Nanotechnology-Based Membranes for Water Purification
Eric M.V. Hoek1, Mary Theresa M. Pendergast1 and Asim K. Ghosh2, 1Department of Civil and Environmental Engineering and California NanoSystems Institute, University of California, Los Angeles, CA, USA, 2Desalination Division, Bhabha Atomic Research Centre, Trombay, Mumbai, India
Herein we present a critical review of nanotechnology-enabled materials touted as low-energy replacements for conventional reverse osmosis (RO) membranes in desalination and water reuse applications. Zeolite coatings promise a highly selective material that have the chemical, thermal, and mechanical stabilities of conventional ceramic membranes. Nanocomposite membranes exhibit up to three times higher permeability than current commercial polymeric membranes, with no change in salt rejection, and can be fabricated with antimicrobial and photoreactive functionalities. Biomimetic membranes can produce highly selective membranes potentially useful in both forward osmosis and RO applications. Aquaporin (AQP)-based lipid bilayer vesicles exhibit nearly 100 times higher water permeability than commercial membranes with near perfect salt rejection. Carbon nanotube (CNT)- and graphene-based membranes (theoretically) exhibit acceptable salt rejections with water permeabilities between 5 and 1000 times higher than commercial membranes. Self-assembled block copolymer membranes represent a fully polymeric approach to forming highly selective structures reminiscent of AQP- or CNT-based materials.
Keywords
Desalination; water reuse; membrane; nanotechnology; zeolite; thin film nanocomposite; biomimetic; carbon nanotube; self-assembled block copolymer; grapheme
9.1 Introduction
Almost 150 years ago, the physicist James Clerk Maxwell conceived his “sorting demon,” a fictitious being that discriminates perfectly between molecules without the expenditure of work—the ideal membrane [1]. Since that time, various membrane processes have been developed, each with specific applications in mind. Membrane-based water purification processes are now among the most important and versatile technologies for conventional drinking water production, wastewater treatment, ultrapure water production, desalination, and water reuse. Commercially available membrane processes for water purification include electrodialysis, electro-deionization, reverse osmosis (RO), nanofiltration (NF), ultrafiltration, and microfiltration. Electrodialysis, electro-deionization, and NF are useful in demineralization, softening, and organic separations, but RO and ultrafiltration membranes are the workhorse technologies for desalination and water reuse. Other membrane-based processes such as forward osmosis, membrane distillation, and pervaporation are emerging, but have found limited application in practice.
In principle, intrinsic advantages of membrane processes include continuous, chemical-free operation, low-energy consumption, easy scale-up and hybridization with other processes, high process intensity (i.e., small land area per unit volume of water processed), and highly automated process control. General disadvantages of membrane processes are short membrane lifetime, limited chemical selectivity, concentration polarization, and membrane fouling [1]. Polarization and fouling of RO membranes require extensive physical and chemical feed water pretreatment (e.g., chlorination, in-line coagulation, flocculent aid addition, membrane filtration), low recovery operation, extensive chemical cleaning, and frequent operator intervention. RO processes further suffer from high intrinsic energy consumption, environmental issues associated with feed water intake and brine discharge, and the need for chemical conditioning of product water.
Nanotechnology promises to dramatically enhance many water purification technologies, such as adsorption, ion exchange, oxidation, reduction, filtration, membranes, and disinfection processes [2]; however, one key issue related to nanotechnology is the question of how to best apply it. Specifically, it is not clear how to interface nanoparticles with contaminants. At present, many expensive nanoparticles cannot be added to water like commodity chemicals and some nanoparticles could present new hazards to human health and the environment [3]. Thus, additional separation processes are required to recover nano-materials for risk avoidance and reuse. A promising approach is to immobilize nano-materials on or within a solid matrix, such as a membrane. The resulting membrane may exhibit improved separation performance, chemical, thermal, or mechanical stability, interfacial properties, or advanced functionality depending on the nano-material selected.
This chapter presents a review of six emerging nanotechnology-based membrane material concepts intended for use in water purification: (1) zeolite-coated ceramic membranes, (2) inorganic–organic thin film nanocomposite (TFN) membranes, (3) hybrid protein–polymer biomimetic membranes, (4) aligned carbon nanotube (CNT) membranes, (5) self-assembled block copolymer membranes, and (6) graphene-based membranes. We have selected these membrane nanotechnologies because each is touted as a possible low-energy replacement for conventional RO membranes. Our objective is to review the basic concepts underlying each technology, the respective materials and methods of fabrication, published separation performance, and current commercialization efforts.
