Possible Applications of Fullerene Nanomaterials in Water Treatment and Reuse
So-Ryong Chae, Ernest M. Hotze and Mark R. Wiesner, Department of Civil and Environmental Engineering, School of Engineering, Duke University, Durham, NC, USA
Fullerenes are a class of molecules composed entirely of carbon. The first of these molecules, Buckminsterfullerene, was discovered in 1985 and contains 60 carbons in the form of a hollow spherical cage consisting of 12 pentagonal and 20 hexagonal faces. Other spherical fullerenes or “buckyballs” have since been synthesized as well as nonspherical fullerenes that include cylinders (carbon nanotubes-CNTs), lobed structures, and bowls to name a few. Further variations on fullerenes include the addition of an almost infinite variety of functionalities ranging from simple hydroxylation to the grafting of deoxyribonucleic acid (DNA) molecules. Driven by immediate applications and the utility of undifferentiated material for subsequent modification, there has been a significant commercial emphasis placed on the production of buckyballs and CNTs. In environmental engineering, fullerenes have been proposed as a basis for developing new technologies for nanomaterial-enabled oxidation and disinfection, improved membrane processes, adsorbents, and biofilm-resistant surfaces. This chapter details recent progress toward the development of these proposed applications. We examine the development of fullerene composite materials using CNTs to strengthen membranes and modify membrane surface chemistry. We also explore the use of fullerene nanomaterials to generate reactive oxygen species (ROS) as the basis for a range of new technologies including in situ generation of oxidants to destroy trace organic compounds, new strategics for disinfection, the inhibition of biofilm development, and reduced biofouling. The use of fullerenes in conjunction with ultraviolet (UV) irradiation is considered as an advanced disinfection process (ADP) for viral inactivation.
Keywords
Fullerenes; Buckminsterfullerene; buckyballs; carbon nanotubes-CNTs; CNTs; fullerene nanomaterials; reactive oxygen species; ROS; advanced disinfection process; ADP
21.1 Introduction
Nano-engineered materials are likely to find numerous applications that will improve environmental technologies and help protect public health, including industrial separations, potable water treatment, chemical synthesis, energy generation and transmission, ground water remediation, and air quality control, to name a few.
Fullerenes are a class of molecules composed entirely of carbon. The first of these molecules, Buckminsterfullerene, was discovered in 1985 and contains 60 carbons in the form of a hollow spherical cage consisting of 12 pentagonal and 20 hexagonal faces [1]. Other spherical fullerenes or “buckyballs” have since been synthesized, the smallest containing 20 carbons. Nonspherical fullerenes have also been synthesized including cylinders (carbon nanotubes-CNTs), lobed structures, bowls, and dendrimers, to name a few. Further variations on fullerenes include the addition of an almost infinite variety of functionalities ranging from simple hydroxylation to the grafting of deoxyribonucleic acid (DNA) molecules. Driven by immediate applications and the utility of undifferentiated material for subsequent modification, there has been a significant commercial emphasis placed on the production of buckyballs and CNTs.
Fullerene-based nanomaterials are emerging in a variety of potential applications, including cosmetics, energy production [2], semiconductors [3], and medical treatments [4]. Estimates of the size of the current nanotechnology market range from 30 to 45 billion dollars [5]. In environmental engineering, fullerenes have been proposed as a basis for developing new technologies for nanomaterial-enabled oxidation and disinfection, improved membrane processes, adsorbents, and biofilm-resistant surfaces [6].
This chapter details recent progress toward the development of these proposed applications. We examine the development of fullerene composite materials using CNTs to strengthen membranes and modify membrane surface chemistry. We also explore the use of fullerene nanomaterials to generate reactive oxygen species (ROS) as the basis for a range of new technologies including in situ generation of oxidants to destroy trace organic compounds, new strategies for disinfection, the inhibition of biofilm development, and reduced biofouling. The use of fullerenes in conjunction with ultraviolet (UV) irradiation is considered as an advanced disinfection process (ADP) for viral inactivation.
