13
Solid Waste (SW) Leachate Treatment using Constructed Wetland Systems
K.B.S.N. Jinadasa1, T.A.O.K. Meetiyagoda2 and Wun Jern Ng3
1Department of Civil Engineering, University of Peradeniya, Sri Lanka
2R&D Unit, CETEC Pvt Ltd, Kandy, Sri Lanka
3Nanyang Environment and Water Research Institute, and School of Civil and Environmental Engineering, Nanyang Technological University, Singapore
13.1 The Nature of Solid Waste (SW) and SW Leachate
Solid wastes are any discarded or abandoned materials and can be solid, semi-solid, or containerized gaseous and liquid materials. In the urban context, Municipal Solid Waste (MSW), commonly referred to as trash or garbage, comprises everyday items used and then discarded. Such items include product packaging, grass clippings, furniture, clothing, bottles, food scraps, newspapers, appliances, paint, and batteries. These can originate from homes, schools, hospitals, and commercial premises. A typical MSW gross composition is given in Table 13.1, where the largest component is organic materials. MSW composition varies even at a particular site; Tables 13.2 and 13.3 show such a variation at a site in Punjab, India. In Table 13.2, the largest component is the compostable materials and water, while nitrogen, phosphorus and potassium percentages are relatively low (Table 13.3) [1, 2].
Table 13.1 Typical MSW gross composition [1].
| Component | Percentage (wt. %) |
| Organic materials | 40 |
| Unrecyclable plastics | 10 |
| Unrecyclable materials | 30 |
| Agriculture waste | 20 |
Table 13.2 Composition of MSW at Punjab site, India [2].
| Category | Item | % |
| Recyclable material | Paper, plastic, rags | 3–5 |
| Leather, rubber, synthetic | 1–3 | |
| Glass, ceramics | 0.5–1 | |
| Metals | 0.2–2 | |
| Compostable material | Food articles, fodder, dung, Leaves, Organic material | 40–60 |
| Inert material | Ash, dust, sand, building material | 20–50 |
| Moisture | 40–80 | |
| Density | 250–500 kg/m3 |
Table 13.3 Chemical Composition of MSW at a Punjab site, India [2].
| Item | % |
| Nitrogen | 0.56–0.71 |
| Phosphorus | 0.52–0.82 |
| Potassium | 0.52–0.83 |
| C/N | 21–30 |
Solid wastes have become a sanitation threat in much of the developing world. The most common method of solid waste disposal is landfilling. Proper sanitary landfill sites have liners and covers, and operations such as compaction, leachate management, and gas collection to protect human health and the environment. Unfortunately, not all landfills have been appropriately constructed and operated, and in South and Southeast Asia more than 90% of all landfills are non-engineered disposal facilities [3].
Leachate is water, which has passed through the filled material and leached (i.e., extracted) material such as dissolved and suspended organic and inorganic matter from the landfill. The water is often the result of precipitation infiltrating the landfills. In addition, the water originally present in the wastes can be released and so contributes to leachate generation [1].
The quantity of leachate production and leachate composition are affected by the climatic factors at the landfill site. Tropical countries have year round temperatures of above 18°C. There is also often high precipitation and hence humidity. Therefore, during rainy seasons (especially in monsoon seasons), leachate production can be expected to increase. While during the hot and dry season, accelerated degradation of wastes can occur although leachate volume is reduced. For example, leachate is formed mostly in the period from November to April in Poland, with the maximum leachate volume occurring in December. During dry periods, leachate would not be noted from May to October [4]. In the tropical environment where rain can occur year round, such distinct periods of leachate production may not occur.
The volume of leachate requiring management would very much depend on the waste's initial moisture content and composition, biochemical and physical transformations, and inflow of water from outside the landfill [5, 6].
13.2 Characteristics of SW Leachate in Tropical Developing Countries
Tropical regions, in the lowlands, have mean annual temperatures exceeding 20°C and these can even exceed 25°C. There are tropical regions which are affected by distinctly seasonal rainfalls, the monsoons. Therefore, leachate production can have distinct variations in the year.
Many landfills in developing countries are in effect open dumpsites where the solid wastes are dumped in an uncontrolled manner. Composition of landfill leachates is influenced by internal and external factors. External factors include solid waste composition, operational mode of landfill, climate, and hydrological conditions. The internal factors are biochemical activities, moisture, temperature, pH and the age of the landfill [5]. MSW leachates are concentrated and complex effluents, which contain inorganic and organic compounds including possibly toxic and hazardous chemicals [7–9]. The organics can include aromatic compounds, halogenated compounds, phenols, pesticides and nitrogenous compounds [10, 11].
Properties of a leachate are often categorized as physical, chemical and biological. Physical properties include solids content, turbidity, temperature, conductivity, color, and density. Chemical properties can be further divided into two categories: inorganic and organic constituents. Chloride, pH, alkalinity, gases, metals, nitrate, phosphate, ammonium are among the inorganic constituents. Proteins, carbohydrates, and oil and grease are among the organic chemical constituents. Pathogens and other microorganisms are among the biological constituents.
The amount of organic pollutants in landfill leachate can be measured in terms of biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total organic carbon (TOC), inorganic carbon (IC) and total carbon (TC). Leachate quality is often also defined with parameters such as dissolved oxygen (DO), pH, oxidation reduction potential (ORP), electrical conductivity (EC), suspended solids, total nitrogen (TN) and total phosphorous (TP).
The composition of landfill leachate at different landfills in Sri Lanka is given in Table 13.4. The highest BOD5 and COD values were recorded at the Kolonnawa site.