9.2 Zeolite-coated ceramic membranes
Ceramic membrane development is currently seeking to form membranes with water permeability on the range of ultrafiltration with the solute selectivity of NF or even RO membranes [4]. In 2001, molecular dynamics simulations showed that zeolite membranes—previously applied for gas separations—may be applicable for aqueous solution separations, in particular forward osmosis and RO [5]. Since then thin zeolite membranes have been studied for RO desalination of brackish water and a variety of wastewaters, showing promising rejection of salts and organics [6–11]. The basic ceramic membrane concepts and synthesis procedures are considered conventional; however, novel applications involve the use of nanoparticulate zeolites. Hydrophilic zeolite membranes applied for gas separations, such as pervaporation, are composed of a loose, thick zeolite film through which separation occurs; however, RO membranes require an ultrathin, dense layer and so pains must be taken to form nanoscale zeolite coating films for this technology to prove practical for water treatment applications.
Zeolites are naturally occurring aluminosilicate minerals with highly uniform subnanometer and nanometer scale crystalline structures. Typical zeolite membranes are amorphous silicate, aluminosilicate, or aluminophosphate crystalline structures formed via hydrothermal synthesis [9,10]. Other synthesis methods include in situ layer-by-layer crystallization and dry gel conversion in the presence of a template-water vapor [11]. Aluminosilicate crystals are intrinsically inert, imbuing these membranes with extreme thermal and chemical stability [12]. Zeolite crystals consist of a three-dimensional (3D) cross-linked (Si/Al)O4 tetrahedral framework, which contains cavities that allow for the movement and containment of ions and water molecules [13]. The containment of molecules in a given zeolite framework is a function of temperature, water content, ion type, and the ratio of Si to Al atoms in the matrix [14]. Many natural zeolites can be produced synthetically, while additional structures, with no natural occurrence, have been synthesized and are characterized as zeolites based on their structures, such as zeolite-A produced by Linde Corporation [14].
Common zeolite materials employed in membranes include Mordenite Framework Inverted (MFI)-type, sodalite (SOD), and linde type A (LTA). Zeolite ZSM-5 (MFI)—the most commonly applied zeolite in membranes—contains straight channels in one direction and sinusoidal channels in the other direction [12]. Since these channels are not interconnected, orientation of the crystals with respect to the flow path is required. Hydroxyl SOD has also been applied in membrane materials [9]. SODs are not mineralogically defined as zeolites, but felspathoids because in nature salt molecules are contained in their frameworks. The SOD cage, however, is quite common to zeolite structures and when crystalline networks are created with this cage structure, zeolitic properties are exhibited. The LTA structure is composed of SOD cages connected by truncated cubo-octahedron, forming an interconnected flow channel [14]. Zeolitic particles with interconnected cage structures like those in LTA offer simplified fabrication since alignment is unnecessary.
Pore size and framework density are the primary factors of concern when considering zeolites for water separations; pore size determines ion selectivity and framework density determines water permeability (Figure 9.1). Atoms other than Si and Al can be substituted into the cage structures of zeolites via ion exchange to imbue alternate charge and structural properties. Since the ability to act as a molecular sieve is due to the channel widths, changing the atoms in the framework, and thus the channel widths, will change the sieve properties [15]. Additionally, both the ion and water molecule mobility through a zeolite depend upon the relative density of the framework structure; open porous structures will facilitate less hindered transport [14]. This is indicated by the framework density, i.e., the number of Si or Al atoms per 1000 Å. The framework density (FD) values (normalized for ideal Si frameworks) are 18.4, 16.7, and 14.2 for MFI, SOD, and LTA, respectively [16], implying that LTA would be expected to have the largest water mobility.

The Si to Al ratio of a zeolite cage is the most important factor affecting chemical stability, hydrophilic properties, and occurrence of inter-crystalline defects. An increase in Si:Al implies a decrease in the overall surface charge on the framework [14]. The MFI-type zeolites are capable of a large range of Si:Al, from ~30 in the ZSM-5 form to nearly pure Si for the isomorphous silicate-type MFI. Noack et al. [11] find that as the Si:Al decreases in MFI-type zeolites, water permeability and selectivity for water increase; however, defects simultaneously increase until a point where selectivity decreases. Additionally, the Si:Al allows for tuning of the surface properties and the resultant electrostatic double layer; such membranes could also be tuned for specific ion-selective applications [7].
