Dye Nanoparticle-Coated Test Strips for Detection of ppb-Level Ions in Water
Yukiko Takahashi, Department of Civil and Environmental Engineering, Nagaoka University of Technology, Nagaoka, Niigata, Japan
Governments throughout the world are continuing to tighten contamination concentration limits and guidelines of harmful inorganic substances for industry and environmental water. For example, the World Health Organization recommends the standard allowance for water quality to be < 50 ppb for cadmium, lead, chromium(VI), mercury, selenium, and arsenic. Despite the increasing demands for simple and rapid monitoring of water quality, particularly for harmful ions, the sensitivities of commercial test kits and other existing methods are insufficient to meet the concentration guidelines.
We have proposed next-generation simple methods for water analysis with high sensitivity to trace ions at ppb levels, named “dye nanoparticle-coated test strip” (DNTS). The DNTS is loaded with a thin layer (400 nm–2 μm) of indicator dye nanoparticles on the top surface of a membrane filter, providing a remarkably concentrated color signal. Therefore, almost all signals can be available, but in contrast to conventional test paper that dyes are distributed over the entire support. The DNTS is applicable not only to dip test but also to filtration enrichment in which target metal ions are concentrated by passing sample solutions through it. In addition, preparative procedures of DNTSs are simple and versatile based on the simple filtration of nano-dispersion of indicator dyes.
Keywords
Test paper; nanoparticle; harmful ions; indicator dyes; colorimetry; trace analysis; thin layer of dye nanoparticle
4.1 Introduction
Harmful inorganic substances have toxic and nondegradable properties that pose a particular hazard to bioorganisms and the ecosystems [1]. The World Health Organization (WHO) has recommended the water quality guidelines to be <50 ppb (μg/L) for toxic inorganic substances, including cadmium, lead, chromium(VI), mercury, selenium, and arsenic [2]. In line with this guideline, strict regulations for industrial effluents, drinking water, and natural waters have been adopted worldwide. High performance analytical instruments, such as atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectrometry (ICP–OES), inductively coupled plasma mass spectrometry (ICP–MS), are extensively used as standard methods to determine trace levels of contaminant concentration. However, in addition to the costly initial/running expenses of instruments, specific technical skills are required for machine operation. Moreover, sample collection, transportation to the analytical laboratories, and quite-complicated sample pretreatment may take time. On-site analytical methods for harmful ions are desired for quick monitoring of industrial wastewater, evaluation of drinking water, urgent assessment of well water in times of disaster, as well as in environmental education in schools [3]. Despite the increasing demands for a simple test method of inorganic ions, the sensitivities of commercial test kits are insufficient to meet the criteria of water quality [4], limiting their usages to qualitative or semi-quantitative tests. Additionally, real water samples frequently contain interfering substances, and therefore high selectivity is required. Some of the optical sensors and ion-selective electrodes show quick response and high sensitivity, but are insufficient in selectivity for a target metal ion particularly in the presence of high levels of interfering ions [5]. Pretreatment of samples, including selective separation and enrichment of trace analyte, is recommended to attain high sensitivity and more reliable monitoring of water quality [6–8]. Solid phase extraction is the preferred technique for concentration of analytes. This technique combined with colorimetric determination has been proposed as a simple analytical method, where analyte ions form intensely colored complexes with the reagent fixed on the solid support [9,10].
4.2 Fundamental concept of dye nanoparticle-coated test strip
4.2.1 Structural features of dye nanoparticle-coated test strip
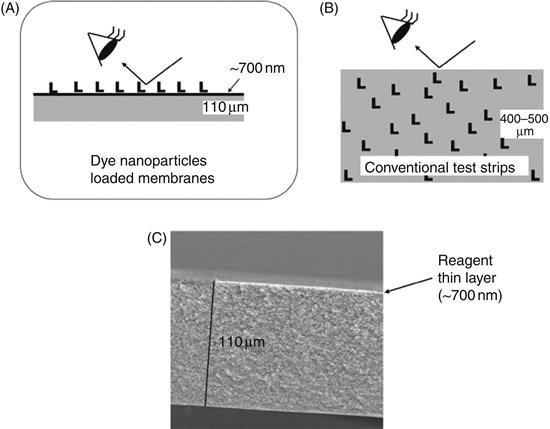
Recently, we reported a simple and versatile method for preparation of dye nanoparticle-coated test strips (DNTSs) for detection of inorganic ions [11]. A thin dye nanoparticle/nanofiber layer (400 nm–2 μm) located on the top surface of the support membrane provides a remarkably concentrated signaling surface composed of a 100% reagent layer. Therefore, almost all signals are available efficiently (Figure 4.1A), but in most conventional test papers, indicator dyes are distributed over the entire support (Figure 4.1B). For that reason, the signal obtained from the top surface must be less intense. It is noteworthy that the present membranes were prepared without any additives such as coating polymers or modifiers. Yet the reagent is not removed from the support by rubbing with a finger or by immersion into water. The cross-sectional SEM image indicates that the thickness of the dye layer is <1 μm (Figure 4.1C). Thus it provides a remarkably concentrated signaling surface composed of 100% pure indicator dye. In contrast, the surface concentration of dye is rather low in conventional test strips prepared by soaking of dye solution.