Table 13.4 Leachate composition at Sri Lankan solid waste dumpsites [12].
| Sample | BOD5 (mg/L) | COD (mg/L) | BOD5/COD | TN (mg/L) | TP (mg/L) |
| Matale | 71 | 4236 | 0.02 | 1549 | 43 |
| Hambantota | 49 | 2475 | 0.02 | 224 | 12 |
| Kataragama | 38 | 1047 | 0.04 | 180 | 20 |
| Bandargama | 938 | 9279 | 0.1 | 305 | 24 |
| Kolonnawa | 49600 | 82577 | 0.6 | 664 | 11 |
| Gampola | 41 | 1249 | 0.03 | 1212 | 6 |
| Gohagoda | 19 | 714 | 0.03 | 684 | 8 |
| Wennappuwa | 86 | 2405 | 0.04 | 361 | 18 |
| Rathnapura | 3757 | 13951 | 0.27 | 558 | 35 |
| Negombo | 294 | 18328 | 0.02 | 703 | 86 |
Leachate characteristics at three landfilling sites in Ludhiana City, Punjab (India) are shown in Table 13.5. The variability needs to be noted and this is also related to age of the site. The concentrations of leachate contaminants at Jamalpur and Noorpur belt are higher than that at Jainpur, which is the oldest one.
Table 13.5 Leachate characteristics at sites in Ludhiana City, Punjab, India [2].
| Parameters | Jainpur | Jamalpur | Noorpur Belt |
| Appearance | Brownish | Brownish | Brownish |
| Odor | Sewage smell | Sewage smell | Sewage smell |
| pH (–) | 9.3 | 9.8 | 9.5 |
| TS (mg/L) | 5,963 | 7,695 | 6,579 |
| SS (mg/L) | 615 | 1,132 | 886 |
| TDS (mg/L) | 5,348 | 6,563 | 5,693 |
| Turbidity (NTU) | 43 | 79 | 68 |
| Hardness (mg/L) | 585 | 638 | 621 |
| BOD5 (mg/L) | 329 | 495 | 406 |
| COD (mg/L) | 1,335 | 2,535 | 2,018 |
| BOD5/COD | 0.24 | 0.19 | 0.20 |
| Chloride (mg/L) | 1,448 | 1,836 | 1,653 |
| Nitrate (mg/L) | 12.5 | 18.6 | 15.9 |
| Total phosphorus (mg/L) | 52.8 | 83.5 | 64.3 |
| Sulphate (mg/L) | 48.7 | 65.1 | 53.8 |
The age of a landfill significantly affects the quantity and quality of the leachate produced. The water retaining capacity is affected due to mineralization of organic substances [13]. The relationship between leachate properties and landfill age is given in Table 13.6 [11, 14, 15] and [16].
Table 13.6 Leachate characteristics at various landfill ages [13].
| Parameter | |||||||
| Age (years) | BOD (mg/L) | COD (mg/L) | BOD: COD | NH3-N (mg/L) | SS (mg/L) | pH | Reference |
| Young (<5) | >2000 | >10000 | >0.3 | – | – | 6.5 | [11, 14] |
| Intermediate (5–10) | 150–2000 | 4000–10000 | 0.1–0.3 | – | – | 6.5–7.5 | |
| Old (>10) | <150 | <4000 | <0.1 | – | – | >7.5 | |
| Young (5) | 2031.62 | 3641.2 | 0.31 | 288.6 | – | 6.52 | [15] |
| Old (15) | 196.83 | 875.44 | 0.20 | 260.03 | – | 5.72 | |
| Young (<5) | 32790 | 41507 | 0.79 | 1896 | 1873 | 6.6 | [16] |
| Intermediate (5–10) | 2684 | 5348 | 0.50 | 1826 | 143 | 7.9 | |
| Old (>10) | 145 | 1367 | 0.11 | 892 | 17.2 | 8.2 | |
Landfilled organic materials undergo anaerobic degradation and this goes through two phases of biological transformations – the acidogenic phase and methanogenic phase. Old landfills would have largely passed the acidogenic phase and so release methanogenic leachate, while producing methane and carbon dioxide [17]. In the acidogenic phase, acidogens convert soluble organic material mainly to acetate, propionate, butyrate, hydrogen, and carbon dioxide.
Less than 5 year-old landfills are largely acidogenic and so can have leachate pH of 3.7–6.5, reflecting the presence of carboxylic acids and bicarbonate ions. As the landfills age, leachate becomes neutral or weakly alkaline (pH of 7.0–7.6) and eventually alkaline (pH 8.0–8.5) [18].
13.3 Treatment Methods for SW Leachate
Commonly, leachate is characterized by high values of COD, pH, ammonia nitrogen and heavy metals, as well as strong color and odor. However, these values are time dependent in relation to a landfill's age [19, 20]. It is not only the quantities that change with age, but also how a particular leachate component is formed can also change with age. Ammonia nitrogen in the leachate from young landfills results from the deamination of amino acids during destruction of organic compounds [21], but high concentration of ammonia nitrogen can also be found in leachate of older landfills due to hydrolysis and fermentation of the nitrogenous fractions of biodegradable substrates [22]. Such behavior makes leachate treatment potentially difficult.
Inanc et al. [23] identified methods to treat landfill leachate including:
- Aerobic treatment such as aerated lagoons, and activated sludge.
- Anaerobic treatment such as anaerobic lagoons.
- Physicochemical treatment such as air stripping, pH adjustment, chemical precipitation, oxidation, and reduction.
- Coagulation using lime, alum, and ferric chloride.
- Advanced techniques such as carbon adsorption, and ion exchange.
Biological processes are often used to treat landfill leachate because of their overall reasonable costs, and especially their operational costs (compared to chemical methods). Biological methods can serve as “pretreatment” to reduce high concentrations of BOD, COD and ammonium before polishing with physico-chemical methods. Biological treatments are, therefore, suitable for application on young leachates (<5 years), which would have higher organic concentrations and BOD/COD ratio. However, mature landfill leachates not only have lower organic concentrations, but the biodegradable organic fraction declines. Therefore biological treatment is potentially less effective for old landfill leachate [11].
