Life Cycle Inventory of Semiconductor Cadmium Selenide Quantum Dots for Environmental Applications
Hatice Sengül1 and Thomas L. Theis2, 1Department of Environmental Engineering, Hacettepe University, Ankara, Turkey, 2Institute for Environmental Science and Policy, University of Illinois at Chicago, Chicago, IL, USA
Nanotechnological innovations offer promise to solve many of the challenging global environmental problems facing humanity. One important class of nanostructured materials that have drawn particular attention consists of various semiconductors that have either direct or indirect environmental benefits ranging from advanced purification of air and water to improved devices for cleaner energy production. However, the advantages of such applications must ultimately be balanced against the environmental costs associated with their complete life cycle: manufacture, use, and disposal. Such an analysis also assists in identifying the most material, energy, and toxicity intensive aspects of the application, and provides a basis for improvement. To date, the literature on cleaner manufacturing of nanodevices and end-of-life behavior of semiconductor materials is sparse. This chapter explores the various applications of semiconductor nanodevices for environmental applications, and quantifies the cradle-to-grave life cycle impacts of a specific nanostructure: cadmium selenide quantum dots. Raw material, energy use, and emissions associated with their synthesis are quantified and a particular application, photovoltaic solar panels, is examined.
Keywords
Nanotechnology; global warming; climate change; semiconductors; cleaner manufacturing; nanodevices; cadmium selenide quantum dots; photovoltaic solar panels
41.1 Introduction
Advanced technologies to improve the quality of the environment require manufacturing and employment of a wide range of materials, chemicals, and equipment. Such improvements can be direct, for example, removal or transformation of one or more contaminants from air, land, or water, or indirect, in which contaminant emissions are reduced as new nano-based technologies are implemented, for example, more efficient energy production, transmission, or use. Yet, life cycle environmental impacts of these technologies may sometimes be overlooked. The same holds true for environmental applications of nanotechnology and determining the tradeoffs of adopting advanced technologies represents a significant challenge. Life cycle assessment is an effective method to compile and evaluate inputs, outputs, and potential environmental impacts of a system through its life cycle, carried out in four consecutive steps: goal and scope definition, inventory analysis, impact analysis, and interpretation [1].
Current research regarding nanotechnologies applied to improve environmental quality is mainly dominated by studies focused on understanding the potential benefits and impacts of applications. The integration of environmental impact assessment into decision-making at the inception of a technology—during research, development, and design phases—presents significant opportunities for reducing wider life cycle impacts. To date, however, such research is almost nonexistent and the potential benefits that may be gained from such an approach remain largely unexplored. This is especially true for nano-based manufacturing methods, for example, there are many alternative methods for synthesizing nanostructured materials and products with nanoscale features. The range of alternative precursors and solvents that can be employed for nanomanufacturing is also extensive (see, e.g., Cushing et al. [2] for a review of alternative liquid phase synthesis methods for nanostructured materials). The environmental impacts of these alternative pathways are likely to vary considerably. There is also a need to address comparative assessment of manufacturing of alternative products (both nano and micro/macro) on an application basis.
Nanostructured materials and products with nanoscale features can be produced from macroscopic scale materials using top–down manufacturing methods such as lithography, etching, and milling, or from lower (atomic) scale materials using bottom–up methods such as sol–gel processing, chemical vapor deposition, arc discharge, and electrostatic self-assembly or a combination of both [3]. Both top–down and bottom–up methods can have significant environmental impacts for several reasons: (i) strict purity requirements and less tolerance for contamination during processing than more conventional manufacturing processes, (ii) low process yields or material efficiencies, (iii) repeated processing, postprocessing, or reprocessing steps of a single product or batch during manufacturing, (iv) use of toxic/basic/acidic chemicals and organic solvents, (v) need for moderate-to-high vacuum and other specialized environments such as high heat or cryogenic processing, (vi) use of or generation of greenhouse gases, (vii) high water consumption, and (viii) chemical exposure potential in the workplace and through technological/natural disasters [4].