By normalizing reported zeolite membrane permeabilities by their film thicknesses, comparisons can be drawn against conventional polymeric RO membranes on the basis of specific hydraulic resistance [17]. While most zeolite films (~3–50 μm thick) formed to date do not have overall permeabilities comparable to thin film composite (TFC) (~50–250 nm) RO membranes, the intrinsic permeabilities per thickness are comparable; in fact, membranes produced by Kazemimoghadam [9] appear to have intrinsic permeabilities three orders of magnitude higher than commercial seawater RO membranes. This implies that zeolite films could provide an economically viable alternative material for high-flux RO membranes with dramatically enhanced chemical, thermal, and mechanical stability. The challenge remains to improve control over crystal nucleation and growth to ensure defect-free ultrathin zeolite films, which may require abandoning or substantially modifying traditional hydrothermal synthesis methods [10]. No commercialization efforts have yet been seen.
9.3 Inorganic–organic TFN membranes
In general, nanocomposite materials are created by introducing nanoparticle materials (the dispersed “filler”) into a macroscopic material (the continuous “matrix”) [18]. The resulting nanocomposite material may exhibit drastically enhanced properties, such as mechanical properties (e.g., strength, modulus, and dimensional stability); chemical and thermal stabilities; permeability for gases, water, and hydrocarbons; electrical and thermal conductivities; surface properties, optical properties, or dielectric properties. For example, dispersing molecular sieve nanoparticles into polymers can produce mixed matrix membrane materials with improved gas mixture permselectivity [19]. In general, the nano-phase is dispersed into the matrix during processing and the mass fraction of nanoparticles introduced is very low (generally <5%) due to the incredibly high surface area-to-volume ratio of nanoparticles.
Research and development of mixed matrix membranes is most extensive for gas separations, pervaporation, and fuel cell applications where the effort focuses on developing more efficient combinations of matrix and filler materials and into better controlling membrane formation. More recent research also focuses on incorporating nano-materials into microfiltration and ultrafiltration membranes to achieve a variety of goals, including enhanced permeability, decreased propensity for fouling, targeted pollutant degradations, and increased thermal and mechanical stabilities [20–26], while maintaining the ease of fabrication and low cost of their fully polymeric counterparts.
Nanoparticle additions have also been made to the selective layers of TFC membranes in order to take advantage of the properties of the nano-materials. The addition of nanoparticles into the polymerization process and through coatings and self-assembly atop membranes has brought about TFN membranes, which offer potential benefits of enhanced performance, reduced fouling, and other novel functionality [27–32]. As with TFC membranes, the two layers of TFN membranes can be independently tuned with nanoparticle additions to the support structure, selective thin film, or both.
In the earliest known study of TFN technology, Jeong et al. [27] describe the synthesis and characterization of zeolite–polyamide TFN membranes formed by interfacial polymerization. The general approach to TFN membrane formation is similar to that of traditional polyamide TFC membranes, but nanoparticles are dispersed in the initiator solution prior to interfacial polymerization, as depicted schematically in Figure 9.2. TFN membranes offer new degrees of freedom in designing NF and RO membranes because the nanoparticle and polymer phases can be independently tuned to impart a wide array of separation performance and novel functionality.
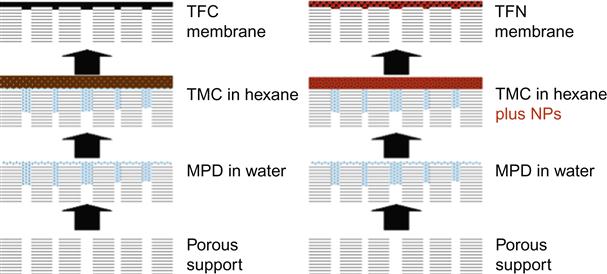
Jeong et al. [27] demonstrate increasing water permeability as a function of nanoparticle loading in MPD–TMC thin films with no observed decrease in rejection of salts and low molecular weight organic solutes. In addition, as nanoparticle loading increased, TFN membranes became increasingly more hydrophilic, negatively charged, and smooth than pure polyamide TFC counterparts [27,28]. Lind et al. [30] evaluated the sizes of the nanoparticles used and found that larger nanoparticles produce membranes with highly favorable surface properties, while smaller nanoparticles produce increased permeability by causing larger characteristic pores. All TFN membranes are found to be less cross-linked than the TFC counterparts, suggesting a more open structure and implying that the nanoparticles used can be tailored to particular membrane applications to enhance membrane performance.
