety of fields due to their unique, beneficial chemical, physical, and mechanical properties. They have recently been proposed to address a variety of environmental problems including the treatment of surface water, groundwater, and industrial wastewaters containing a range of organic, inorganic, and microbial contaminants [23]. With regards to contaminated site remediation they have tremendous potential to remediate a wide variety of common subsurface contaminants. For example, carbon nanotubes have been found to have a heavy metal adsorption capacity five times greater than that of granular activated carbon [24]. Their ability to adsorb organic contaminants such as PAHs [25] and dichlorobenzene [26], both US EPA priority pollutants, has also been evaluated. Reactive nanometals, such as nanoscale zero valent iron (nZVI), are also the focus of significant research for the remediation of environmental contaminants (e.g., [27–35]). A number of studies have found that reaction rates are much faster for nanometer scale metals when compared to larger, micrometer or millimeter scale metals and that the nanometer scale metals can degrade a wider range of contaminants (e.g., [28,34,36]). Furthermore the incorporation of a second noble metal catalyst in the zero valent nanometal has been shown to significantly improve degradation rates beyond that of a single zero valent nanometal [36,37]. Contaminants that can be remediated using nanometals include PCBs, chlorinated ethenes (e.g., TCE), chlorinated ethanes [e.g., hexachloroethane (HCA), pentachloroethane (PCA)], and heavy metals (e.g., ASIII) [28,33,34,38,39]. However, there are still a number of common contaminants found at sites requiring remediation that, thus far, cannot be degraded using nanoparticles. For example, 1,1-dichloroethane and 1,2-dichloroethane, both EPA priority pollutants, to date have not been dechlorinated using reactive nanometals [39].
Much of the work investigating the applicability of nanoparticles for remediation has been completed in small bench scale experiments under ideal conditions (e.g., using DI water and dissolved phase single component contaminants) and low contaminant concentrations. At many sites, however, NAPLs were not disposed of as pure liquids, but in acidic or basic mixtures sometimes containing surface active compounds [12,13,40]. A review of Sloat [13] provides a good perspective of common disposal practices in the 1960s. Sloat [13] discusses, in great detail, the composition of waste liquids (e.g., fabrication oil comprised of 75 percent carbon tetrachloride and 25 percent lard oil) that were improperly disposed of to the subsurface at the Hanford Low Level Waste Management site. Another complicating factor that needs to be investigated regarding contaminated site remediation using nanoparticles is the presence of chemical heterogeneities in the subsurface (e.g., natural anions and cations dissolved in water as well as soil surface constituents). A limited amount of studies have been published related to the field application of reactive nanometals and their associated complexities for chlorinated hydrocarbon remediation (e.g., [30,41,42]). Each study reported significant chlorinated hydrocarbon reductions; however, significant questions remained at the completion of these field trials including the mobility of the nanoparticles in the field, the premature passivation of the reactive nanometal and the potential appearance of more toxic degradation products. The complexities associated with nanometal remediation under field conditions have also been the focus of controlled laboratory experiments [35,43]. For example Lien et al. [35] investigated the ability of nZVI to remediate a mixture of carbon tetrachloride and heavy metals. As discussed earlier, mixtures of chlorinated hydrocarbons and heavy metals are common at many DOE contaminated facilities [12]. Lien et al. [12] found that different heavy metals affected the dechlorination rate of carbon tetrachloride as well as the degradation products. In another study Liu et al. [43] found that aqueous phase anions commonly found in the field, such as nitrate, decrease TCE dechlorination rates due to surface passivation of nZVI. In their study Liu et al. [43] also investigated nZVI promoted dechlorination rates as TCE concentrations approached their solubility limit. TCE concentrations would be expected to approach the solubility limit near the contaminated source zone. They found that dechlorination rates were slightly reduced as TCE approached the solubility limit. Significant work is needed beyond these studies to fully understand remediation via reactive nanometals under the complex conditions found at the field scale to fully take advantage of the tremendous opportunities nanotechnology offers.
28.5 Example of contaminated site remediation via reactive nanometals
An understanding of the subsurface contaminant architecture is also important for the design of efficient remediation alternatives. One significant challenge faced by those developing site remediation plans is actually locating the contaminated source zone, if one exists. As an example, consider the migration of a chlorinated solvent, a DNAPL, from a leaking underground storage tank (Fig. 28.1). Chlorinated solvents are the most common groundwater contaminants at US hazardous waste sites [44]. The migration and ultimate entrapment of NAPLs are governed by gravitational, viscous, and capillary forces [45]. Chlorinated solvents are denser than water and thus migrate vertically downward through the unsaturated and saturated zones following their release to subsurface environments and only coming to rest once they have reached an impervious layer (e.g., clay or bedrock), as illustrated in Fig. 28.1A. However, a fraction of the disposed DNAPL may reside in high saturation pools on lenses of impervious soils above the impervious layer or may be retained in residual ganglia between the DNAPL release point and the impervious layer. Remediation strategies need to target both residual ganglia as well as pooled DNAPLs. The application of reactive nanoparticle remediation for contaminated DNAPL source zones such as this can be broken down into a series of steps: the transport of the nanoparticles, with the bulk aqueous phase (or some delivery fluid) (Fig. 28.1A), to the DNAPL contaminated zone, their partitioning to the DNAPL/aqueous phase interface (Fig. 28.1B) and their reaction with and degradation of the DNAPL while avoiding the generation of more toxic daughter products (Fig. 28.1C). It is clear that reactive nanoparticles have significant potential to complete step three of this process as the work completed to date investigating remediation using reactive nanometals has shown they can significantly reduce dissolved aqueous phase contaminant concentrations. It should be pointed out that they have, thus far, been unable to significantly reduce source zone free phase NAPL mass [46]. Significant reductions in source zone free phase NAPL mass are necessary to significantly reduce the risk to downstream groundwater receptors. Further work is necessary for the optimized delivery of nanoparticles to the source zone (step one) and their partition to the NAPL/water interface (step two).