For bottom–up techniques, which are usually the preferred methods for the synthesis of most nanoparticles and nanostructured materials, the literature contain a wealth of empirical data and analysis about process design, material precursors and solvents, and operating parameters [2,5]; however, only a small percentage takes into account environmental impacts. One class of nanostructured materials that have considerable potential for a wide variety of environmental applications is quantum dots, crystalline semiconductors of small enough radius to confine the motion of conduction band electrons, valence band holes, or excitons. Figure 41.1 compares number of sources over time in the scientific literature on the investigation of quantum dots, number of studies on a specific type of quantum dot (cadmium selenide, CdSe), and the number of sources that refer to environmental aspects of CdSe quantum dot manufacturing. There are more than twenty-five thousand sources in the literature investigating synthesis and applications of quantum dots (three thousand related to CdSe quantum dots) but only thirty-five refer to greener, cleaner, or environment-friendly synthesis, and only one [6] solely addresses environmentally benign synthesis of CdSe quantum dots. Whereas the number of quantum dot sources has increased markedly over time, there is no clear trend on environmental aspects of manufacturing.

Candidate quantum dot materials are usually compound semiconductors (e.g., CdSe, PbTe, ZnS, InAs) made up of elements of group II and VI, IV and VI, or III and V. Unfortunately, many of these substances are highly toxic and nonrenewable elements, negative attributes that may be exacerbated by their nanoscale dimensions, which may significantly increase their toxicity and environmental impacts [7]. The synthesis of quantum dots also requires highly toxic precursors and solvents with significant upstream and downstream environmental impacts.
41.2 Applications and synthesis of quantum dots
For bulk semiconductors, the carriers (electrons and holes) are free in all three dimensions and have a continuous valence band. When the size of a material becomes smaller than its exciton (electron–hole pair) radius, carriers are confined in space. Quantum dots are a unique group of semiconductor particles that are small enough to exhibit quantum confinement effects. As a result, the density of states that carriers can occupy becomes quantized. Due to quantum confinement, electronic and optical properties of materials dramatically change [8].
Some applications require a core-shell structure (e.g., for sharper luminescence) in which case the core compound semiconductor is surrounded by another compound semiconductor (e.g., CdSe/ZnS). Colloidal quantum dots are bound to stabilization agents (also called passivation or capping agents) that give the dot its stability in solution. Quantum dots are being investigated for a wide variety of applications, including:
• single-electron transistors,
• infrared/near infrared photodetectors,
Some of these applications have already been commercialized or are in the process of being introduced in the market [9]. Enhanced luminescence and band gap tunability of quantum dots enable them to be applied as nanosensors for environmental analysis and monitoring (screening, diagnostic applications, and monitoring). Several pollutants and pathogens in a sample can be detected simultaneously with high sensitivity [10]. Recent applications include detection of single cells of E. coli using CdSe/ZnS quantum dots [11], detection of Cryptosporidium parvum and Giardia lamblia quantum dot–antibody conjugates [12], and detection of E. coli O157:H7 and S. typhimurium [13]. Goldman et al. have performed multiplexed sandwich immunoassays by conjugating CdSe/ZnS quantum dots to antibodies to simultaneously detect four toxins that eliminate the need for numerous excitation sources/emission windows and complex processing [14].
Chemical sensors based on quantum dots are used to detect ions in aqueous solutions. Chen and Rosenzweig [15] reported the analysis of Cu(II) and Zn(II) ions by CdSe quantum dots capped with polyphosphate, L-cysteine, and thioglycerol in water samples. Jin et al. [16] reported detection of cyanide ions in water samples by a CdSe quantum dot nanosensor. Konishi and Hiratani [17] used an oligo (ethylene glycol) capped cadmium sulfide quantum dot nanosensor to detect copper ions (Cu[II] and Cu[I]). Gattas-Asfura and Leblanc [18] reported the detection of Cu(II) and Ag(I) with a peptide-coated cadmium sulfide quantum dot nanosensor. Sirinakis et al. [19] used a CdSe/ZnS quantum dot nanosensor to detect aromatic hydrocarbons.
Indirect application of quantum dots toward improving environmental quality includes the use of quantum dot films as active layers for solar cells. As part of the novel so-called third generation photovoltaics that aim to eliminate the shortcomings of conventional solar cells, quantum dot solar cells offer a dual solution for advancing the solar technology by increasing the efficiency and allowing roll-to-roll production and thus increasing energy production and throughput and lowering manufacturing costs [20,21]. Quantum dot solar cells can absorb nearly all of the incident solar radiation for wavelengths above their absorption onset with a film of only 200 nm thickness [22]. Candidate materials as quantum dots for solar cells are mostly compound semiconductors of group II–VI, IV–VI, or III–V of the periodic table, including CdSe, CdTe, CdS, InP, InAs, InSb, ZnS, ZnO, ZnSe, ZnTe, PbSe, PbTe, PbS, HgTe, GaN, GaP, GaAs, GaSb, Si, Ge, AlAs, and AlSb.