Where the activated sludge method is used, leachate is aerated in an open tank with diffusers or mechanical aerators. This is a suspended growth process [24, 25]. Aerobic treatment in its most basic engineered configuration is the oxidation lagoon. This is a naturally aerated pond. However, to achieve satisfactory treatment results, oxidation lagoons require a large amount of land area to ensure sufficient oxygen transfer and, hence, avoid septic conditions. Unlike the activated sludge process, which is dependent on flocculated biomass, the active biomass in the oxidation lagoon largely accumulates as a biofilm on the lagoon bottom and sides.
Landfill leachate has been effectively treated with the rotating biological contactor (RBC). RBC consists of large disks supporting biofilms and with radial and concentric passages slowly rotating in a trough. While rotating, 40% of the support media surface area is in the leachate. The continuous rotation and alternating exposure to air and leachate allows microorganisms to metabolize substrates aerobically [24, 25].
The Sequencing Batch Reactor (SBR) is a complete-mix batch activated sludge system without a secondary clarifier. The SBR differs from the conventional activated sludge plant in its temporal-frame process arrangement in contrast with the spatial-frame arrangement in the latter [26]. The SBR operating cycle typically comprises fill, react, settle, draw and idle.
It should be noted that aerobic processes, as those described above, are unlikely to be able to degrade all the types of organic compounds found in leachate, as these may be persistent in the face of aerobic metabolic activity. Consequently, the treated leachate can still have a significant residual organic content. Often the aerobic process is preceded by an anaerobic process, which then serves as a pretreatment stage to reduce organic strength. Such an approach is often necessary to reduce both the capital and operating costs of an aerobic process only system. A high degree of waste stabilization is possible with low production of excess biological sludge. The anaerobic process also has lower nutrient requirements (making nutrients supplementation unnecessary), no oxygen requirements, and the process by-product of methane is a useful energy source.
There are many types of anaerobic systems incorporating suspended or attached growth, fixed and moving film systems, and combinations of these. In the absence of oxygen (free and combined), organic matter is converted to carbon dioxide and methane gas (Figure 13.1). Since such metabolism yields relatively little energy to the microorganisms, their growth rates and cell yields are lower than those in aerobic processes.
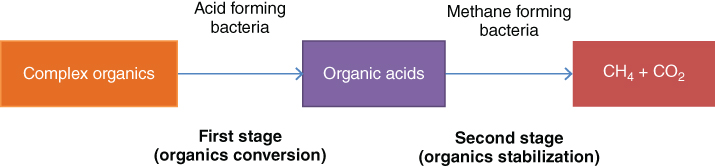
Figure 13.1 Organic matter conversion in the absence of oxygen [1].
Volatile fatty acid intermediates are primarily acetic and propionic acid. During acidogenesis, pH can be reduced due to fatty acid production if there is inadequate buffering capacity. Low pH has the potential to inhibit further acidogenesis and would inhibit methanogenesis. Typically pH values are maintained between 6.4 and 7.2, to allow both acidogenesis and methanogenesis to proceed satisfactorily.
In engineering terms, the simplest of the anaerobic systems to construct would be anaerobic ponds. These are designed deep (deeper than aerobic ponds) and operated with high organic loadings, and such high loadings are used to maintain anaerobic conditions in the pond beneath the top liquid layer. Anaerobic ponds are typically operated with long hydraulic detention times and would have no induced mixing. Consequently the design and placement of the inlet and outlet works must be such that short-circuiting is avoided.
The upflow anaerobic sludge blanket (UASB) process is a sludge blanket system. UASB technology has been applied in tropical countries where the relatively high ambient temperatures make it easy to operate a mesophilic process. In the UASB, the leachate enters the reactor from the bottom and flows upward through an anaerobic sludge blanket. The three key engineered elements in the reactor are the influent distribution system, the three phase (gas, liquid, and solids) separator, and effluent withdrawal system.
Ion exchange resins, synthetic and natural, have had wide application in water and wastewater treatment [27] and can also be expected to potentially applicable in leachate treatment.
Ammonia dissolved in the leachate can be removed as a gas, using air stripping. Air stripping can be carried out in tanks or packed towers, or in counter-current, multi-stage reactors. The pH value is important and when it is lower than 7, ammonia tends to remain in the solution. However, when pH is raised to 11, ammonia tends to come out of the solution and enters the gas phase. Therefore, in a gas stripper, pH is adjusted to 11–12 by adding NaOH.
Coagulation can be used to remove suspended and colloidal matter, and some dissolved matter as well. Its efficiency can, however, be influenced by the appropriateness of the coagulant in relation to the target pollutants, dosage of coagulant, pH, mixing speed and duration, temperature and dissolved solids content [28]. Inorganic coagulants such as aluminum sulfate (Alum) and ferric chloride (FeCl3) are most commonly used [29]. Poly aluminum chloride (PAC) has also been found an effective coagulant and can be used in place of alum [30]. Typically PAC dosages are lower than alum and this would result in lower quantities of aluminum sludge produced, which would require subsequent disposal.
Residual organics remaining after biological and chemical treatments can be removed with activated carbon adsorption. The disadvantage of this method is its associated operating costs, since the activated carbon should be replaced (or regenerated) at regular intervals [25].
Given the complex nature of leachate, a single unit treatment process is unlikely to be adequate. Leachate treatment systems would typically begin with an anaerobic process, followed by aerobic, chemical, and final physical processes (e.g., sorption).
Constructed wetlands (CWs) do inherently include many of the processes described above. CW systems have aerobic and anaerobic processes. These are mediated by macrophytes and microphytes. Additionally, the soil on which the macrophytes grow, can also mediate physical and chemical processes.
13.3.1 Advantages of Constructed Wetlands for Leachate Treatment Under Tropical Climate
Constructed wetlands (CWs) are engineered systems that are used as a secondary or tertiary treatment process for wastewater treatment. Compared to other treatment systems, CWs are low cost, easily operated and maintained, and have a strong potential for application in developing countries [31].
