Sensors Based on Carbon Nanotube Arrays and Graphene for Water Monitoring
Dan Du1,3, Weiying Zhang1,3, Abdullah Mohamed Asiri2 and Yuehe Lin3,4, 1Key Laboratory of Pesticide and Chemical Biology of Ministry of Education, College of Chemistry, Central China Normal University, Wuhan, PR China, 2Chemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia, 3School of Mechanical and Materials Engineering, Washington State University, Pullman, WA, USA, 4Pacific Northwest National Laboratory, Richland, WA, USA
Nanomaterials, particularly carbon nanomaterials, have shown great promise in environmental applications. In this chapter, we focus only on the use of two carbon nanomaterials, carbon nanotubes (CNTs) and graphene-based sensors for water monitoring and detection of hazardous chemicals. The excellent electrochemical behaviors of CNTs and graphene indicate that they are promising electrode materials in electroanalysis. Sensors based on CNTs and graphene have shown excellent performance in electrochemical detection of metal ions, pesticides, and other pollutants.
Keywords
Sensors; carbon nanotubes; arrays; graphene; metals; pesticides
1.1 Introduction
Environmental monitoring of toxic pollutants in surface and subsurface water sources and wastewaters presently relies on the collection of discrete liquid samples for subsequent laboratory analysis. Sensors that are field-deployable and able to measure part-per-billion (ppb) or nanomolar levels of toxic pollutants will reduce time and costs associated with environmental monitoring of hazardous chemicals. Electrochemical sensors appear to be a very promising technique that offers desired characteristics such as field-deployability, selectivity, sensitivity, robustness, and inexpensiveness [1–3].
Nanomaterials, particularly carbon nanomaterials, have a significant role to play in new developments in each of the biosensor size domains [4–6]. They have shown great promise in many applications, such as bioscience and biotechnology, energy storage and conversion, environmental and biomedical applications. In this chapter, we focus only on the use of two carbon nanomaterials, carbon nanotubes (CNTs) and graphene-based sensors for water monitoring and environmental applications.
CNTs are well-ordered, hollow graphitic nanomaterials made of cylinders of sp2-hybridized carbon atoms. They have high aspect ratios, high mechanical strength, high surface areas, excellent chemical and thermal stabilities, and rich electronic and optical properties [7]. The latter properties make CNTs important transducer materials in biosensors: high conductivity along their length means they are excellent nanoscale electrode materials [8–10]. These materials are classed as single-walled carbon nanotubes (SWCNTs) (Figure 1.1A), which are single sheets of graphene “rolled” into tubes, or multiwalled carbon nanotubes (MWCNTs), each of which contains several concentric tubes that share a common longitudinal axis [11]. As one-dimensional (1D) carbon allotropes, CNTs have lengths that can vary from several hundred nanometers to several millimeters, but their diameters depend on their class: SWCNTs are 0.4–2 nm in diameter and MWCNTs are 2–100 nm in diameter. An important part of the success of CNTs for these applications is their ability to promote electron transfer in electrochemical reactions.
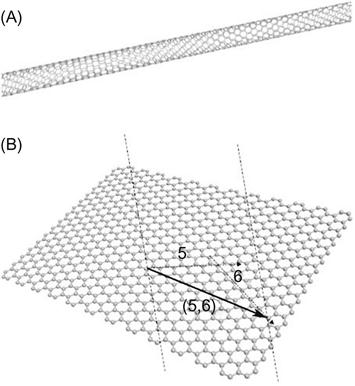
Graphene is a 2D sheet of carbon atoms in a hexagonal configuration with atoms bonded by sp2 bonds. These bonds and this electron configuration are the reasons for the extraordinary properties of graphene, which include a very large surface area (at 2630 m2/g, it is double that of SWCNTs), a tunable band gap, room-temperature Hall effect, high mechanical strength (200 times greater than steel), and high elasticity and thermal conductivity [12]. Graphene is the most recent member of the multidimensional carbon-nanomaterial family, starting with fullerenes as a 0D material, SWCNTs as 1D nanomaterials, and ending with graphite as a 3D material. Graphene fills the gap for 2D carbon nanomaterials (Figure 1.1B). Isolation of individual graphene sheets was long sought, but only in 2004, it was achieved by a surprisingly simple technique [13]. Since then, fundamental research and research on applications have increased rapidly. Graphene is an ideal material for electrochemistry [14–17] because of its very large 2D electrical conductivity, large surface area, and low cost. The use of graphene in electrochemical sensors and biosensors is particularly interesting, with the first articles emerging in 2008. Since then, their number has grown explosively.