21.2 Chemistry of fullerene nanomaterials
Fullerenes may exist in a number of geometries such as nanotubes and spherical cages (C60), both of which have been tested for use in environmental application research (Fig. 21.1). The latter form has an extremely low solubility in water [7] and therefore they must be modified on the surface [8], clustered (nC60) [9], or mixed with a surfactant or stabilization agent [10] for significant concentrations to be reached in water. As a variation on fullerenes, CNTs are limited by similar solubility constraints and also need to be functionalized or coated to increase their affinity for water [11]. This is a primary consideration when adapting fullerene nanomaterials for environmental applications.
Two varieties of CNTs are multi-walled carbon nanotubes (MWCNT) and single-walled carbon nanotubes (SWCNT). SWCNT can be visualized as graphite sheets that have been rolled up and seamlessly attached via carbon bonds. The nature of this rolling (chirality) determines whether these materials are metallic (conducting electricity) or are semiconductors [12]. MWCNT are made up of two or more such tubes wrapped around each other, similar to the layers of an onion. Of the two, SWCNT are more attractive for use in applications due to their purity and uniformity where tensile strength (stronger than steel), electrical conductivity (comparable to copper), and thermal conductivity are all improved over MWCNT. However, functionalizing the surface of the SWCNT to improve its affinity for water (or for some other reason) may entail a sacrifice in the strength or conductivity of the SWCNT.
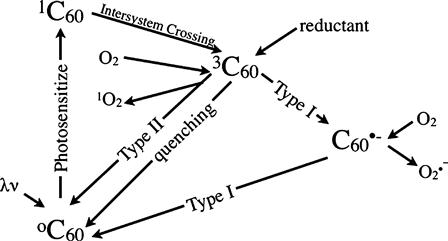
While the surface chemistries of nanotubes and C60 are quite similar in the aqueous environment their photochemistries are very different. In particular, C60 is a photoactive molecule. Photoactive materials may be classified as photocatalysts such as titanium dioxide [13] or photosensitizers such as the dye Rose Bengal (RB) [14] and the molecule C60. Photocatalytic behavior is governed by the band gap that can be described as the energy difference between the valence band (Fermi level of the highest energy electrons) and the conduction band (lowest energy unoccupied molecular orbitals). Light striking a photocatalyst will promote an electron across this band gap creating holes in the molecular orbitals it leaves behind. Both the promoted electrons and the holes can react in solution to form ROS. In contrast, electrons in photosensitizers are excited within their molecular orbitals. Sensitized electrons can behave according to two mechanisms: type I electron transfer involving a donor molecule and type II primarily involving energy transfer to a ground state oxygen. Both of these pathways will potentially produce ROS in solution. The net effect of both these processes is to convert light energy into oxidizing chemical energy. This chemical energy can then potentially be used to oxidize contaminants or disinfect microorganisms. In the case of pure C60 the photosensitization process happens efficiently due to its ability to perform intersystem crossing with close to zero loss of energy [15]. However, in aqueous systems C60 will form clusters. Cluster formation will tend to decrease the lifetime of the triplet state and therefore the formation of ROS [16]. The addition of functional groups and coatings that reduce clustering may increase ROS production [16–18] although the addition of functional groups to the C60 cage tends to reduce the quantum yield of the triplet state that is most responsible for ROS formation. A diagram of possible C60 photosensitization pathways is given in Fig. 21.2.
21.3 Applications of fullerene nanomaterials
21.3.1 Membrane fabrication using fullerene nanomaterials
Membrane technologies are playing an increasingly important role as processes for removing salts, particles, organic matter, or gases from water. The performance of membranes is intimately linked to the materials they are made from. The composition of the membrane will determine important properties such as rejection (selectivity), propensity to foul, mechanical strength, and reactivity.