There are two general types of wetlands: surface flow systems and subsurface flow systems [31]. Subsurface flow CWs are further divided into two types according to flow direction: vertical subsurface flow (VSSF CWs) and horizontal subsurface flow (HSSF CWs) [31]. Surface flow CWs are further divided into three types based on the plants used (Figure 13.2). Hybrid constructed wetlands combine the different types of CWs to enhance treatment efficiency. Hybrid CWs tend to have higher pollutant removal efficiency than the single type CW systems [31]. Hybrid CWs can be seen as analogs of the anaerobic–aerobic treatment systems discussed earlier. For example, the VSSF CW would act as an aerobic system, while the HSSF CW system would act as an anaerobic system. Constructed wetlands have ecological conditions similar to natural wetlands [32].

Figure 13.2 Constructed Wetlands classification [31].
Microorganisms in the wetlands system are in suspension, adhering to the plants, and in the soil. These then use the pollutants in the leachate as substrates. Aerobic heterotrophic bacteria degrade BOD5 and COD, while nitrogen compounds are converted by autotrophic bacteria.
The processes that affect nitrogen conversion include ammonia volatilization, nitrification, denitrification, nitrogen fixation, plant and microbial uptake, mineralization (ammonification), anaerobic ammonia oxidation, and, sorption [31, 33]. CWs have ability to remove heavy metals in the leachate by binding to soil or sediments due to positive charge of the heavy metal cations, precipitation as insoluble salts (CO32–, HCO3–, and OH–), and uptake by bacteria and plants. Various processes are also involved in the removal of pathogens in CWs and these include adhering to particulates and sedimentation, filtration, UV radiation, unfavorable water chemistry, temperature, predation, and antibiotics that are produced by the plants [31].
CWs are more sustainable in terms of energy requirements compared to mechanical systems such the activated sludge, since the primary energy requirement for macrophyte maintenance would be fulfilled by solar energy [31]. CWs can also be aesthetically landscaped and have often served as wildlife sanctuaries. Moreover, treated effluent from CWs can be used as irrigation water for agriculture.
13.4 Experimental Methodology for Plant Species and CW Performance Evaluation
Evaluation of CW performance with different plant species can be performed at three levels. Analysis can be initiated with batch tests to assess leachate treatment efficiency. For this, laboratory-scale sub-surface flow CW units have been used with three wetland plant species: narrow leaf cattail (Typha angustifolia), green bulrush (Scirpus atrovirens) and umbrella palm (Cyperus alternifolius). Replicates of each plant species are fed with various dilutions leachate, e.g., 25%, 50%, 75% and 100% leachate and with the challenges lasting 7 days. This allows for quick screening and determination of suitable plant species to use.
The second level of tests could be a pilot scale experiment in the field. For example, subsurface flow hybrid wetlands with 7 days HRT have been used with each CW test unit comprising a VSSF followed by a HSSF sub-unit and planted with Typha angustifolia (Plate 1; Figure 13.3). The length, width, and height of the VSSF sub-unit are 1 m, 0.7 m and 0.6 m, respectively. The effective height was 0.5 m. The configuration of the HSSF sub-unit is 1 m × 0.7 m × 0.45 m.

Figure 13.3 Plate 1: Pilot scale hybrid constructed wetland units planted with Typha angustifolia.
In the VSSF sub-unit, the bottom layer (5 cm) comprised 32 mm coarse gravel to disperse the flow and to allow for drainage of the treated leachate. The next 20 cm of the bed is filled with 19–20 mm medium gravel, and on top of that is 20 cm of 6–8 mm fine gravel. The top layer is a 5 cm soil layer (Figure 13.4).

Figure 13.4 Layer arrangement of the pilot-scale VSSF CWs.
A 45 cm high layer of 32 mm coarse gravel is used to distribute the influent flow into the HSSF sub-unit and to collect the treated effluent at the other end of the system. The middle layer is filled with 6–8 mm fine gravel up to 40 cm in height and the remaining 5 cm is filled with soil (Figure 13.5). If the HSSF sub-units are not receiving effluent from the VSSF sub-units, then the leachate may be applied at the following dilutions: 10%, 25%, and 50% of leachate.

Figure 13.5 Layer arrangement of the pilot-scale HSSF CWs.
While second level tests may be conducted with synthetic leachate so that consistent test conditions may be obtained, third level tests should be performed with the real leachate. The example shown in Plate 1 (Figure 13.3) had performance analysis based on real leachate collected from the “Gohagoda dumpsite” in Kandy, Sri Lanka (Plate 2; Figure 13.6) and with three different wetland plant species (Plates 3–5; Figure 13.7): narrow leaf cattail (Typha angustifolia) (Plate 3; Figure 13.7a), green bulrush (Scirpus atrovirens) (Plate 4; Figure 13.7b) and umbrella palm (Cyperus alternifolius) (Plate 5; Figure 13.7c). In such tests, the plants were challenged with 5% and 25% dilutions of the real leachate. Narrow leaf cattail (Typha angustifolia) (Plate 3; Figure 13.7c) belongs to the Typhaceae family. This is a commonly used plant for wastewater treatment. This plant is a helophyte and can survive in marshy conditions. There have been reports in the literature of this plant having been used for wastewater and leachate treatment. Green bulrush (Scirpus atrovirens) (Plate 4; Figure 13.7b) belongs to the Cyperaceae family. This plant has also been used in CWs for wastewater treatment. This perennial plant is about 2½–4' tall, un-branched and more or less erect. The culm is green, glabrous, and terete. It can survive flooding and drought conditions. Umbrella palm (Cyperus alternifolius) (Plate 5; Figure 13.7c) belongs to the Cyperaceae family and is commonly grown as a pond plant especially in shallow water. A favorable condition for growth is when plant is submerged up to 10 cm and so this plant has a higher tolerance to flooded conditions.