1.2 CNT-based electrochemical sensors
1.2.1 Various methods for preparation of CNT-based sensors
Although CNTs are relatively new in analytical fields, their unique electronic (electron transfer rate similar to edge-plane graphite), chemical (biocompatibility and ability to be covalently functionalized), and mechanical properties (3 times stronger than steel) make them extremely attractive for chemical and biochemical sensors [18,19]. Various methods have been used to prepare CNT-based sensors: (a) casting of CNT thin films, from the suspensions of CNTs in solvents [20–28], such as sulfuric acid [21], Nafion [25], dihexadecyl hydrogen phosphate (DHP) [26], DMF [27], and acetone [28], prior to being coated on electrode surfaces, (b) using CNTs as paste electrodes or electrode composites [29–31], and (c) using aligned CNT as electrode substrates [32–40]. With specific to metal ion sensors, DHP [26] and Nafion [25] have been used to disperse MWCNTs under ultrasonication prior to being drop-coated on glassy carbon electrodes. The CNT film enables the development of mercury-free electrodes that can detect from 10−9 to 10−6 M of Cd and Pb.
Most CNT-based sensors take advantage of the bulk properties of CNTs, including increased electrode surface area [41], fast electron transfer rate [42], and good electrocatalytivity in promoting electron transfer reactions of many important species [43,44]. Using CNTs as nanoelectrodes have been increasing explored since conventional macroelectrodes (having diameter in millimeters), such as glassy carbon and carbon paste, are known to have slow mass transport. Using nanoelectrodes (with diameter in nanometers) can enhance mass transport [37,38,45]; as electrodes decrease in size, radial (3D) diffusion becomes dominant and results in fast mass transport and fast electron transfer, and promotes rapid electrochemical detection. Nanoelectrodes also have higher responsiveness (or higher mass sensitivity) than macroelectrodes, attributed to their lower background (charging) currents [46]. Additionally, they are less influenced by solution resistance due to lower ohmic drop [38]. The high diffusion rate at nanoelectrodes enables the study of fast electrochemical and chemical reactions [47]. Despite its advantages, a single nanoelectrode offers extremely low capacitive current (in pico-amperes), thereby requiring expensive signal amplifiers. To solve this issue, nanoelectrode arrays (NEA) consisting of millions of nanoelectrodes have been developed at Pacific Northwest National Laboratory (PNNL) in collaboration with the Boston College, in order to provide magnified signals without the need for a signal amplifier.
High-density aligned CNT electrode arrays (i.e., dense CNT forest) have been reported to show fast electron transfer and electrocatalytic characteristics [48], but they do not maintain the properties of individual nanoelectrodes due to the overlapping of their diffusion layers [49–53]. To make each CNT on the array work as an individual nanoelectrode, the spacing must be sufficiently larger than the diameter of the nanotubes. A nanoscale in size of the electrode and the million numbers of electrodes will potentially result in an improved signal-to-noise ratio (and hence improved detection limits) by three to four orders of magnitude compared with that of single nanoelectrode.