Fullerene nanomaterials have unique properties of strength, ability to tailor size, flexibility in modifying functionality, and electron affinity that have created much excitement around their potential for new membrane-based technologies. The small and controllable diameter of fullerene nanotubes suggests that membranes made from these materials in a fashion where fluid flows through the center of the CNT might be highly selective. The small diameter of the CNT also implies a high resistance to flow through a membrane composed of such nanometer-sized pores. Surprisingly, molecular modeling indicates that water should be able to flow much faster through hydrophobic CNTs due to the formation of ordered hydrogen bonds [19]. The hydrophobic surface in the interior of a defect-free CNT appears to allow for a nearly frictionless flow [19]. Visualization of water within CNTs confirms the lack of interaction between water molecules and the interior surface of CNTs [20] and experiments using membranes composed of aligned CNTs have confirmed that flow through the CNTs is orders of magnitude greater than that predicted by Poiseuille flow through tubes composed of conventional materials [21,22].
There are also promising applications for fullerene–polymer composites in pressure-driven membranes. The strength of CNTs, coupled with reported antibacterial properties, suggest that fullerene–polymer composites may find use in creating membranes that resist breakage or inhibit biofouling. The incorporation of C60 into polymeric membranes has been observed to effect membrane structure and rejection [23].
We have explored the use of MWCNTs in the development of advanced composite membranes for applications in water treatment. MWCNT (4% w/w) were incorporated as a composite into polysulfone ultrafiltration (UF) membranes, prepared according to the wet-phase inversion method. The dispersion of the nanotubes and the morphology of the membranes were observed by scanning electron microscopy (Fig. 21.3, adapted from Brunet et al. [24]). The membranes were characterized for surface roughness, contact angle, permeability, and mechanical properties. A partial de-aggregation of the nanotubes leads to individual nanotubes within the polymer as well as bundles nested in the pores. After addition of MWCNTs, the asymmetric structure of the membrane and the permeability were not disturbed, neither was the hydrophobicity, but the roughness increased. However, the tensile strength of the composite membrane was not improved in this case, suggesting the need to match CNT functionalization to the polymer to more evenly distribute the nanotubes throughout the composite.

Future convergence between nanochemistry and membrane science will likely yield a generation of active membrane systems. Nanomaterials might also be incorporated into membranes to impart properties that are activated by an electrical or chemical signal. Living organisms are the ultimate nanotechnology. The ability of cell membranes to selectively transport materials, often against concentration gradient, and to avoid fouling is impressive. As the field of nanochemistry advances, engineered biomimetic systems based on selective transport or rejuvenating layers of self-organizing materials may be developed for performing critical separations in energy and environmental applications.
21.3.2 Oxidation of organic compounds
The ROS producing properties [11,15,25] of fullerenes might be harnessed to generate oxidizing species to enhance destruction of organic compounds in water [26]. We are exploring the oxidation of probe organic compounds by ROS generated from a suspension of hydroxylated fullerene (fullerol, C60(OH)22–24)) prepared using a sonication method that rapidly produces stable suspensions of fullerol aggregates [27]. The efficiency of contaminant destruction under UV irradiation can be compared with that of a known photosensitizer that produces singlet oxygen, RB [28]. Results to date show proof of concept for this approach, although the degree of compound destruction is modest and compound-specific. Specificity of the reaction may be exploited to achieve destruction of one compound while avoiding undesirable oxidation by-products that arise from reactions with other materials. Figure 21.4 illustrates the relative destruction of 2-chlorophenol (2-CP) by equal concentrations of fullerol and RB when irradiated with UV light. For the conditions applied, approximately 17 percent of the 2-CP was destroyed after 30 minutes of irradiation. RB produced a slightly greater degree of photo-induced degradation (28 percent). For further study, it is important to understand oxidation kinetics of various organic compounds at various temperatures and pHs. Finally, the formation of ROS from fullerol suggests the possibility of engineering fullerol-sensitized systems to destroy organic compounds or possible disinfection in water and wastewater.