Figure 13.6 Plate 2: Gohagoda dumpsite, Kandy, Sri Lanka.

Figure 13.7 (a) Plate 3: Typha angustifolia; (b) Plate 4: Scirpus atrovirens and (c) Plate 5: Cyperus alternifolius.
The Gohagoda dumpsite (Plate 2; Figure 13.6) is otherwise known as the Thekkawatta Landfill and is located in Kandy district at 7°18′47.33′′N, 80°37′17.19′′E Sri Lanka. This area receives annual rainfall of 2,500 mm. Gohagoda dumpsite has been in operation since the 1970s. The Mahaweli River flows 200 m below the dumpsite and there is a threat of leachate contamination.
13.5 Effect of Plant Species on Leachate Components
13.5.1 Effect on Organic Compounds
Organic compounds in landfill leachate include fatty acids, humic acids and fulvic-like substances [34]. The molecular weights of these three groups are low, high and intermediate respectively. The biodegradable fraction of the organics is measured by the BOD, while the non-biodegradable fraction is measured in terms of the difference between the BOD and COD values [35]. Aerobic and anaerobic degradation of soluble organic substances are responsible for removal of BOD. Hydrolysis and catabolic activities of autotrophic and heterotrophic bacteria contribute to the biodegradation of these compounds. The following reaction shows the biodegradation of organic compounds involving aerobic heterotrophic organisms:
Anaerobic degradation of organic compounds can also occur in CWs, going deeper into the soil supporting the macrophytes, and in thick biofilms occurring elsewhere on the macrophytes and suspended particles. This process has different stages of reactions involving facultative and obligate anaerobic heterotrophic microorganisms. The first step is fermentation performed by facultative microorganisms.

Then, anaerobic microbes use the above end-products and convert these into methane and carbon dioxide. Where there are oxidized species present such as nitrates and sulphates, then there can also be end-products such as nitrogen and hydrogen sulphide.

The BOD5/COD ratio indicates the biodegradability of a leachate. At young landfills (landfilled wastes not older than 3–5 years), the BOD5/COD ratio is high (0.7), indicating high biodegradability of organics in the leachate. In mature landfills (5–0 years) the BOD5/COD ratio decreases to 0.5–0.3 and the proportion of easily biodegradable organic matters decreases proportionately, while the non-biodegradable matter would have remained intact. Landfills over 10 years are considered as old landfills and these typically have low BOD5/COD ratios of possibly less than 0.1. As the BOD5/COD ratios decline, biological treatment processes become less effective [36]. Portions of the Gohagoda dumpsite may be considered an intermediate stage landfill site (given the continuous use) and its BOD5/COD is about 0.3. This would suggest dilution at 25% leachate would be appropriate for the tests. Examples of test results at various organic (in terms of BOD and COD) loading rates on CWs are shown in Table 13.7.
Table 13.7 Organic loading rates of synthetic leachate applied to CWs.
| Organic loading rate (g/m3/d) | ||
| Dilution at X% Synthetic leachate | BOD | COD |
| 100% | 63.24 | 371.2 |
| 75% | 41.08 | 280.5 |
| 50% | 23.34 | 190.8 |
| 25% | 11.69 | 90.7 |
The BOD, COD, and TC removal efficiencies by the three plant species tested are given in the Table 13.8. All three plant species performed well for BOD, COD, and TC removal with 25% leachate and Umbrella Palm was the most effective. The pilot-scale tests results (using synthetic leachate) are given in Table 13.9. The good performances indicated by the laboratory tests were reproduced in the pilot tests. In addition, Table 13.10 shows the average COD concentration profiles at various stages in the CWs.
Table 13.8 BOD5 Removal efficiency (%).
| 100% | 75% | 50% | 25% | |||||||||
| Plant type | BOD5 | COD | TC | BOD5 | COD | TC | BOD5 | COD | TC | BOD5 | COD | TC |
| Narrow leaf cattail | 86.4 | 83.1 | 77.3 | 86.3 | 88.7 | 84.7 | 89.0 | 96.2 | 91.2 | 89.5 | 99.1 | 90.3 |
| Green bulrush | 85.3 | 79.7 | 68.0 | 79.8 | 85.6 | 72.0 | 89.9 | 87.8 | 82.8 | 92.9 | 98.2 | 77.7 |
| Umbrella palm | 87.9 | 89.6 | 82.7 | 93.4 | 96.5 | 88.7 | 97.7 | 98.7 | 94.0 | 99.3 | 99.6 | 97.3 |
Table 13.9 Organic loading rates (OLR) and hydraulic loading rates (HLR) of different leachate-fed CW systems.
| Dilution | Type of CW | OLR (COD g/m3/d) | HLR (m/day) |
| 10% Leachate feeding system | VSSF CW HSSF CW | 17.6 0.9 | 0.071 0.064 |
| 25% Leachate feeding system | VSSF CW HSSF CW | 35.4 9.6 | 0.071 0.064 |
| 50% Leachate feeding system | VSSF CW HSSF CW | 99.1 43.1 | 0.071 0.064 |
Table 13.10 COD concentration profile in the leachate-fed CW systems.
| Stage | 10% system | 25% system | 50% system |
| Inlet (mg/L) | 246.4 | 496 | 1386.3 |
| Effluent of VSSF CWs (mg/L) | 13.7 | 142 | 635.7 |
| Effluent of HSSF CWs (mg/L) | 7.4 | 86 | 160.1 |
COD removal efficiencies of the hybrid systems with the 10%, 25% and 50% synthetic leachate feeding rates were 97.0, 82.6 and 88.5%, respectively, while the values for the VSSF alone CWs were 94.4, 71.3 and 54.1%, respectively.