Quantum dots can be synthesized through two major routes: vapor-phase and liquid-phase deposition, or colloidal synthesis. In vapor-phase synthesis, quantum dots are grown through epitaxial self-assembly by deposition on the surface of a semiconductor layer that has a lattice structure compatible with the quantum dot-compound semiconductor. In colloidal synthesis, precursors of groups II and VI are separately dissolved in organophosphorus solvents such as trioctyl phosphine (TOP), tributyl phosphine (TBP), or triisopropyl phosphine (i-TPP) and injected into a solution of heated solvent (usually a coordinating solvent such as trioctyl phosphine oxide [TOPO]) or a solvent mixture. Quantum dots nucleate and grow instantaneously upon injection of both precursors—or only group V or VI precursors—into a flask containing the solvent—or a mixture of solvent plus group II or III precursors. Further growth occurs through Ostwald ripening (i.e., small particles are absorbed by bigger ones).
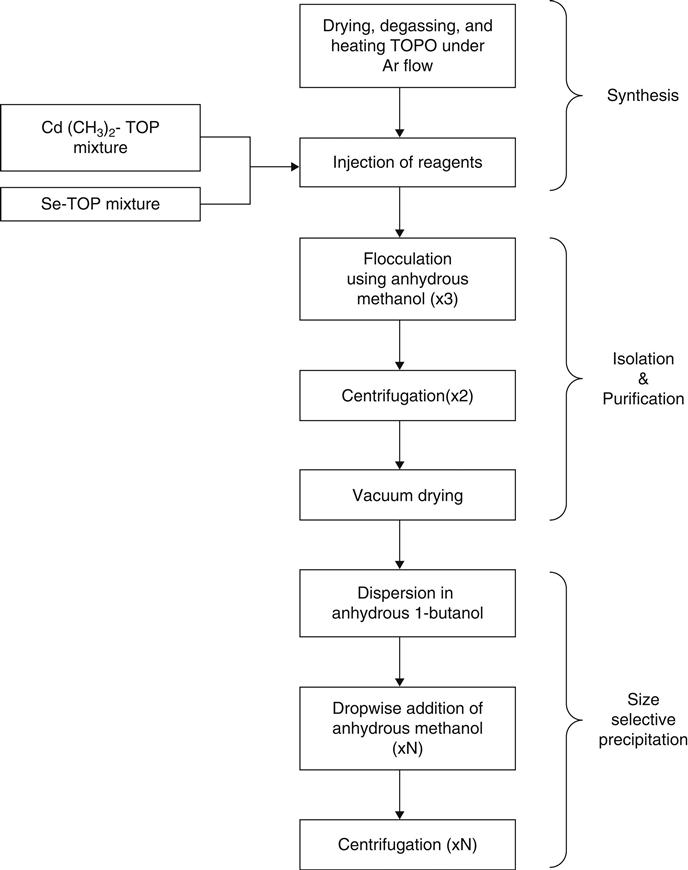
Colloidal synthesis of CdSe is the most well-known method for the synthesis of quantum dots, and has been extensively researched [23–27]. It has become “a model system” to study colloidal synthesis of quantum dots in general [28]. Figure 41.2 shows the flow for synthesizing CdSe quantum dots using the conventional and most widespread method pioneered by Murray et al. [23].
Colloidal synthesis is a batch process with low energy and process requirements. The vast majority of environmental impacts are likely to occur in connection with raw material acquisition; thus the choice of precursors and solvents is quite important in reducing the cumulative impact. For the dual precursor route, the source of selenium is selenium powder—there are no alternatives; however, it can be dissolved in different solvents before injection. For cadmium, alternative cadmium compounds can be employed. Dimethyl cadmium, an organometallic compound, is used as the cadmium precursor in conventional synthesis; however, dimethyl cadmium is an extremely toxic, expensive, and unstable solvent that limits its suitability for large-scale synthesis [29]. This has led researchers to search for alternative cadmium compounds including other organometallics such as alkyl/alkoxy cadmium compounds (e.g., dineopentylcadmium), cadmium salts of fatty acids (cadmium acetate, cadmium oleate, cadmium laurate), and inorganic forms (cadmium oxide, cadmium carbonate). Instead of using dual precursor sources, single source precursors may also be used although they are less commonly known and applied [30–32].