Additionally, resistance to physical compaction has been imparted to RO membranes by including nanoparticles both in the polysulfone support of TFC membranes and in the polyamide coating of TFN membranes [31,32]. In general, nanocomposite membranes have higher initial water permeation and less flux decline during compaction, implying lower overall operating energy is required. Permeability of the TFN membranes is intermediate between commercial seawater and brackish water RO membranes, while TFN membrane NaCl rejection is the highest. The seawater desalination performance of laboratory prepared TFC and TFN membranes was evaluated (Figure 9.3) along with commercially fabricated seawater RO membranes. Hand-cast TFC and TFN membranes rival commercial membranes (characterized in flat-sheet form by the authors and in spiral wound element by the manufacturers) simultaneously exhibit salt passages equivalent to commercial seawater RO membranes with one to three times higher permeability.

Li et al. [8] formed TFN membranes containing silver and titania nanoparticles with the goal of producing potentially antimicrobial and UV-active NF membranes. TFN NF membranes were prepared following the method of Jeong et al. [27]. Silver nanocomposite membranes exhibited virtually identical water flux and salt rejection as pure polyamide equivalents. Most of the Ag0 particles remained on the surface even after the performance test as confirmed by scanning electron microscopy (SEM), X-ray photoelectron microscopy (XPS), and atomic force microscopy (AFM). The authors describe “anti-biofouling tests of silver nanoparticle immobilized membranes,” in which Pseudomonas (species unspecified) was deposited on as-synthesized silver–TFN membranes, and cultivated in the incubator at 37 °C and at 90±5% humidity for 24 h. From visual inspection of SEM images, the authors speculate “colonies of Pseudomonas were formed in the membrane without Ag nanoparticles, whereas those in the Ag incorporated membrane were almost killed.” However, no analyses are presented to quantify cell adhesion or to cell growth on the membranes. The same authors report that incorporation of titania nano-particles in MPD–TMC thin films increases water flux due to the enhanced hydrophilicity of the TFN membranes. Optimum membrane performance and good mechanical properties were achieved at a titania loading up to 5% (w/w). Above this critical concentration, poor mechanical properties and separation performance were reported.
A company was formed in California (see www.nanoh2o.com) to commercialize nanocomposite membranes for desalination and water purification. According to the company’s web site, NanoH2O’s nanostructured membranes exhibit a wide array of advantageous membrane characteristics, including “improved permeability while maintaining requisite salt and contaminant rejection, both passive and active fouling resistance, as well as “tunable” membrane performance to address specific water chemistries. By leveraging existing membrane synthesis techniques, NanoH2O’s advanced membrane technology requires few modifications to existing commercial manufacturing facilities—and fits within current desalination pressure vessels without alteration.” Laboratory data presented above (Figure 9.3) suggests that nanocomposite RO membranes can be fabricated with similar rejection, but twice the permeability of commercially available seawater RO membranes. In addition, various nanoparticles can be selected to impart a wide array of antimicrobial and photo-catalytic capabilities, which may improve fouling resistance.
9.4 Hybrid protein–polymer biomimetic membranes
Biomimetic membranes are designed to imitate the highly selective transport of water and solutes across biological membranes. The general approach to forming biomimetic membranes is to incorporate active transport proteins (isolated from cell cultures) within a vesicular or planar lipid bilayer or a more stable synthetic analog, e.g., ABA triblock copolymer monolayers [33]. The protein may facilitate diffusion, cotransport, or counter-transport in the presence of concentration gradients, light energy, or chemical energy [34]. While single channel measurement in lipid bilayers has served as the basis for understanding membrane protein function, building functional devices incorporating such proteins requires high yield insertion and integration within a stable matrix [33,35]. Further stability of lipid or block copolymer membranes can be achieved by cross-linking the matrix via UV irradiation or by adding free-radical initiators such as azobisisobutyronitrile [1].
Perhaps the most promising approach for water purification is the development of aquaporin (AQP) protein–based biomimetic membranes. The interior of, for example, AQP-1 presents a 15 Å long, narrow channel through which water molecules line up and permeate by hopping along in single file [36–38]. Molecular dynamics simulations have been used to characterize osmotic and diffusion permeability coefficients for water through AQP-1 by applying chemical potential and hydrostatic pressure differences, respectively, across a single protein. Simulations agree well with previously published experimentally derived permeability coefficients and they reveal the osmotic permeability to be about an order of magnitude (~12–13 times) larger than the diffusion permeability. If this relationship is universal, AQP protein-based membranes might be better suited for forward osmosis separations (concentration-driven) rather than RO separations (pressure-driven). These and other simulations also suggest that water hopping rates in narrow CNTs are 7–14 times faster and thus potentially more promising for creating low-energy desalination membranes [39]. This is discussed in more detail below.