HLRs for the VSSF CWs and HSSF CWs were 0.071 and 0.064 m/d, respectively (Table 13.9). When the hydraulic loading rate was < 0.01 m/d, organic matter removal was reduced, although removal of other pollutants had increased with the increase in HRT. Increased loading rates reduced the pollutant removal capacity of the CWs. For Hybrid CWs (VSSF-HSSF), an HLR < 0.045 m/d and OLR 100 g/m3/d were found effective for leachate treatment. Pilot-scale experiments using hybrid CWs and real leachate also showed high BOD, COD and TC removals. Mean influent BOD5 was mg/L (for 5% leachate) and 381 mg/L (for 25% leachate). Standard deviation was ±36.4 and ±162.8 for 5% and 25% leachate, respectively. Mean influent COD was 564 mg/L and 1,135 mg/L for the 5% and 25% leachate, respectively. Total carbon (TC) was 184 mg/L and 368 mg/L for the 5% and 25% leachate, respectively, with respective standard deviations ±31.8 and ±70.6. Table 13.11 shows the organic loading rates based on 5% and 25% leachate.
Table 13.11 Organic loading rates (OLR) with 5% and 25% real leachate.
| Leachate concentration | OLR (BOD g/m3/d) | OLR (COD g/m3/d) |
| 5% Leachate | 7.3 | 16.4 |
| 25% Leachate | 24.2 | 48.7 |
COD and BOD5 removal efficiency of the VSSF CWs was above 94% for all plant species. Supradata [37] reported oxygen in the media could be supplied by the plant roots. The oxygen is a by-product of photosynthesis. Aerobic conditions around the plants' root system could therefore be maintained and hence contributing to the high organic removals.
Figures 13.8 and 13.9 show the residual BOD and COD profiles with respect to time in the hybrid CWs planted with the various macrophytes.

Figure 13.8 BOD5 profiles with respect to time for hybrid CWs planted with four different plant species.

Figure 13.9 COD profiles with respect to time for hybrid CWs planted with with four different plant species.
13.5.2 Effect on Removal and Transformation of Nitrogen Compounds
Common nitrogen forms in landfill leachate are ammonia, organic nitrogen and possibly some nitrite (NO2–) and nitrate (NO3–). Cyclic processes that occur in “conceptual compartments” such as the water column, sediments, plant roots, biofilms, and stem and leaves can participate in the removal of the nitrogenous compounds from the leachate.
Nitrification (13.1) and denitrification (13.3) are processes that are important in nitrogen removal [31, 38].
The different biochemical transformations of nitrogen in wetlands are given in Table 13.12.
Table 13.12 Biogeochemical transformation of nitrogen in wetlands [31, 33].
| Process | Transformation |
| Volatilization | NH3(aq) → NH3 (g) |
| Ammonification | Organic-N (aq) → NH3 (aq) |
| Nitritation | 2NH4+(aq) + 3O2 → 2NO2–(aq) +2H2O (aq) + 4H+(aq) |
| Nitrification | 2NO2–(aq) + O2 → 2NO3–(aq) |
| Denitrification | 2NO3– (aq)→ 2NO2–(aq) → 2NO (g) → N2O (g)→ N2 (g) |
| Dissimilatory nitrate reduction | 2NO3– (aq) → NH3 (aq) |
| N2 fixation | N2 (g) → Organic-N (aq), NH3 (aq) |
| Biological assimilation | NH3 (aq), NO2–(aq), NO3– (aq) → Organic-N (aq) |
| Ammonia adsorption | NH3 (aq) → NH3 (s) |
| ANAMMOX | NH3 (aq) + NO2–(aq) → N2 (g) |
The Hybrid Constructed Wetlands can have more stable removal rate of nitrogen in comparison to that of one-stage systems. The average removal rate was 7.8 kg Ntot/ha/d [39]. One stage system would not be able to effectively achieve both nitrification and denitrification processes in the same compartment. Pilot-scale experiments for leachate treatment using hybrid CWs with natural leachate have clearly revealed the transformation and removal of nitrogen compounds within wetland beds with the help of plant species. Ammonia nitrogen is one of the major components in the leachate. Average influent concentrations are 73.24 mg/L and 131.5 mg/L for 5% and 25% diluted real leachate.
VSSF CWs are dominated by aerobic conditions that enhance the aerobic microbial activities. Aerobic bacteria convert NH4+-N into NO3–-N by nitrification process. Therefore considerable reduction of NH4+-N can be observed in VSSF CWs. The best average NH4+-N removal efficiency of 88.7% was found for the green bulrush plant and 87.3%, 79.9% for umbrella palm and narrow leaf cattail respectively. Vymazal [33] has also mentioned that NH4+-N removal in VSSF CWs was 84.2% and 48.3% in HSSF CWs.
Intermittent feeding can be helpful in nitrogen removal as substrate pores can then be periodically re-supplied with oxygen (in the entrained air) and this could supplement the oxygen supplied by the roots in the plant rhizosphere, hence stimulating nitrification [31]. Thus, purposefully pulse loaded VSSF wetlands have been noted to promote NH4+-N oxidation [31, 40, 41].
In HSSF CWs normally saturated with water, anaerobic conditions occur. This therefore would restrict the nitrification process and enhance the denitrification process. Low average NH4+-N removal efficiencies can be observed in HSSF CWs [31]. HSSF CWs can remove NO3–-N by the denitrification process. The anoxic conditions in the saturated HSSF CWs enhance the anaerobic microbial activities. The Umbrella palm in the HSSF CW model has shown the best average removal efficiency of 61%.
Influent NO3–-N concentrations were very low in the leachate at 0.64 mg/L and 1.18 mg/L for 5% leachate and 25% leachate, respectively. But NO3–-N concentrations in VSSF CWs had increased due to the nitrification process. Umbrella palm VSSF CWs model showed the best average NO3–-N production of 18.3 mg/L and 34 mg/L for 5% and 25% leachate, respectively.