Recent work has focused on the development of surface modified nanometals (e.g., [27,29,33,46–49]) for optimum transport of reactive nanometals to the contaminated source zone. Although reactive nanometals are quite reactive they are not stable in aqueous phase solutions without some modification (e.g., [27,46,49]). These studies suggest that it will be possible to develop surface modified reactive nanometals that can be transported, with the aqueous phase, to the contaminated source zone. Further work, however, is necessary as many of these studies were conducted in simplified systems and their transport through permeable media systems has not been adequately evaluated. For example controlled nanometal transport column experiments are typically on the decimeter scale (e.g., [27,48]) however nanometals will likely have to travel at least one or two orders of magnitude more at the field scale. Therefore even a small fraction of nanometal loss from the aqueous phase at the decimeter scale may become quite important at the field scale as it could translate into significant nanometal loss from the aqueous phase prior to reaching the contaminated source zone. In addition to experimental transport studies the ability to predict nanoparticle mobility is a prerequisite to the design of nanometal delivery systems for source zone remediation. The limited nanoparticle mobility studies published to date (e.g., [27,50]) have used predictive models originally developed for colloidal transport or filtration in porous media systems (e.g., [51,52]). Further study is necessary to determine if these models are appropriate for reactive nanometal transport at the field scale. Reactive nanometals may be removed from the aqueous phase due to deposition on the soil surface and straining (retention at grain/grain intersections). Traditional colloid filtration theory predicts colloid removal due to deposition on the solid surface but does not incorporate straining. A recent study by Saleh et al. [29] found that straining is an important nanometal removal mechanism and suggested that traditional colloid filtration theory may not be appropriate for the prediction of nanometal transport in the subsurface.
The second important step in the application of reactive nanoparticle remediation for contaminated DNAPL source zones, partitioning to the NAPL/water interface, has received much less attention than the other two steps. For reactive nanometals to realize their full DNAPL source zone remediation potential, they must either partition to the DNAPL/water interface or reside in the immediate vicinity. To address this issue Saleh et al. [46] synthesized nZVI on which surface active triblock copolymers were anchored. This structure facilitates nanoparticle transport to the NAPL/aqueous phase interface where the zero valent iron core can degrade the chlorinated solvent. In a subsequent study Saleh et al. [29] emplaced a NAPL in their column transport experiments and suggested that selection of a polymer with an appropriate hydrophobe/hydrophile ratio is vital for optimal partitioning. The encapsulation of nZVI in a nonionic surfactant sorbitan triolate emulsifier has also been used at a field trial to enhance partitioning to the NAPL/water interface [41]. In addition the emulsifier was designed to protect nZVI from reacting with groundwater constituents that would decrease its reducing capacity. To date our ability to precisely design and synthesize reactive nanometals such that they partition to the NAPL/water interface, while still being mobile and able to degrade the contaminants of choice, is lacking and further research is necessary.