1.2.2 Fabrication of aligned CNT NEA
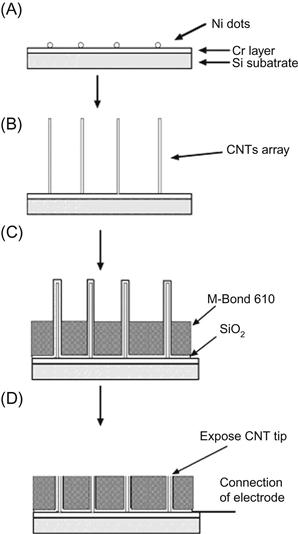
The fabrication procedure for low-site density aligned CNT NEA has been refined [54,55]. Figure 1.2 shows the scheme of the fabrication procedure. After the electrochemical deposition of Ni nanoparticles (shown in Figure 1.2A), an aligned CNT array was grown (shown in Figure 1.2B), and then a thin layer of SiO2 was coated on the surface by magnetron sputtering to insulate the Cr layer. After that, 5 m thick M-Bond 610 (two-component, solvent-thinned, epoxy-phenolic adhesive from Vishay Intertechnology, Inc.) was coated and cured at 170°C for 2 hours, which further insulates the Cr and also provides mechanical support to the CNTs. After these steps, the CNTs were half embedded in the polymer resin as shown in Figure 1.2C. In the next step, a fiber-free cloth was used to polish the surface that mechanically breaks the top part of the CNTs and exposes the tip of the CNTs as shown in Figure 1.2D. Finally, the sample surface was rinsed in deionized water, and an insulated copper wire (0.5 mm in radius) was attached to the corner of the substrate by applying a drop of conductive silver epoxy followed by insulating epoxy. The copper wire NEAs assembly was left to cure in air at room temperature for several hours. Figure 1.3 shows the structure of the final CNT NEAs.
1.2.3 Applications of CNT-based sensors for metal ion monitoring
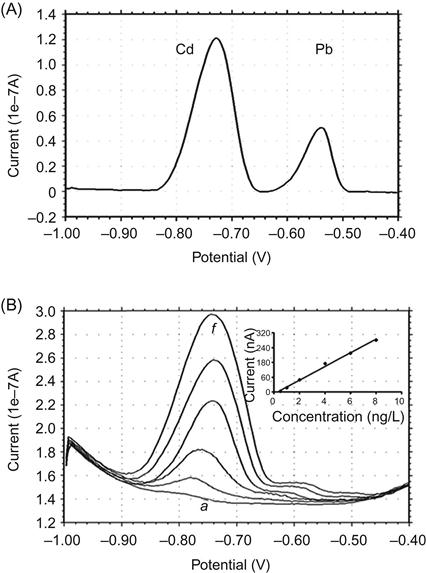
The CNT NEAs have been applied for detection of toxic metal ions. With Hg-coated CNT NEA, linear relationship between the Pb signals and the Pb concentration in the solutions ranging from 1 to 100 ppb (g/L) has been obtained [55]. Because Hg is highly toxic and not suitable for field-deployable use, the relatively benign bismuth, Bi(III), has been evaluated as a Hg substitute [56,57]. Bi-based electrodes performed as well as Hg-based electrode for Cr(VI) quantitation after the accumulation of Cr(VI)-diethylenetriammine pentaacetic acid (DTPA) chelate [56]. Highly responsive voltammetric analysis of Cd and Pb at the Bi-based CNT NEA was obtained [34]. Figure 1.4A shows well-defined peaks of 5 g/L of Cd and Pb obtained after only 2 min of accumulation, in which Bi was accumulated in situ with the target metals at −1.2 V. Figure 1.4B shows a linear calibration curve of Cd achieved at a very useful concentration range of 0.5–8 g/L of Cd. The detection limit of 0.04 g/L was obtained under optimum experimental conditions.
The attractive behavior of the new CNT NEA sensing platform holds great promise for on-site environmental monitoring and biomonitoring of toxic metals.
1.3 Graphene-based sensors
1.3.1 Graphene-based electrochemical sensors
The excellent electrochemical behaviors of graphene indicate that graphene is a promising electrode material in electroanalysis. Graphene, together with its various derivatives, such as graphene oxide (GO), graphene nanoribbon, chemically reduced graphene oxide (rGO), or nitrogen doped graphene [58–62], has shown fascinating advantages in electrochemistry such as electrochemical devices, capacitors, or transistors due to its remarkable electrochemical properties [63–66]. The graphene-based materials have been used to construct various biosensors based on different sensing mechanisms. Because their 2D structure provides a large area for the immobilization of enzyme, and enriched oxygen-containing groups are apt to react with amino-acid residues, graphene derivatives are ideal substrates for immobilization of certain biomolecules. Enzymes can be immobilized on graphene surfaces through the following three methods: (1) electrostatic interaction between positively charged graphene and a negatively charged enzyme, (2) covalent linkage of the enzyme molecule to the graphene surface, and (3) entrapment of the enzyme using the polysaccharide chitosan or another polymer. Electrochemical approach is considered as one of the best methods for biomolecule detection due to its high sensitivity, low cost, quick response, and easy operation. The excellent electrochemical behaviors of graphene make it a promising electrode material to improve the detection of biomolecules [67]. Graphene-based materials enhance the direct electron transfer that is characteristic of some redox enzymes and retain bioactivity because of their favorable electronic properties and biocompatibility [68–70].