During CdSe quantum dot synthesis the cadmium and selenium precursors are dissolved in TOP, TBP, or i-TPP and form complexes with cadmium and selenium. The solvent precursors are injected in TOPO (termed “the hot matrix”) at high temperatures (250–300°C) [33]. Multicomponent solvents where phosphonic acids act as cosurfactants may also be used. Addition of tetradecylphosphonic acid (TDPA) to a hexadecyl amine (HDA)-TOPO-TOP stabilizing mixture slows nanocrystal growth resulting in good crystallinity and improved size distribution [34]. Addition of phosphonic acids also enables morphological control of quantum dots and leads to synthesis of rod- or tetrapod-shapes that show better performance for specific applications (e.g., solar cells) [35]. Trioctyl phosphine oxide surfactant also acts as a capping agent and ensures the solubility of quantum dots in nonpolar solvents, that is, quantum dots do not exist as stand-alone particles, TOPO molecules are attached to CdSe in solution [36]. Methods are also available to render CdSe soluble in aqueous solvents [37,38].
The need to search for alternative solvents stems from the cost of organophosphorus solvents. The price of quantum dots per gram is currently about $2,000 with solvents accounting for up to 90 percent of the cost [39,40]. Recent trends are to use saturated and unsaturated fatty acids (e.g., oleic acid, stearic acid, lauric acid) [41] and heat transfer fluids based on phenyls [33]. The variety of capping agents is far more extensive and application-dependent, including alkylthiols, alkylamines, peptides, and carboxylic acids in addition to organophosphorus ones. Figure 41.3 presents alternative precursors and solvent pathways for the synthesis of CdSe quantum dots. Table 41.1 provides a listing of sources with varying precursors and solvents/ligands.
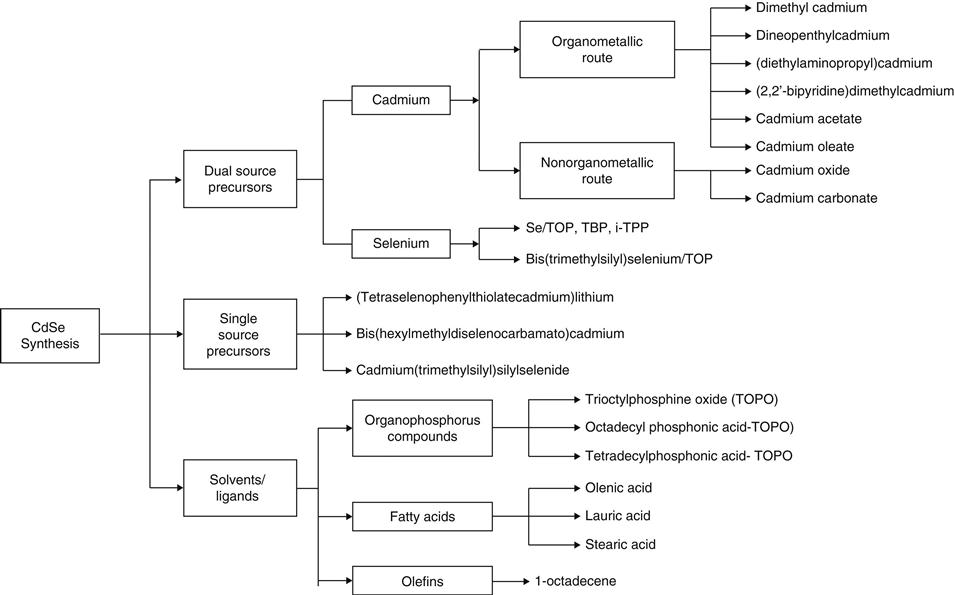
Table 41.1
Alternative Precursors and Solvent Systems for the CdSe Synthesis-Dual Source Precursors
| Precursors | ||
| Cadmium Precursor/Solvent | Selenium/Solvent | Solvent(s) |
| Dimethyl cadmium/TOP/HPA/TDPA [23,35] | Bis(trimethylsilyl) selenium/TOP [90] | Dichloromethane [90] |
| Cadmium oxide/TDPA-TOPO/ ODPA-TOPO* [29] | Se/TOP [23] | TOPO [23] |
| Cadmium methoxide/ HAD-TOPO* [85] | Se/TBP [71] | TOPO/TBPA [35] |
| Cadmium oleate/Squalane-Oleyl amine-TOP [41] | Se/i-TPP [71] | 1-octadecene [40] |
| Cadmium 2,4-pentanedionate/Squalane-Oleyl amine-Oleic acid [76] | Se/TOP- squalane [76] | Dowtherm A, Therminol 66 (T66)-Oleic acid [90] |
| Cadmium acetate, cadmium carbonate/SA/SA-TOPO/Lauric acid/Technical TOPO/TDPA-TOPO* [86] | Sodium selenosulfate [89] | |
| Dineopentylcadmium, bis (3-diethylaminopropyl) cadmium and (2,2-bipyridine)dimethylcadmium/TBP [87] | Se/TOP-CTAB/ TOP-DDAB [90] | |
| Cadmium laurate, Cadmium myristate, Cadmium palmitate [88] | ||
| Cadmium oxalate/ Ethylenediamine* [89] | ||

Notes: (1) Syntheses designated with “*” are one-pot approaches meaning only selenium solution is injected to the solution for the growth of quantum dots, cadmium precursors are already dissolved in the solvent prior to the injection of selenium containing solution. (2) Dowtherm A and Therminol 66 are commercial names for two heat transfer fluids containing phenyls.