4.2.2 Simple yet versatile fabrication methods of DNTSs
We designed two fabrication methods of DNTSs for the purpose of producing a large number of test strips with various organic indicator dyes. These methods, including two different preparation processes of nano-dispersion solution and simple filtration process of the dispersion with a fine membrane filter having microscopic pores, are shown in Figure 4.2.
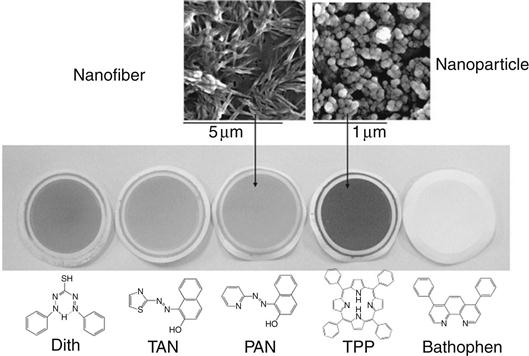
In order to fabricate DNTSs with hydrophobic indicator dyes, a simple process referred to as the “reprecipitation method” [12,13] was proposed for the preparation of aqueous dye nano-dispersion (Figure 4.2, top). The typical preparative process involves injection of a water miscible organic solution of a dye compound into water under vigorous stirring. Dispersion of nano-sized dye compounds is formed immediately upon combining the aqueous solution. The growth time of the nano-dispersion solution was determined to be the time until the average size of dye nanoparticles become more than ca. 100 nm. After the growth period ended, the nano-dispersion is simply passed through with a fine membrane filter by suction. Then, by surface filtration mechanism, dye nanoparticles are uniformly and firmly attached to a membrane filter. The thicknesses of dye nanoparticle layers were experimentally observed to be between 400 and 700 nm, which depend largely on the amount of reagents loaded as well as on the molecular size (weight). The morphology of the nanoparticles varies from particle to fibrous depending on the molecular structure (Figure 4.3).
On the other hand, the “reprecipitation method” is inapplicable to hydrophilic charged dyes such as reagents having sulfone groups and pyridinium groups. For these hydrophilic indicator dyes, nanoscale ion exchangers are used as dye absorbents to produce dispersions of hydrophilic dye/nano absorbent nanocomposites. In principle, a charged dye adsorbs onto the surface of nano-adsorbent having the opposite charges (1–100 nm in diameter), followed by decrease in the surface charge of the adsorbent, and then aggregation occurs (Figure 4.2, bottom). A preparative procedure of hydrophilic dye/nano absorbent loaded DNTS is technically easy, only by mixing individual solutions under the appropriate pH.
For example, 10 mL of TMPyP/silica nanocomposite aqueous solution was prepared by mixing 100 μL of 2 mM TMPyP and 4×10−5 wt% of silica (ca. 10–14 nm in diameter) at pH 7.8 with 0.01 M Tris buffer solution [14]. Similarly, the growth time of nanocomposite in the aqueous solution was decided as the time until the average size of nanocomposites become more than ca. 100 nm. Then, by filtration in the similar way to that of hydrophobic dyes, roughly 100% of the nanocomposite was coated as a thin layer onto the surface of the membrane filter through surface filtration. Some metal oxides and modified latex nanoparticles, which are smaller than 100 nm in diameter and having sufficient positive or negative surface charges in aqueous solution, are promising candidates as nano-adsorbents for fabricating nanocomposite-coated DNTSs. The thicknesses of dye nanoparticle layers were experimentally observed to be ca. 1–2 μm, which depends on the amount and the size of the nano-adsorbent used.
A wide variety of analytical dye compounds can be converted as nanoparticles or nanocomposites in aqueous solution by these two methods and then DNTSs can be simply fabricated from them. The versatility of the preparative procedures of DNTSs enables a screening test of reagents against one target ion. Another outcome of the versatility is that DNTS is a novel platform technology to produce highly sensitive test strips for any target with common dye indicators.