1.3.2 Graphene sensors for pesticides
Because of the high toxicity of organophosphorus pesticides (OPs), they still remain a serious health threat to humans across the world. Rapid detection of these toxic agents has become increasingly important for health protection [71,72]. Although gas or liquid chromatography and mass spectrometry are accurate and routinely performed for analyzing OPs [73,74], these methods have a number of disadvantages, limiting their applications for rapid analyses under field conditions. Development of a simple, rapid, sensitive, and inexpensive method with real-time output to detect OPs is of considerable interest. Graphene has been developed as an enhanced sensing platform for on-site detection of OPs, based on immobilization of acetylcholinesterase (AChE) or OP hydrolase (OPH). Our group has reported a self-assembly of AChE on a gold nanoparticles (Au NPs)–graphene nanosheet hybrid using a long-chain polyelectrolyte, poly-(diallyldimethylammonium chloride) (PDDA) as a linker for detection of OP [75]. As shown in Figure 1.5, GO was used as the precursor to produce chemically reduced graphene oxide nanosheets (cr-Gs) and PDDA strands could terminate at the cr-Gs surface due to the electrostatic interaction between positive charged PDDA and negative charged GO. By adding NaBH4, chemical reduction and Au NPs decoration were realized in one step. By using PDDA as a linker, AChE could be enriched and stabilized on the nanohybrid. Self-assembling was easy and fast to be realized between negatively charged AChE and positively charged PDDA. As a result, nanoassembly AChE/Au NPs/cr-Gs was formed in the presence of PDDA and was utilized as the catalyst platform of the electrochemical biosensor. Under the optimized condition, ultrasensitive detection of paraoxon (a model compound of organophosphate pesticides) was realized with lab-built flow injection analysis system. Figure 1.6 is a typical i–t curve acquired at AChE/Au NPs/cr-Gs incubated with different concentrations of paraoxon. Signal (a) is from AChE/Au NPs/cr-Gs for 2 mM acetylthiocholine (ATCh), signals (b to h) are generated from 2 mM ATCh after the AChE/Au NPs/cr-Gs incubated with different concentrations of paraoxon: 0.1 pM, 0.25 pM, 2.5 pM, 25 pM, 0.25 nM, 2.5 nM, and 5 nM paraoxon for 15 min, respectively. Catalysis activity of AChE to ATCh is inhibited apparently after exposure to paraoxon, and even a very low concentration of paraoxon (0.1 pM) could cause the apparent inhibition. Oxidation current of enzymatic product decreased sharply as paraoxon concentration increased. The results demonstrate that the developed approach provides a promising strategy to improve the sensitivity and enzyme activity of electrochemical biosensors.


In another approach, we prepared a graphene–ZrO2 nanocomposite (GZN) used for both enrichment and detection of methyl parathion (MP) [76]. GZN was synthesized by one-step electrodeposition onto a glass carbon electrode (GCE) and served as a selective sorbent to enrich MP in garlic samples due to the strong affinity between ZrO2 and phosphoric group. The determinations included two main steps: (a) MP adsorption and (b) electrochemical stripping detection of adsorbed MP. Fast solid-phase extraction (SPE) was shown by the effective extraction of MP to the GZN modified electrode within 5 min without taking any special measures. Compared to plain graphene or a ZrO2 nanoparticle modified electrode, GZN is more sensitive and selective to MP due to a synergistic effect. The improved performance combines the advantages of a large surface area and the fast electron transfer of graphene with the strong affinity of ZrO2 to the phosphate group to open new opportunities for a simple, sensitive, selective, and fast analysis of OPs.