Abbreviations: CTAB: Cetyltrimethylammonium bromide; DDAB: Didodecyldimethylammonium bromide; HPA: Hexylphosphonic acid; Me: Methyl group; ODPA: Octadecyl phosphonic acid; SA: Stearic acid; TBP: Tri-n-butylphosphine; TDPA: Tetradecylphosphonic acid; TOP: Trioctylphoshine;TOPO: Trioctylphosphine oxide; i-TPP: Triisopropylphosphine.
41.3 Methodology
This chapter addresses the environmental impacts of nanostructured semiconductor quantum dots as applied to the production of solar energy, using CdSe quantum dots as an example. A life cycle approach is used, facilitated by application of the life cycle assessment software SimaPro 7.1.4 [42] and data from the Swiss national LCI database Ecoinvent [43]. Materials were modeled in SimaPro using information and data from the Ecoinvent database and other sources referenced in the following paragraphs.
For infrastructure and transport of materials, generic data are employed. Infrastructure use was approximated by the “chemical plant, organics, RER (Country code for Europe in the database)” module and the transport was approximated by the “transport, lorry 16t” module in the database assuming an average distance of 100 km for transportation of quantum dots from the production site to the use site. The waste disposal option is selected as hazardous waste incineration for which a life cycle inventory (LCI) already exists. Energy consumption for some materials is based on estimates for similar chemicals in the database. Primary energy consumption for TOP is based on unit process inventory for organophosphorus compounds in the Ecoinvent report No. 15 [44]. The energy consumption during synthesis (drying and heating TOPO before and during synthesis) is estimated assuming 3 J/g K is a representative value for heat capacity of TOPO and the reaction mixture [45]. A similar figure is obtained using power consumed by a heating mantle for the average reaction duration. Since LCI of materials are based on electricity mix for Europe, emission values reported here are conservative as the share of coal in the electricity mix for Europe has a much lower value than that of United States and share of nuclear energy is much higher [46].
41.4 Life cycle inventory of synthesis of CdSe quantum dots
As expressed earlier, the acquisition of raw materials for quantum dot synthesis is likely to be a major contributor to environmental impacts. Twenty-two materials are required for the synthesis of CdSe quantum dots. The LCI data for six materials are not available in the Ecoinvent database. These material processes were modeled in SimaPro using LCI data for other materials available in the Ecoinvent database and other sources referenced in the following paragraphs. Figure 41.4 presents the material flows for the synthesis of CdSe quantum dots. Gray filled boxes represent materials modeled in SimaPro using the Ecoinvent database and the literature/patents; other boxes represent materials for which data is already available in the Ecoinvent database.