Water purification membranes built from AQP proteins embedded within lipid or copolymer films have been published in both the patent and open literature. For example, a patent was awarded in 2007 for methods of incorporating biological membrane proteins into a copolymer matrix to produce biomimetic membranes with a wide variety of functionalities [40]. In one form of the invention, a composite membrane incorporates two different proteins that cooperate to produce electricity from light. In another form, water transport proteins are embedded in a membrane to enable water purification. The patent claims, “The preferred form described has the form of a conventional filter disk. To fabricate such a disk, a 5 nm thick monolayer of synthetic triblock copolymer and protein is deposited on the surface of a 25 mm commercial ultrafiltration disk using a Langmuir–Blodgett trough. The monolayer on the disk is then cross-linked using UV light to the polymer to increase its durability. The device may be assayed by fitting it in a chamber that forces pressurized source water across the membrane.” However, there is no guidance as to how one should select a synthetic triblock copolymer nor is there any data in support of the actual function of the embedded AQP.
Kumar et al. [41] characterize transport characteristics of AQP-Z containing symmetric poly-(2-methyloxazoline)–poly-(dimethylsiloxane)–poly-(2-methyloxazoline) (PMOXA15–PDMS110–PMOXA15) triblock copolymer vesicles. AQP-Z is selected based on the ability for high water permeation and high selectivity. In addition, it is easy to purify and multiply using a recombinant Escherichia coli strain. The symmetric triblock copolymer with a high hydrophobic to hydrophilic block ratio was selected, reminiscent of a lipid-bilayer membrane. The authors report water permeability coefficients through AQP-containing vesicles that are almost 100 times higher than commercial RO membranes, whereas vesicles without AQPs exhibit permeabilities 10 times lower than RO membranes. The authors also claim high solute selectivity reporting, “The calculated reflection coefficients of salt, glycerol, and urea were greater than one, indicating higher relative rejection of these solutes (data not shown).” The concept of a “reflection coefficient” derives from nonequilibrium thermodynamic arguments, which give rise to values of the reflection coefficient ranging from 0 to 1 [42,43]. A value larger than one is theoretically impossible, which suggests that the authors’ measurements or calculations may be improperly communicated in the chapter; regardless, this shows the functionality of AQPs in synthetic applications.
Transport across biological membranes is driven by osmotic pressure gradients, rather than mechanically applied pressure gradients as in industrial filtration processes. Kaufman et al. [44] demonstrate that lipid bilayers were formed atop NF membranes that can be operated under a mechanical driving force as RO membranes. NF membranes are chosen as the support because of their high permeability and low surface roughness that allow for minimal distortion of the lipid bilayer. AQP solutions (of protein PM28, the integral protein of a spinach leaf plasma membrane) are deposited onto commercially available NF membranes via vesicle fusion. Electrostatic interactions are tailored to optimize surface coverage with the lipid bilayer, and full, defect-free coverage is implied by the drastic decrease in permeability of the composite membrane.
Currently, scale-up is the primary concern for biomimetic membrane development. Some fabrication methods form free-standing lipid/polymer membranes and hydrogel-supported lipid layers [45–49]. Others, following the lead of Kaufman et al. [44], demonstrate the approaches to form AQP films atop robust conventional supports [50–52]. A recent approach has been shown by Zhao et al. [53] in which AQPs are first incorporated into proteoliposomes and the resulting structures are included in a cross-linked polyamide coating film (Figure 9.4). Similar to the zeolitic TFN membranes discussed above, here, the proteoliposomes form highly selective preferential flow paths for water passage through the selective layer. These membranes were shown to produce ~40% higher permeability than commercial brackish water RO membranes with similar or greater salt rejection. In addition, these membranes have been produced in 200 cm2 sheets and hold promise for further scale-up, thanks to their utilization of the conventional interfacial polymerization method.