4.2.3 Detection characteristics with DNTS
The DNTS is applicable not only to immersion test but also to filtration enrichment in which target ions are concentrated by passing a sample solution through it (Figure 4.4). Immersion test is just the same detection way as using any ordinary test papers, such as urine, pH, and ions, but it takes longer to complete sufficient color development with DNTS than those with other test papers. The major reason for this is that DNTSs detect a very low concentration of a target ion at ppb level. In addition, DNTSs are water permeable and insoluble in aqueous samples, and hence trace ions are enriched on them simply by filtration of the sample solution. Filtration enrichment is a unique approach provided by DNTS and based on the fact that the dye nanoparticle layer acts as a solid phase feasible to extract a target ion mainly by complex formation between a target ion and indicator dye. Generally, filtration enrichment is more sensitive than immersion test, and one of the examples performed by PAN (1-(2-pyridylazo)-2-naphthol) nanofiber DNTS is introduced in Section 4.4.1. Moreover, filtration enrichment enables highly selective detection by optimizing the reaction conditions, such as solution pH, masking reagent, and flow rate, and the example carried out by dithizone nanofiber DNTS, described in Section 4.4.2.
4.3 The strategy to produce a suitable DNTS for a target ion
The versatility of the preparative procedures of DNTSs enables a screening test of a lot of reagents against a particular target. Figure 4.5 explains how to select a suitable DNTS among prospective DNTSs consisting of various candidate reagents. Candidate dyes have been known and reported as sensitive indicators for a target ion in aqueous solution or organic solvent. For example, there were 15 candidates at the start of development of DNTSs for Cd(II) detection, finally 5-Br-PADAP nanoparticle DNTS and TMPyP/silica nanocomposite DNTS were selected through seven evaluation standards.
4.4 Detection of harmful ions in water with DNTSs
4.4.1 PAN nanofiber DNTS for Zn(II) detection
Membranes coated with PAN nanofibers were applied as test strips for the detection of Zn2+ in a test solution (pH 8.4) [11]. Figure 4.6A shows the color changes of PAN-coated strips in immersion test. Notably 65 ppb of Zn2+ was detected by a naked eye color test. Sub-ppb concentrations of Zn2+ were successfully detected by filtration enrichment of 100 mL of the sample solution (Figure 4.6B). This filtration enrichment procedure amplifies the signal intensity capable of eye detection of ppb-level metal ions. Practically, leaking of reagents out of the sample solution was observed to be negligible during dip test and sample filtration procedures. We also monitored the color change and the relative color intensity with reflectance absorption spectrometry. The peak at λmax=550 nm indicates that neutral [Zn(PAN)2] is formed on the membrane filter as the major species. The increasing peak intensity with increasing Zn(II) concentration enables quantitative determination of test samples by comparison with the calibration curve.

4.4.2 Dithizone nanofiber DNTS for Hg(II) detection
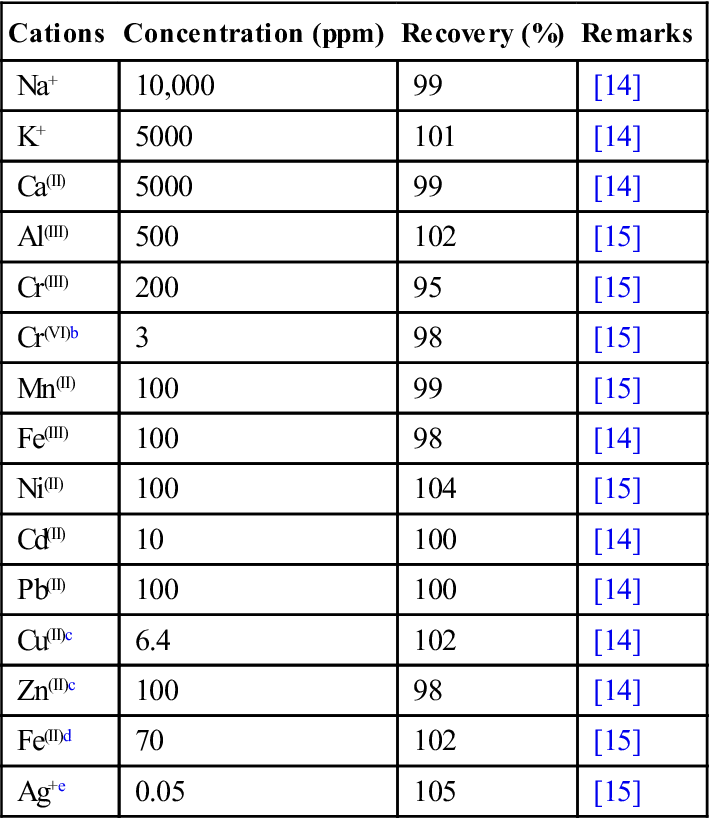