Life cycle inventory for the synthesis of CdSe quantum dots is based on the original and widely used method of Murray et al. [23]. Cadmium precursor is dimethyl cadmium [Cd(CH3)2]. Cadmium compounds are included in the list of 189 chemicals listed as hazardous air pollutants under the 1990 Clean Air Act. Cadmium is also on the list of chemicals appearing in the Emergency Planning and Community Right-To-Know Act of 1986 [47]. Cadmium is produced as a by-product of zinc (80 percent) and lead (20 percent) mining and refining. Sources include:
• purification sludge from electrolytic zinc plants;
• fumes and dusts collected in ESPs (electrostatic precipitators) from zinc, lead, lead–zinc, or copper–lead–zinc ore processing;
• recycled zinc metal containing cadmium [48];
• recycled nickel–cadmium batteries [49];
• recycling cadmium from municipal solid waste (MSW) has also been proposed [50].
Approximately 3 kg of cadmium is produced for each ton of zinc produced. Environmental impacts from zinc processing allocated to cadmium production are small, 0.5 percent and 0.58 percent of environmental impacts are contributed by cadmium based on mass and economic value allocation [51]. Cadmium is refined in four major steps: leaching, precipitation, reduction or cadmium plating, and casting. During cadmium refining, cadmium is released from melting furnaces, retorting, casting and tapping, and packaging [48].
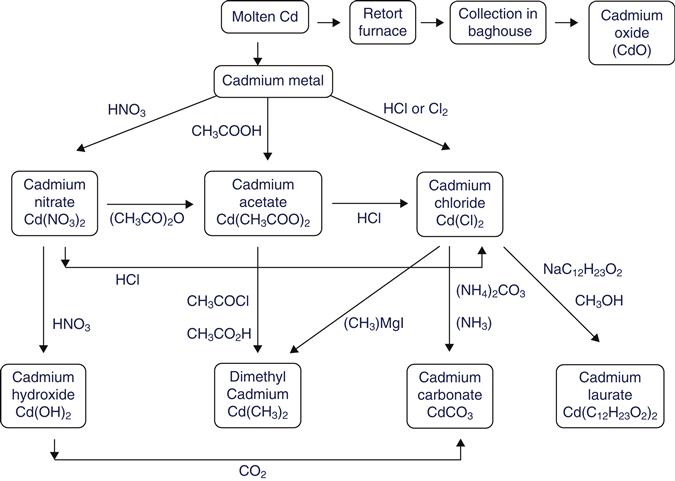
Cadmium precursors can be produced through a number of pathways and from different starting materials [48,52,53]. Figure 41.5 presents the network for the synthesis of alternative cadmium compounds used as precursors for the synthesis of CdSe quantum dots. Cadmium oxide has the shortest route and is probably the one with the lowest environmental impact in terms of energy and materials consumption as cadmium can be easily oxidized upon exposure to air in furnaces. But cadmium oxide vapors are more toxic than cadmium nitrate, cadmium acetate, or cadmium chloride. Therefore, the toxicities of alternative cadmium compounds must be evaluated for a comparative assessment. In the case of dimethyl cadmium, as can be seen in Fig. 41.5, it can be synthesized either from cadmium acetate or cadmium chloride. Its synthesis from cadmium acetate avoids the extra steps of synthesizing cadmium chloride, which is more toxic than cadmium acetate, using Grignard reagents (CH3MgI). However, it involves the use of another chlorinated substance, acetyl chloride (CH3COCl). This is the case if cadmium chloride is to be produced from cadmium acetate. If cadmium chloride is produced directly from cadmium using hydrochloric acid or chlorine gas, the relative impacts of synthesizing dimethyl cadmium using cadmium acetate or cadmium chloride as the starting material are difficult to compare. Further, cadmium chloride is usually the commercially available compound for the synthesis of other cadmium compounds. No information exists for the toxicity of dimethyl cadmium but parallels can be drawn with other organometallic compounds, for example, organocadmium compounds show similar toxicities to organomercury compounds [54].