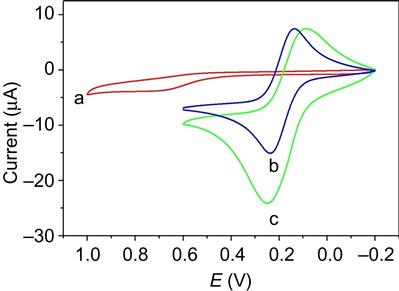
Different kinds of metal nanoparticles have been introduced into graphene, such as Au [77,78], TiO2 [79,80], and SnO2 [81], to strengthen the activity. Graphene-based nanocomposites have been developed as an enhanced sensing platform for biosensors, because these kinds of nanocomposite films may generate synergistic effect to enhance the sensitivity. As shown in Figure 1.7, we demonstrated a facile procedure to efficiently prepare Prussian blue nanocubes/reduced graphene oxide (PBNCs/rGO) nanocomposites by directly mixing Fe3+ and (Fe(CN)6)3− in the presence of GO in polyethyleneimine aqueous solution, resulting in a novel AChE biosensor for detection of monocrotophos [82]. Figure 1.8 compares the cyclic voltammograms of bare electrode and AChE–PBNCs/rGO/SPE in the presence of acetylthiocholine chloride (ATCl). The oxidation of thiocholine, a hydrolysis product of ATCl by AChE, reaches maximum oxidative current at 680 mV obtained at AChE modified SPE (curve a). In the case of AChE–PBNCs/rGO/SPE in PBS buffer, a pair of reversible peaks typifying the oxidation and reduction of PB is clearly observed (curve b). The small distance between peak potentials indicates its adsorption on the electrode surface. When adding 3.0 mM ATCl to the PBS, the oxidation current obtained at AChE-PBNCs/rGO/SPE (curve c) obviously increases compared to that observed in the absence of ATCl (curve b) and the peak potential shifts negatively to 220 mV (curve c). The over-potential decreases ~460 mV compared to curve a, suggesting that PBNCs/rGO has an electrocatalytic activity toward the oxidation of thiocholine. We further studied the amperometric response of ATCl by i–t measurements at the potential of 0.2 V obtained at different AChE modified electrodes including bare SPE, PB/SPE, and PBNCs/rGO/SPE. The oxidation current of thiocholine at bare SPE reached the maximum steady-state current (1.4 mA) with 26 s, while it increased to 10.7 mA at PB/SPE with response time of 20.6 s due to the good electron conductivity of PB and its high electrocatalytic activity. However, when PBNCs/rGO nanocomposite was introduced onto the electrode, further increased current (15.2 mA) and decreased response time (10.9 s) were observed. The results demonstrate that the largest catalytic current is observed at the PBNCs/rGO electrode because of the synergistic effects between the reduced GO and the PB nanocubes on thiocholine oxidation. Other researchers explained the observed synergy by the hypothesis that introducing CNTs into the polymer matrix containing redox mediators improved its electronic and ionic transport capacity and electron self-exchange in the polymer film [83,84]. This hypothesis can also be applied to our system of a PBNCs/rGO nanocomposite modified electrode. The reduced GO in the nanocomposite improves the electronic and potassium ionic transport capacity, resulting in an enhancement of the electrocatalytic activity. The general reactions on the electrode surface could be as follows:
(1.1)
(1.2)
(1.3)

The catalytic thiocholine oxidation in the case of PB can be achieved with the redox center active at ~220 mV, which greatly lower the oxidation potential to thiocholine detection. The AChE biosensor shows rapid response and high sensitivity for detection of monocrotophos with a linear range from 1.0 to 600 ng/mL and a detection limit of 0.1 ng/mL. These results suggest that the PBNCs/rGO hybrids nanocomposite exhibited high electrocatalytic activity toward the oxidation of thiocholine, which lead to the sensitive detection of OP pesticides.