We demonstrated dithizone nanofiber thin film for highly sensitive and selective detection of Hg(II) [15,16]. Dithizone nanofiber was prepared by the reprecipitation method. Simply by filtration of the dispersion through the membrane filter dithizone nanofiber thin film of ca. 440 nm in thickness was deposited firmly and uniformly on one side of the membrane filter. Determination of Hg(II) at ppb level was achieved by filtration enrichment of a sample solution and successive colorimetric analysis. Consequently, Hg(II) was concentrated in the film as reddish brown complexes at pH 2.7. More than 90% of 10 ppb Hg(II) was retained in the film at the filtration rate of 1.3–9.3 mL/min. The presence of Na+ (10,000 ppm), K+ (5000 ppm), Ca(II) (5000 ppm), Al(III) (500 ppm), Cr(III) (200 ppm), Mn(II) (100 ppm), Fe(III) (100 ppm), Ni(II) (100 ppm), Cd(II) (10 ppm), and Pb(II) (100 ppm) in the acidic solution at pH 2.7 did not interfere with the detection of 10 ppb of Hg(II) at all (Table 4.1). The addition of the masking reagent, EDTA was very effective for Cu(II) and Zn(II) by forming water-soluble complex and passing through dithizone DNTS. Prefiltering, in combination with the addition of iodine ion, also effectively masked Ag(I) interference. The interference from Cr(VI) and Fe(II) was also eliminated based on oxidation–reduction reaction. The present method succeeded in determining simulated wastewater, river water, and seawater spiked with 10 ppb Hg(II). Calibration curves for Hg(II) were obtained when the sample volume was 100 mL, and the 3σ detection limit was calculated to be 0.057 ppb (n=10) by a thin layer chromatography (TLC) scanner at 500 nm.
Table 4.1
Tolerance Limits on the Determination of 10 ppb of HgIIa
| Cations | Concentration (ppm) | Recovery (%) | Remarks |
| Na+ | 10,000 | 99 | [14] |
| K+ | 5000 | 101 | [14] |
| Ca(II) | 5000 | 99 | [14] |
| Al(III) | 500 | 102 | [15] |
| Cr(III) | 200 | 95 | [15] |
| Cr(VI)b | 3 | 98 | [15] |
| Mn(II) | 100 | 99 | [15] |
| Fe(III) | 100 | 98 | [14] |
| Ni(II) | 100 | 104 | [15] |
| Cd(II) | 10 | 100 | [14] |
| Pb(II) | 100 | 100 | [14] |
| Cu(II)c | 6.4 | 102 | [14] |
| Zn(II)c | 100 | 98 | [14] |
| Fe(II)d | 70 | 102 | [15] |
| Ag+e | 0.05 | 105 | [15] |
a0.01 mol/dm3 glycine buffer (pH 2.2–2.9) was added.
bWhen 0.01 mol/dm3 ascorbic acid was added, the allowance value in the presence of Cr(VI) was identical to that of Cr(III).
c2×10−4 mol/dm3 EDTA was added.
d5 mM KBrO3 was added.
ePretreatment of a sample solution by filtration with a membrane filter (0.1 μm open pores) after the addition of 1×10−4 mol/dm3 NaI.
Source: Reproduced from Ref. [15]. Copyright: The Japan Society for Analytical Chemistry. Reproduced with permission.
Some other DNTSs were also effective in detecting targets at ppb level, for instance, TMPyP/silica nanocomposite DNTS for the determination of Cd(II) and Pb(II) ions by filtration enrichment [14], 5-Br-PADAP nanoparticle DNTS for Cd(II) by improved immersion test [17].
4.5 Conclusions and future perspectives
The attractive features of DNTS with a thin layer composed of dye nanoparticles or nanocomposites are its simplicity, extremely high sensitivity, and versatility. The versatility in particular means that DNTS is a novel platform to produce highly sensitive test strips for any target with common indicator dyes. In order to realize an “on-site” detection system by the present nanoparticle/fiber-based chemical sensors, a compact kit could be created, which would include a water sampler, pre-filtration membrane to remove insoluble particles, appropriate buffer, and masking reagents. A syringe type sampling system sandwiched with the membrane type color sensor is a primary candidate for simultaneous achievement of sampling, removal of interfering ions and signalization. In combination with a handy reflectometer or diffuse reflectance spectrometer, present nanostructured chemical sensors can provide a quantitative monitoring method of trace heavy metals (ppb level) that has not been achieved by conventional test kits.
Acknowledgments
This work was financially supported by the Environment Research and Technology Development Fund (B-1005) of the Ministry of the Environment, Japan and a grant (Kakenhi 25281038) from the Japan Society for the Promotion of Science (JSPS).