Average influent TN concentrations are 93.9 mg/L and 171.3 mg/L for 5% and 25% diluted leachate, respectively. Average total efficiency was also highest in Umbrella palm (74.6%). According to Vymazal [33], TN removal efficiencies were 44.6% and 42.3% for VSSF CWs and HSSF CWs, respectively. Laboratory-scale synthetic leachate treatment models have revealed high removal efficiency for TN (Table 13.13). The Umbrella palm was the most effective.
Table 13.13 TN removal efficiency for laboratory-scale CW batch experiment.
| Plant type | 100% | 75% | 50% | 25% |
| Narrow leaf cattail | 73.5 | 83.3 | 90.6 | 94.9 |
| Green bulrush | 69.3 | 71.3 | 89.6 | 84.8 |
| Umbrella palm | 76.4 | 87.4 | 93.5 | 98.8 |
Influent concentrations of NH4+-N in the hybrid CWs ranged from 22 mg/L to 102 mg/L when using synthetic leachate. The average removal efficiency of NH4+-N in VSSF beds was better (up to 56% removal) than in HF beds (at 29% removal). In a hybrid system, the combined removal was 69% in the VSSF-HSSF CWs.
NO3–-N concentrations in the outlet of the hybrid systems (15.4, 18.0, and 47.2 mg/L, respectively) are generally higher than that in the inlet (1.6, 2.9, and 10.0 mg/L, respectively) under different feeding loading rates (Table 13.14). This would suggest inadequate denitrification.
Table 13.14 Nitrate profile at various synthetic leachate dilutions applied to the CW.
| Leachate concentration | |||
| 10% | 25% | 50% | |
| Inlet | 1.62 (±0.4) mg/L | 2.9 (±1.5) mg/L | 10 (±5.9) mg/L |
| VSSF effluent | 28.2 (±20.2) mg/L | 43.5 (±32.7) mg/L | 89.3 (±46.5) mg/L |
| Outlet | 15.4 (±9.9) mg/L | 18.0 (±17.8) mg/L | 47.2 (±24.0) mg/L |
Negative rates of nitrate removal can occur, implying high nitrification potential in the VSSF systems and inadequate denitrification in the HSSF systems. A higher TN removal efficiency can be observed in hybrid systems, compared to individual VSSF or HSSF CW. The TN removal efficiency at 10% synthetic leachate concentration is 70.1%, while the values are 30.6 and 58.4% in VF CW and HF CW respectively.
13.6 Summary
When considering the organic compounds removal (i.e., BOD, COD, and TC) at different influent concentrations, the batch experiment with all three plant species showed higher performance with 25% leachate. In addition, among the three selected plants, Umbrella palm had the highest efficiency. Pilot-scale experiments of the hybrid system cited better performance compared to the values of VSSF CWs. Furthermore, the experiments with real leachate showed similarly good performance with the hybrid system being better than the VSSF CW and HSSF CW applied individually.
When considering the removal and transformation of nitrogen compounds with different concentrations, removal efficiency for TN was recorded highest in all concentration groups. Similar to organic removal, Umbrella palm showed the highest average removal efficiency among the three selected plant species. Apart from that, the average removal efficiency of NH4+-N and NO3–-N in VSSF-HSSF CWs systems showed an improvement in the efficiency figures compared to VSSF and HSSF CWs. Field scale experiments for leachate treatment also revealed that nitrogen removal and transformations within the hybrid CWs are comparably higher compared to other treatment systems.
The three plant species selected are tolerant to the concentrations of COD, BOD and NH4+-N present in the diluted leachate. Among these, the green bulrush plant shows the best removal efficiencies for many of the parameters in one-stage systems. However, the highest overall performance in hybrid systems is achieved with the Umbrella palm.
Hybrid constructed wetlands are capable of more stable removal of organics and nitrogen in comparison to the one-stage systems, when treating landfill leachate. The average removal rates of organics and nitrogen compounds are higher than those in the conventional systems. This would be due to the sequence of aerobic and anaerobic conditions in the hybrid CW system.
References
- 1 Raghab SM, El Meguid AMA, Hegazi HA. Treatment of leachate from municipal solid waste landfill. J HBRC 2013; 9(2):187–192.
- 2 Bhalla B, Saini MS, Jha MK. Characterization of leachate from Municipal Solid Waste (MSW) Landfilling Sites of Ludhiana, India: A Comparative Study. Int J Eng Res Applic. 2012; 2(6):732–745.
- 3 Visvanathan C. Solid Waste Management in Asian Perspectives. Environmental Management Tools 2006, Bangkok, pp. 1–7.
- 4 Jędrczak A. Quantity and chemical composition of landfill leachates (In Polish). Mat. III Konferencji Szkoleniowej. Budowa bezpiecznych składowisk. Fundacja PUK, Wisła, 1993.
- 5 Johansen OJ, Carlson DA. Characterization of sanitary landfill leachates. Water Res. 1976; 10(12):1129–1134.
- 6 Jędrczak A, Haziak K. Quantity, chemical composition, and treatment of landfill leachates. In: Mat. Konferencyjne IV Konferencji Szkoleniowej nt. Budowa bezpiecznych składowisk odpadów. Fundacja PUK, Poznań, 1994.
- 7 Plotkin S, Ram NM. Multiple bioassays to assess the toxicity of a sanitary landfill leachate. Arch Environ Cont Toxicol. 1984; 13(2):197–206.
- 8 Rojíčková-Padrtová R, Maršálek B, Holoubek I. Evaluation of alternative and standard toxicity assays for screening of environmental samples: selection of an optimal test battery. Chemosphere 1998; 37(3):495–507.
- 9 Ward ML, Bitton G, Townsend T, Booth M. Determining toxicity of leachates from Florida municipal solid waste landfills using a battery-of-tests approach. Environ Toxicol. 2002; 17(3):258–266.