Life cycle inventory of dimethyl cadmium is not available in the Ecoinvent database. The synthesis of dimethyl cadmium from methyl magnesium chloride and cadmium chloride were modeled in SimaPro with data and information from the literature and patents. Synthesis of Grignard reagent, methyl magnesium chloride (CH3MgCl), required to produce dimethyl cadmium is also modeled in Simapro [55–61].
Selenium powder is used as selenium precursor. Usually a solution of selenium dissolved in TOP is prepared and used as a stock solution. Selenium is an element regulated under the Safe Drinking Water Act. It is a widely distributed mineral produced as a by-product of the electrolytic refining of copper [62]. Selenium can be extracted from copper refinery slime by roasting with soda ash or sulfuric acid. Other methods include wet chlorination, oxidative leaching with sodium hydroxide solution under pressure, and the hydrometallurgical process of chlorination in hydrochloric acid [63]. Production of selenium based on roasting process with sodium carbonate uses hydrochloric acid, soda, and sulphur dioxide releasing hydrogen chloride, sulfur dioxide, chloride, sodium, and sulfate [64] and inventory data for its production is already available in the Ecoinvent database.
The organophosphorus surfactants TOP and TOPO can be produced from elemental phosphorus, phosphines, or halogenophosphines [65]. Trioctyl phosphine is manufactured by the radical-catalyzed addition of 1-octene to phosphine. Trioctyl phosphine oxide can then be produced from TOP by peroxide oxidation, according to Equation 41.1 [54]:
(41.1)
The catalytic synthesis of TOP and TOPO (via cuprous chloride) is initiated with methylethylketone peroxide [MEKP, (C6H5CO)2O2] generated from the oxidation of methylethylketone (MEK) by hydrogen peroxide (H2O2). The complete synthesis was modeled in Simapro using data and information from the literature and patents [54,66–70].
The reaction chemistry and stoichiometry of CdSe synthesis is one of the poorly understood parts of CdSe quantum dot synthesis [71]. Also, the thermal decomposition products of dimethyl cadmium in TOPO are currently unknown. Thermal decomposition releases free methyl radicals that recombine and decompose to form hydrocarbons. Gas-phase decomposition yields methane, ethane, propane, ethylene gases [72]. In the TOPO solvent, ethers may form as the methyl radicals combine with the oxygen in TOPO [73]. Ethers may subsequently form complexes with unreacted selenium. To date, no record exists for mass spectrometric analysis of individual compounds. The reaction yield for the synthesis of quantum dots is somewhat difficult to control, ranging from 25 to 97 percent depending on nanocrystal size, heating time, and precursor [34,74–76]. Murray et al. report that the waste from isolation and purification steps is composed mostly of elemental cadmium and selenium as analyzed by powder X-ray diffraction and energy dispersive X-ray [23]. The waste also includes excess TOPO, TOP, and alcohols used to precipitate quantum dots. The waste from the size selective precipitation step contains primarily methanol and 1-butanol.
The subsequent application of this information to solar collectors was conducted as part of this study. A “functional unit” of 1 watt of peak power was selected. The actual power a solar cell can produce depends on the installation location, the tilt angle, the energy conversion efficiency, and the energy loss in the inverter. Solar spectra are defined by an air mass (AM) value, which is a measure of the length of the path through the earth’s atmosphere that the solar radiation travels. The value is calculated as 1/cos z, where z is the zenith angle between a line perpendicular to the earth’s surface and a line intersecting the sun. AM 1 describes the case in which the sun is directly overhead. The AM 1.5 (at an angle 42.8°) spectrum is commonly used for testing and reporting solar cell devices meant for terrestrial use [77]. The peak power is calculated from efficiency, solar cell area, and input light irradiance using Equation 41.2:
(41.2)
where η=energy conversion efficiency; E=1000 W/m2 for AM 1.5 at 25°C; Ac=surface area of solar cell.