1.3.3 Graphene sensors for other pollutants
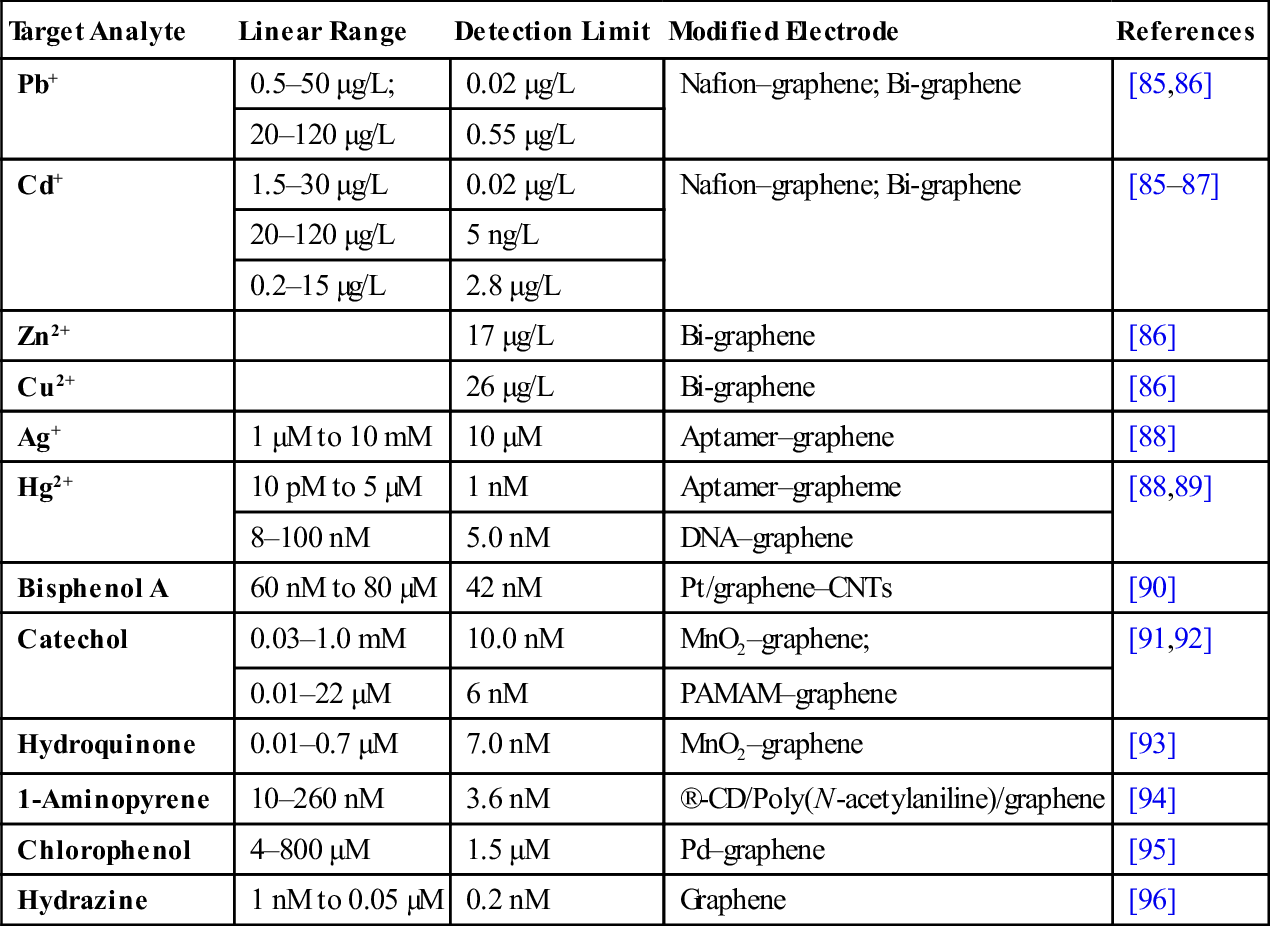
Graphene-based electrochemical sensors have been developed for environmental analysis. Table 1.1 collected some quantitative detection of heavy metal ions and environmental pollutants based on graphene sensors [85–96]. For example, Li et al. [85,87] report that Nafion–graphene composite film-based electrochemical sensors not only exhibits improved sensitivity for the metal ion (Pb2+ and Cd2+) detections, but also alleviates the interferences due to the synergistic effect of graphene nanosheets and Nafion [85]. The stripping current signal is greatly enhanced on graphene electrodes. As shown in the differential pulse anodic stripping voltammograms (DPASV) in Figure 1.9, the stripping current signal is well distinguished. The linear range for the detection of Pb2+ and Cd2+ is wide (0.5–50 and 1.5–30 g/L for Pb2+ and Cd2+, respectively). The detection limits (S/N=3) are 0.02 mg/L for both Cd2+ and Pb2+, which are more sensitive than those of Nafion film modified bismuth electrode [97], and ordered mesoporous carbon-coated GCE [98], and comparable to Nafion/CNT-coated bismuth film electrode [99]. The enhanced performance is attributed to the unique properties of the graphene (nanosized graphene sheet, nanoscale thickness of these sheets, and high conductivity), which endowed the capability to strongly adsorb target ions, enhanced the surface concentration, improved the sensitivity, and alleviated the fouling effect of surfactants [85].