21.3.3 Bacterial and viral inactivation
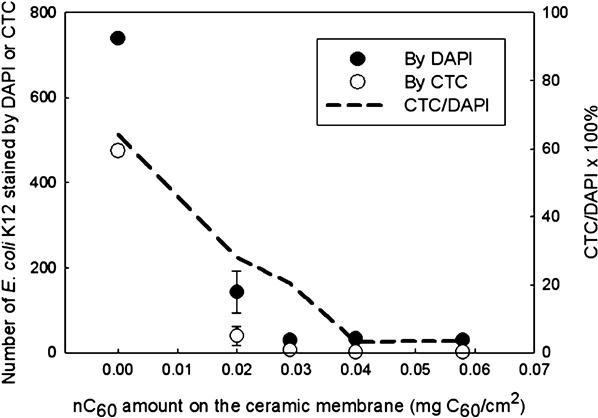
Fullerenes have also been considered for their possible antimicrobial properties. The medical literature details the ability of C60 and C70 fullerenes to cleave DNA and inactivate viruses, bacteria, and kill tumor cells [29,30] suggesting that they might be used for disinfection [28] or to produce surfaces resistant to microbial growth. We consider here the example of inactivation of waterborne bacterial viruses [27] and the development of anti-fouling agents for membranes used in water and wastewater treatment where biofouling is known to be a critical limitation [31]. As an illustration of the latter case, we have modified ceramic membranes by depositing a layer of C60 and then observed impact on bacterial attachment and metabolic activity. Bacterial activity was monitored in terms of the total number of bacteria present (DAPI) to those that were metabolically active (CTC). As shown in Fig. 21.5, the total number of bacterial colonies of E. coli K12 and the fraction of viable bacteria as measured by the CTC to DAPI ratio (CTC/DAPI) decreased rapidly as the amount of C60 on the membranes increased. Thus, both the affinity for bacterial attachment to the membranes and bacterial viability decreased with increasing amounts of C60 present on the membranes. It is possible that extracellular polymeric substances (EPS) and soluble microbial product (SMP) will hinder contact between bacteria and C60 and therefore limit long-term efficiency of C60 in anti-biofouling activity. Such “fouling of anti-foulants” remains an active area of research.
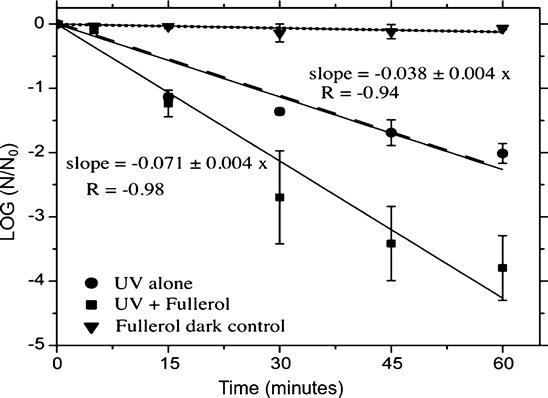
The impact of fullerol on virus was studied using the MS2 bacteriophage, a virus that is used extensively for studies of water pollution and control [32–34], and it is similar in morphology to hepatitis A virus and poliovirus. A simple dilution and enumeration technique allowed for viruses to be counted before and after exposure to fullerol and low pressure UV light (approximately 365 nm). As seen in Fig. 21.6, the addition of as little as 1 μM fullerol into the suspension nearly doubled the log inactivation rate of the MS2 phage in UV light alone.
21.4 Summary
Fullerenes are versatile, new materials with properties that suggest great potential for improving water treatment technologies. However, our ability to manipulate and fully exploit the properties of these materials remains limited, requiring further innovations to develop efficient formats for immobilizing, recovering fullerenes. More revolutionary changes in water treatment using these materials are likely to be based on the ability to conceive of and build devices that actively function at the scale of the material itself. Such devices might combine functions such as energy harvesting and treatment, detection or control.
Acknowledgements
This study was supported in part by ONR grant 05032901, NSF grant BES-0508207, and partial funding by a grant from the Korea Research Foundation (MOEHRD) (KRF-2006-D00125).