A company was formed in Denmark (see www.aquaporin.dk) to commercialize AQP membranes for water production via RO as well as forward osmosis and energy production by pressure retarded osmosis—as indicated by their patents [54,55]. Membranes are currently available in customizable sizes up to 20 cm × 25 cm and the company targets an automated roll-to-roll production by the end of 2013. The patent claims, “The invention primarily aims at developing an industrial water filtration membrane and device comprising AQPs incorporated into a membrane capable of purifying water with the highest purity, e.g., 100%. No techniques or filters known today can perform this task.” Outside of this company’s patent filings, there is little information available about how their membranes are made, tested, or fabricated in commercial-scale systems, but the recent work by Zhao et al. [53] indicates it may be possible. Further concerns about the long-term and chemical stability of these materials remain. Nonetheless, biomimetic membranes may be the closest real-world analog to Maxwell’s sorting demon because of (a) their high selectivity and (b) the strong basis for further development of bio-inspired water purification membranes.
9.5 Aligned CNT membranes
It is not yet clear if robust protein-based membranes can be developed for practical water treatment applications—especially when subjected to hydraulic pressure, membrane fouling, and chemical cleaning over multiple years of service. Another approach toward bio-inspired membranes is the formation of aligned CNT membranes, reported to exhibit fast mass transport of both gases and water [56,57]. The biomimetic inspiration for aligned CNT membranes comes from studies that demonstrate single-walled CNT channels can be designed to mimic the rapid water transport through biological proteins in addition to gated/active transport [58–60]. Tremendous interest in aligned CNT membranes is evident from the plethora of studies that suggest dramatic fluxes of water through individual CNTs or aligned films of CNTs [61–70].
A molecular dynamics study suggests that membranes comprising sub-nanometer diameter CNTs can desalt water when used as RO membranes [71]. The narrow pores reject ions extremely well, but conduct water at 5–1000 times the rate of commercially available TFC RO membranes. These efficiencies may be achieved if aligned CNT membranes can be fabricated with <1 pore in 100 over the size of 10 Å in diameter. The primary causes of salt rejection and water transport in these studies is the narrow, smooth, nonpolar nature of the CNTs; hence, separation performance may not be specific to chirality number of concentric walls. An idealized structure is depicted in Figure 9.5, showing a highly regular and dense coverage of CNTs embedded in a stable polymeric matrix.

While the exceptional transport characteristics and gating potential of aligned CNT membranes are well recognized, the critical challenge for their application to water purification will be scaling up the synthesis of large membrane areas. Methods of fabricating aligned/oriented CNT membranes are well documented [72–78]. Few are likely to produce simple, fast, inexpensive, and scalable methods of preparing oriented CNT membranes. One approach uses an applied magnetic field to align nanotubes during polymer film casting as proposed by Smalley and co-workers [77,79]. Thick macroscopic membranes of magnetically aligned single-walled CNTs were produced via high-pressure filtration of aqueous surfactant-suspended single-walled nanotube in a magnetic field, resulting in membrane thicknesses of 10 μm and surface area of 125 cm2. Field strengths of 7 and 25 T were used. Polarized Raman spectroscopy indicated good nanotube alignment (uniform anisotropy across their surface and throughout their thickness) at both intensities of magnetic field, but the authors did not evaluate filtration performance. Holt et al. [57] produce sub-2 nm diameter aligned double-walled CNT membranes through an automated microelectromechanical catalytic chemical vapor deposition (CVD) process. Pore densities are as high as 0.25×1012 pores per cm2. Water flux through these CNT membranes is found to be at least three orders of magnitude higher than theoretical, Hagan–Poiseuille predictions; however, alignment via CVD is expensive, sensitive, and not yet applicable for large-scale fabrication.
A more practical approach, originally proposed by Deheer et al. [78] to form aligned CNT films for characterizing their optical and electronic properties, simply filters a suspension of CNTs onto a hydrophilic microfiltration membrane. Subsequently, Li et al. [74] used this approach to prepare a partially oriented super-hydrophobic films of CNTs, where CNTs were capped with platinum nanoparticles to facilitate their eventual use as proton-exchange fuel cell membranes. A cathode prepared from the oriented CNT film produced higher single-cell performance in lab-scale polymer electrolyte fuel cell than cathodes comprised of carbon black and disordered CNT films. Oriented CNT films also exhibit improved mass transport. A paper by Kim et al. [80] describes a similar filtration approach for fabricating aligned CNT polymer nanocomposite membranes for high-flux gas transport.