- 10 Oman CB, Junestedt C. Chemical characterization of landfill leachates – 400 parameters and compounds. Waste Manage. 2008; 28:1876–1891.
- 11 Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P. Landfill leachate treatment: Review and opportunity. J Hazard Mater. 2008; 150(3):468–493.
- 12 Sewwandi B, Takahiro K, Kawamoto K, Hamamoto S, Asamoto S, Sato H. Characterization of landfill leachate from municipal solid wastes landfills in Sri Lanka. In: ICSBE. [online], 2012. Available at: www.civil.mrt.ac.lk/conference/ICSBE2012/SBE-12-236.pdf [Accessed 1 December 2017].
- 13 Szpadt R. Characteristics and treatment methods of municipal landfill leachates. Municipal Rev. 2006; 60–66.
- 14 Bhalla B, Saini MS, Jha MK. Effect of age and seasonal variations on leachate characteristics of municipal solid waste landfill. Int J Res Eng Technol. 2013; 2:223–232.
- 15 Lee AH, Nikraz H, Hung YT. Influence of waste age on landfill leachate quality. Internat J Environ Sci Develop. 2010; 1(4):347.
- 16 Kang KH, Shin HS, Park H. Characterization of humic substances present in landfill leachates with different landfill ages and its implications. Water Res. 2002; 36(16):4023–4032.
- 17 McCarty PL. Anaerobic waste treatment fundamentals. Public works. 1964; 95(9):107–112.
- 18 Tałałaj I. Quality of underground waters in the vicinity of municipal landfills. In Jakość wód gruntowych wokół wysypisk odpadów komunalnych. W: II Forum Inżynierii Ekologicznej “Monitoring środowiska”red. Wiatr I, Marczak H. Nałęczów (p. 640), 1998.
- 19 Malina J. Design of anaerobic processes for treatment of industrial and municipal waste (Vol. 7). CRC Press, 1992.
- 20 Im J-H, Woo H-J, Choi M-W, Han K-B, Kim C-W. Simultaneous organic and nitrogen removal from municipal landfill leachate using an anaerobic-aerobic system. Water Res. 2001; 35(10):2403–2410.
- 21 Klimiuk E, Kulikowska D, Koc-Jurczyk J. Biological removal of organics and nitrogen from landfill leachates – A review. Management of pollutant emission from landfills and sludge. Taylor & Francis Group, London, 2007, pp. 187–204.
- 22 Tatsi AA, Zouboulis AI. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv Environ Res. 2002; 6(3):207–219.
- 23 Inanc B, Calli B, Saatci A. Characterization and anaerobic treatment of the sanitary landfill leachate in Istanbul. Water Sci Technol. 2000; 41(3):223–230.
- 24 Goorany O, Oztürk I. Soluble microbial product formation during biological treatment of fermentation industry effluent. Water Sci Technol. 2000; 42(1-2):111–116.
- 25 Aquino SF, Stuckey DC. Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds. Water Res. 2004; 38(2):255–266.
- 26 Poltak RF. Sequencing Batch Reactor Design and Operational Considerations. New England Interstate Water Pollution Control Commission, 2005.
- 27 Wang S, Peng Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J. 2010; 156(1):11–24.
- 28 Wang JP, Chen YZ, Ge XW, Yu HQ. Optimization of coagulation–flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloids and Surfaces A: Physicochem Eng Aspects. 2007; 302(1):204–210.
- 29 Amokrane A, Comel C, Veron J. Landfill leachates pretreatment by coagulation-flocculation. Water Res. 1997; 31(11):2775–2782.
- 30 Mamlook R, Badran O, Abu-Khader MM, Holdo A, Dales J. Fuzzy sets analysis for ballast water treatment systems: best available control technology. Clean Technol Environ Policy. 2008; 10(4):397–407.
- 31 Stefanakis AI, Akratos CS, Tsihrintzis VA. Vertical Flow Constructed Wetlands: Eco-engineering Systems for Wastewater and Sludge Treatment. Elsevier Science: Oxford, 2014.
- 32 Sayadi MH, Kargar R, Doosti MR, Salehi H. Hybrid constructed wetlands for wastewater treatment: A worldwide review. Internat Acad Ecol Environ Sci. 2012; 2(4):204.
- 33 Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci Total Environ. 2007; 380(1):48–65.
- 34 Bricken EC. Constructed Wetlands as an Appropriate Treatment of Landfill Leachate. Doctoral dissertation, University of Natal, 2003.
- 35 Tchobanoglous G, Burton FL, Stensel HD. Wastewater Engineering Treatment and Reuse, 4th edn. McGraw-Hill: New York, 2003.
- 36 Wojciechowska E, Gajewska M, Obarska-Pempkowiak H. Treatment of landfill leachate by constructed wetlands: three case studies. Polish J Environ Stud. 2010; 19(3):643–650.
- 37 Supradata. Domestic Waste Water Treatment by using Cyperus alternifolius, L. with Subsurface Flow Wetland System (SSF-Wetlands). Master Program of Environmental Science, Diponegoro University, Semarang, 2005.
- 38 Redmond ED, Just CL, Parkin GF. Nitrogen removal from wastewater by an aerated subsurface-flow constructed wetland in cold climates. Water Environment Research. 2014; 86(4):305–313.
- 39 Pempkowiak HO, Gajewska M. The removal of nitrogen compounds in Constructed Wetlands in Poland. Polish J Environ Studies. 2003; 12(6):739–746.
- 40 Cooper P. The performance of vertical flow constructed wetland systems with special reference to the significance of oxygen transfer and hydraulic loading rates. Water Science and Technology. 2005; 51(9):81–90.
- 41 Yalcuk A, Ugurlu A. Comparison of horizontal and vertical constructed wetland systems for landfill leachate treatment. Bioresource Technol. 2009; 100(9):2521–2526.