Nanotechnology based remediation technologies offer tremendous potential for contaminated site cleanup. There are, however, challenges that need to be addressed prior to the widespread usage of nanotechnology based remediation technologies. As an example of the conditions that could be encountered at a DNAPL contaminated site the multiphase flow and contaminant transport numerical simulators M-VALOR [53,54] and MISER [55,56] were used to model a hypothetical DNAPL release, redistribution, and subsequent water flood. The water flood simulations give an estimate of the mean arrival time of an injected nanometal at the source zone. The reactive lifetime of nZVI is a concern as they can be rapidly corroded, thereby losing their reactivity (e.g., [37,57]). Furthermore nZVI reacts with natural groundwater constituents, decreasing its reducing capacity prior to reaching the source zone [41,43]. The selected DNAPL was tetrachloroethylene (PCE), a US EPA priority pollutant. PCE was released into a two-dimensional heterogeneous domain (7.925 m wide by 9.754 m deep) at equivalent rates of 0.08 L/d and 0.27 L/d (cases a and b, respectively) for 400 days followed by 300 days of redistribution. Soil properties for the simulations are based on a geostatistical representation of a surfactant enhanced aquifer remediation demonstration site in Oscoda, MI [58–62]. Soil permeabilities of the two-dimensional domain are presented in Fig. 28.2. As illustrated in this figure some soil layering exists at this site and soil characteristics at the site are only moderately heterogeneous. This site was subject to extensive site characterization with 14 vertical and angled cores. Grain size distributions of 167 subsamples, subdivided from the 14 core samples, were quantified and used to estimate soil sample permeability using the Carman–Kozeny equation [58,61,63]. Representative capillary pressure/saturation retention properties were estimated using the Haverkamp and Parlange method [64] and Brooks–Corey retention curve entry pressures [65] were estimated using Leverett scaling [66]. The numerical simulator M-VALOR [53,54] was used to model PCE infiltration and redistribution. In this example the PCE release and redistribution conditions were identical to those of Christ et al. [62] with the exception of the higher injection rate in case b. Following 300 days of PCE redistribution, a water flood was initiated at an equivalent rate of 28.4 L/min (hydraulic gradient of 3.1 percent). These water flood conditions are similar to those during the surfactant flood and recovery activities at the field site [59]. The mean arrival times from the water flood simulations neglect nanometal removal from the aqueous phase due to deposition on the soil surface as well as removal due to straining. Furthermore, this analysis neglects any kind of dispersive flux. As such this simple analysis could be considered a best case scenario regarding nanometal arrival times. The numerical simulator MISER [55,56] was used to model the water flood.
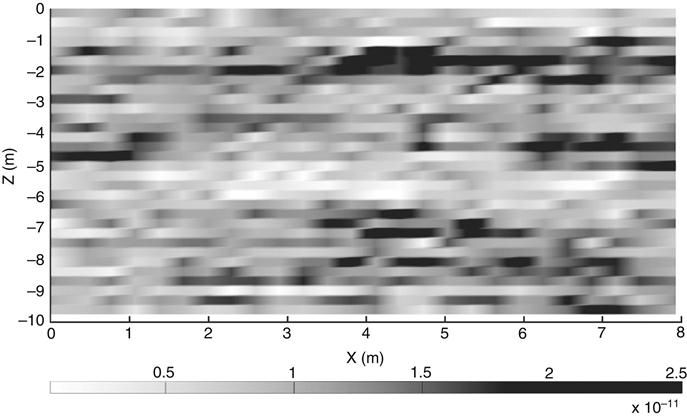
Following 400 days of PCE infiltration and 300 days of redistribution, free phase PCE is present in pools, above low permeability lenses, and at residual ganglia saturations between these pools (Figs. 28.3 and 28.4). PCE contaminates the domain to a greater extent in the simulation with the higher PCE injection rate and PCE saturations are generally higher (i.e., NAPL saturations are generally higher in Fig. 28.4 when compared to Fig. 28.3). Maximum PCE saturations are 0.33 and 0.65 for cases a and b, respectively. Maximum PCE saturations would likely increase if the simulated site had a lower mean soil permeability and a larger variance in soil permeability [67]. Following DNAPL release and redistribution a waterflood was initiated, representing the injection of a nanometal slurry at the left side of the simulated domain. Mean arrival times for the reactive nanometals are shown in Figs. 28.5 and 28.6. Mean arrival times generally increase from the left of the model domain, where the nanometals are injected, to the right side of the model domain. The mean arrival times for the nanometals are generally less than 10 days in these simulations; maximum were 16 and 24 days for cases a and b, respectively. These arrival times are certainly within the expected reactive lifetime of nZVI under ideal conditions (e.g., [37,57]) however further work is necessary to determine reactive lifetimes under conditions found in the field. Due to the horizontal extent of the PCE contamination in case b, numerous pore volumes of nZVI slurries would have to be injected prior to reactive nZVI reaching the downstream PCE contamination, as the nZVI would first react with upstream PCE or natural groundwater constituents. Increasing PCE saturations would also increase the reactive nanometal arrival times due to decreased permeability to water in the PCE saturated zones. Nanometal arrival times may be much greater at field sites with lower permeability sands or soils. The sands at the site used in this example, with a mean permeability of 2.2×10−11 m2, would certainly not be considered low permeability sands. For example, the well-known Borden fieldsite, with medium to fine grained sands, has a mean permeability of 7.6×10−12 m2 [68]. Prior to the implementation of reactive nanometals for DNAPL remediation, an assessment of the reactive lifetime required to reach the source zone is, therefore, necessary on a site-specific basis.


28.6 Summary
A number of exciting, new, and innovative remediation technologies have been made possible through the advent of nanotechnology. These remediation alternatives hold significant promise but require further research and development. An additional hurdle that will require attention is the public and regulatory perception surrounding nanotechnology. For example, although carbon nanotubes have potential for the removal of metal contaminants their toxicity is unknown. Similarly, many of the noble metal catalysts used with nZVI are known contaminants, although the mass fraction of these catalysts is usually quite small. A risk assessment is needed to determine the benefit of using a known or potential contaminant to remediate another contaminant prior to the widespread implementation of nanotechnology-based remediation alternatives. Furthermore, the tremendous benefits of nanotechnology will need to be communicated to the public and regulators prior to its widespread use.