Table 1.1
Graphene-Based Sensors for Environmental Applications
| Target Analyte | Linear Range | Detection Limit | Modified Electrode | References |
| Pb+ | 0.5–50 μg/L; | 0.02 μg/L | Nafion–graphene; Bi-graphene | [85,86] |
| 20–120 μg/L | 0.55 μg/L | |||
| Cd+ | 1.5–30 μg/L | 0.02 μg/L | Nafion–graphene; Bi-graphene | [85–87] |
| 20–120 μg/L | 5 ng/L | |||
| 0.2–15 µg/L | 2.8 μg/L | |||
| Zn2+ | 17 μg/L | Bi-graphene | [86] | |
| Cu2+ | 26 μg/L | Bi-graphene | [86] | |
| Ag+ | 1 μM to 10 mM | 10 μM | Aptamer–graphene | [88] |
| Hg2+ | 10 pM to 5 µM | 1 nM | Aptamer–grapheme | [88,89] |
| 8–100 nM | 5.0 nM | DNA–graphene | ||
| Bisphenol A | 60 nM to 80 µM | 42 nM | Pt/graphene–CNTs | [90] |
| Catechol | 0.03–1.0 mM | 10.0 nM | MnO2–graphene; | [91,92] |
| 0.01–22 μM | 6 nM | PAMAM–graphene | ||
| Hydroquinone | 0.01–0.7 μM | 7.0 nM | MnO2–graphene | [93] |
| 1-Aminopyrene | 10–260 nM | 3.6 nM | ®-CD/Poly(N-acetylaniline)/graphene | [94] |
| Chlorophenol | 4–800 μM | 1.5 μM | Pd–graphene | [95] |
| Hydrazine | 1 nM to 0.05 µM | 0.2 nM | Graphene | [96] |

1.4 Conclusions and future work
This chapter review work is relevant to the two fastest growing carbon nanomaterials: CNTs and graphene, in water monitoring and environmental applications. Due to their excellent electrochemical behaviors, CNTs and graphene have shown excellent performance in electrochemical detection of metal ions, pesticides, and other pollutants.
Since their introduction in 1991, CNTs have been widely investigated for electrochemical sensors of many important biomolecules because of their electrocatalytic and antifouling properties, biocompatibility, high surface, and mechanical strength. For trace metal analysis, CNT thin film created by drop-coating of CNT–solvent suspensions on electrode surfaces has been explored in order to develop mercury-free sensors by exploiting the bulk properties of the CNTs. Array of low-site-density aligned CNTs has been grown on metal substrates by a nonlithographic method. Each CNT serves as a nanoelectrode which normally has greater mass transfer rate and higher mass sensitivity than conventional macroelectrodes. The array of millions of CNT nanoelectrodes provides magnified voltammetric signals for trace metal ions without the need for a signal amplifier.
As a new nanocarbon material, graphene has exhibited excellent performance in electrochemistry applications. Graphene has been synthesized with various strategies. Novel methods for well-controlled synthesis and processing of graphene should be developed. For electrochemical applications, the approach with chemical/thermal reduction of GO looks promising. Better understanding of physics and chemistry at the surface of graphene and interaction of chemicals and biomolecules at the interface of graphene will play an important role in applying graphene as nanoscaffold in water and other environmental pollutants monitoring.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21275062, 21075047) and the Program for New Century Excellent Talents in University (NCET-12-0871). PNNL is operated by Battelle for DOE under Contract DE-AC05-76RL01830.