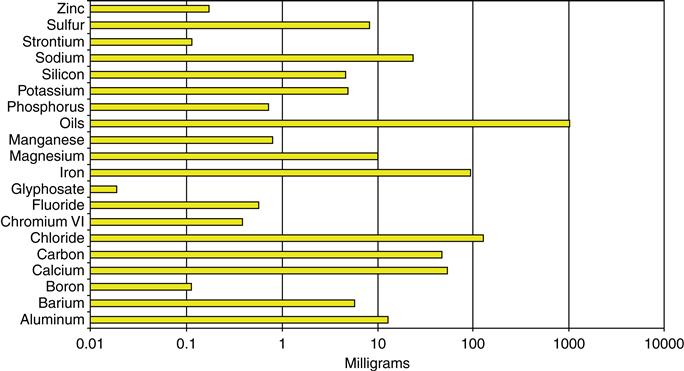
The current performance of quantum dot solar cells does not exceed 5 percent (actually laboratory measurements are less, 5 percent is taken as base scenario) but they are expected to reach a conversion efficiency of 42 percent or higher (best case scenario) [78]. A quantum dot layer requires 120 μl of quantum dot solution for a solar cell area of 220 mm2 [79–82]. Accordingly, for 1 watt of peak power at standard conditions, the required amount of quantum dots ranges from 920 to 2290 mg for the base scenario, and from 110 to 270 mg for best case scenario. To produce 1 watt of peak power, a minimum of 110 mg of quantum dots needs to be synthesized. Material inputs and emissions to soil, water, and air for the synthesis of 1 gram of CdSe quantum dots are provided in Figs 41.6–41.9. On a mass basis, air emissions contribute the most to the cumulative emissions, followed by water and soil emissions. Overall, approximately 110 g of waste materials are produced for the synthesis of 110 mg quantum dots for 1 watt peak of energy, thus the global waste burden of synthesis of CdSe quantum dots for solar cell films is in the range 110–2,225 kg/kWp, corresponding to a conservative waste-to-product ratio of 972 for quantum dots. Note that the waste-to-product ratio was calculated only for the synthesis of quantum dots, not the whole life cycle (i.e. raw materials acquisition and synthesis) and excludes the waste from energy demand and transport, consistent with literature that reports waste-to-product ratio [83]. The waste-to-product ratio is highly dependent on the amount of solvent used for isolation and purification steps. The number of isolation and size selective precipitation steps depends on the material, surfactants, and target application. The application of three to five isolation and purification steps is accepted as reasonable for quantum dot synthesis [84]. In this chapter, we report LCI results for five repetitions of isolation, and size selective precipitation steps. The total volume of solvents (i.e., methanol and 1-butanol) used is 1.1 liters per gram of quantum dots. The waste-to-product ratio drops to 590 for three repetitions of isolation, purification, and size selective precipitation. For comparison, bulk chemical synthesis ratios are typically less than 10, fine chemicals of less than 50, and many pharmaceuticals of the order of 100 [83].



It must be emphasized that synthesis of quantum dots is based on the original organometallic route, other routes may give different results. It must also be stated that the results are based on laboratory studies and that amount of quantum dot used is for deposition of quantum dot layer by spin-coating, wherein most of the solution becomes waste and cannot be recycled; adoption of other deposition techniques may result in less waste. This analysis does not include the material flow analysis of the manufacture of solar cells.
41.5 Conclusions and future perspective
Life cycle analyses of nano-based technologies are essential to identify environmental advantages and disadvantages of nanoproducts and meet the objective of early diagnosis and treatment of environmental risks of technological developments in this area.
Nanosensors and other applications of quantum dots will be in the market soon as the transition from research to markets has been fast for many nanoproducts. Quantum dot lasers and quantum dot biodetection products have already reached markets. Given the prospective market size of quantum dots, the waste burden and environmental impacts linked to the emissions to the environment caused by the production and use of quantum dots may become significant in the future. In an effort to identify associated risks, we used life cycle assessment software SimaPro, the LCI database Ecoinvent and other data sources to establish a LCI for the synthesis of CdSe quantum dots, which are the most frequently used quantum dots so far. Raw material use, energy use, and emissions to air, water, and soil were quantified. On a mass basis, air emissions contribute the most to the cumulative emissions, followed by water emissions contributing to 12 percent of the total emissions. These emission values are specific for the conventional route of synthesis. Further research is required that applies Life Cycle Assessment (LCA)/LCI to raw materials selection to identify alternative raw materials that are less polluting. Our results indicate that the isolation, purification, and size selective precipitation contribute the most to environmental impacts.
Acknowledgments
This work has been supported by grant number 0646336, “Life Cycle of Nanomanufacturing Technologies” of the Division of Chemical, Bioengineering, Environmental, and Transport Systems, National Science Foundation, Cynthia Eckstein project manager.