The lack of experimental desalination performance data for macroscopic aligned CNT membranes fabricated by scalable approaches sheds some doubt on their immediate viability for water purification. Moreover, unrelated research—describing a promising, practical approach to create water soluble CNTs—demonstrates that CNTs become highly carboxylated and sulfonated when exposed for only a few minutes to concentrated nitric and sulfuric acid mixtures (in the presence of heat) [81]. The energy barrier for salt partitioning into CNTs is based on a lack of polar functionality [71]; hence, CNT-based membranes may lose their favorable transport characteristics if exposed to acids. Additional practical considerations remain unclear, including: (1) the long-term stability of CNTs in water, (2) the possibility that solutes may partition into nanotubes and alter water or solute transport, and (3) the long-term fouling resistance and chemical tolerance of aligned CNTs membranes. Despite the lack of experimental desalination performance data, separation characteristics derived from molecular dynamics simulations and small-scale microfabricated films justify continued exploration of aligned CNT membranes.
9.6 Self-assembled block copolymer membranes
Another route to attaining highly porous and ordered structures is self-assembled nanoporous block copolymer membranes. Block copolymers are macromolecules composed of distinct block polymeric segments with the ability to self-assemble into highly ordered structures. By varying the conditions under which self-assembly occurs, various structures can be formed, including densely packed cylindrical pores ideal for water separation membranes, similar to that depicted in Figure 9.5. In the figure, the matrix material would be composed of one of the block segments and the aligned pores composed of a second distinct block segment. Self-assembly occurs when the characteristic differences between polymeric segments on single copolymer chains cause microphase separation to occur. For example, when a selective solvent is added to a solvent–nonsolvent system consisting of macromolecules of two distinct regions—one soluble, the other insoluble—a predictable arrangement will form based on the respective interactions of each polymer with the solvent [82].
Thanks to the tunability of each block, specific functionalities can be attained. Techniques for producing such membranes involve shear aligning (which typically produces thicker than desired films), controlled substrate–polymer interactions (which are effective, but difficult to control in large-scale production), and phase inversion (which is successful, but expensive as the block copolymer is used for both the support and the selective layers) [83]. In theory, aligned cylinders formed through nanostructuring of block copolymers could enable a fully polymeric analog to AQP or aligned CNT membranes, providing an opportunity to take advantage of nanopore performance, while maintaining ease and economy of large-scale polymeric membrane fabrication.
Successful fabrication of ultrafiltration and microfiltration self-assembled membranes has been seen in the literature [83–87]. Yang et al. [88] formed an NF membrane with an 80 nm thick selective layer of well-ordered 15 nm diameter cylindrical pores atop a (250 µm) conventional support, capable of filtering viruses. This approach holds benefits of a highly tunable top layer, with pores ranging from 10 to 40 nm, and the reliability of conventional supports. Using copolymers for the selective layer alone provides large cost savings and may pose an advantage for large-scale production [89]; however, the process is limited in scalability because of the difficulty of transferring films without damage to the porous structure. Other groups have removed the need for a transfer step by forming the self-assembled structures directly atop a conventional support or by forming block copolymer asymmetric membranes [84,90]. Kabuto et al. [91] have shown the ability to form asymmetric self-assembled membranes, from a commercially available copolymer, with a top layer of ordered pores as small as 3 nm. The challenge remains to control the pore size produced and attain nano-ranged pores capable of functioning as RO membranes. Additionally, control of the self-assembly and the ability to produce large, defect-free areas must be achieved in order to consider scale-up.
Membranes with aligned nanopores formed by self-assembly of block copolymers offer a significant promise as fully polymeric analogs to AQP and aligned CNT membranes. In principle, these structures could be fine-tuned for water filtration or RO applications if pore sizes can be accurately controlled and scale-up is made possible. They may also serve as ideal support membranes in high-flux, high-selectivity forward osmosis membranes for osmotic power production. Evidence of large-scale production of self-assembled membranes has not yet been realized.
9.7 Graphene-based membranes
Another carbon-based material that is currently being investigated for desalination membrane materials is graphene [92,93]. Graphene is a 2D sheet of hexagonally arranged sp2-bonded carbon atoms, which is a single to several atomic layers thick, exhibits high mechanical strength, and is impermeable to molecules as small as helium [94,95]. Size-tunable nanopores can be formed in graphene through methods such as oxidation, electron beam irradiation, ion bombardment, doping, diblock copolymer templating, and chemical etching [96–102]. Graphene-based nanoporous films have been demonstrated to function as molecular sieves in applications such as DNA characterization [102]. In theory, regularly sized pores could be formed to create a novel RO membrane material (Figure 9.6).
A recent molecular dynamics study suggests that salt rejection is possible through graphene membranes with pore diameters up to 5.5 Å [93]. The results show potential permeability enhancements of two to three orders of magnitude over conventional seawater RO membranes. It is interesting to note, however, that there is almost no enhancement in permeability per unit thickness relative to conventional polyamide RO membranes, implying that grapheme is not intrinsically more permeable than commercial polymeric membranes. (This assumes a single atomic layer thickness of ~2 Å for graphene [93] and a thickness of ~200 nm for polyamide films.) This means that thin single sheets of graphene need to be produced in mechanically stable forms that can withhold applied pressures in order to realize these extreme flux enhancements. There is promise for large-scale production of graphene, since Bae et al. [103] have shown the ability to produce rectangular sheets as large as 30 in diagonal.
To date, no experimental measurements have been reported to confirm the theoretical transport results. This is an important next step in bringing graphene-based membranes to realization. To break into the membrane market, graphene sheets would need to be manufactured in large, defect-free areas. In addition, the materials will need to be shown to be resistant to fouling and scaling. Only then will it be possible to operate the membranes at higher than normal fluxes, leading to savings on both operating and capital expenses.
9.8 Conclusions
In this chapter, we have discussed a range of nanotechnology-enabled materials touted as possible low-energy replacements for conventional RO membranes in desalination and water reuse applications. Each technology—zeolite-coated ceramic membranes, TFN membranes, protein–polymer biomimetic membranes, aligned CNT membranes, self-assembled block copolymer membranes, and graphene-based membranes—presents an opportunity for performance enhancements if realized; however, each also has unique limitations to overcome. Zeolite coatings promise high selectivity, with separation capabilities only realized with polymeric materials to date, as well as the chemical, thermal, and mechanical stabilities offered by conventional ceramic membranes; however, to date, practical fabrication has not been realized. The intrinsic permeabilities of these materials imply that they could provide economically viable alternatives for high-flux RO membranes with dramatically improved stability and cleanability; however, the challenge remains to improve control over crystal nucleation and growth to ensure defect-free ultrathin zeolite films, which may require abandoning or substantially modifying traditional hydrothermal synthesis methods. Mixed matrix membranes offer mechanically stable, compaction-resistant filtration membranes and support structures for high-pressure applications. TFNs seek to produce compaction-resistant membranes with silica, fouling-resistant membranes with nano-silver, self-cleaning photo-reactive membranes with titania nanoparticles, or highly permeable and selective membranes with molecular sieve zeolites. Data reveal that nanocomposite membranes exhibit up to three times higher permeability than current commercial polymeric RO membranes, with no change in salt rejection, and can additionally be fabricated with antimicrobial and photo-reactive functionality. Multiple authors have demonstrated macroscopic fabrication and practical desalination performance. The major advantage of these materials is the ability to fabricate them through standard practices and with current equipment since only an addition to the casting solutions is required. At this point, long-term stability is undocumented and needs to be explored, in particular for the surface functionalized versions that require exposed surface areas.
Biologically inspired membranes—AQPs, aligned CNTs, and block copolymers—seek to simultaneously improve selectivity and permeability. Each of these innovative materials concepts promises unique performance enhancements and each has unique hurdles to overcome before it is commercially viable. Biomimetic membranes can produce highly selective membranes potentially useful in both forward osmosis and RO applications. AQP-based lipid bilayer vesicles exhibit nearly 100 times higher water permeability than commercial RO membranes with near perfect salt rejection; however, macroscopic fabrication, practical desalination tests, and long-term stability are only beginning to be explored. CNT- and graphene-based membranes (theoretically) exhibit acceptable salt rejections with water permeabilities between 5 and 1000 times higher than commercial RO membranes. Self-assembled block copolymer membranes represent a fully polymeric approach to forming highly selective structures reminiscent of AQP- or CNT-based materials; however, controlling the formation and attaining pores small enough for RO separations remains a challenge. At present, macroscopic fabrication, practical desalination testing, and long-term stability are unproven for these carbon-based and self-assembled membranes; furthermore, all graphene-based membrane projections are based on computational studies alone. Commercial efforts are already under way for both nanocomposite and biomimetic membranes. Currently, there is no evidence of zeolite ceramic, CNT, self-assembled, or graphene membrane commercialization.

