Advanced Nanosensors for Environmental Monitoring
Omowunmi A. Sadik, Department of Chemistry, Center for Advanced Sensors and Environmental Systems, State University of New York at Binghamton, Binghamton, NY, USA
Nanoparticle-enhanced chemical and biosensors offer dynamic and quantitative analysis of toxic compounds in drinking water with potentials to provide rapid, ultrasensitive, and accurate risk assessment. We have developed a number of nanostructured materials as candidates for assessing the occurrence of water contamination. These include polyamic acid–metal nanoparticle composite membranes, polyoxy-dianiline membranes, sequestered metal nanoparticles within electroconducting polymers, and underpotential deposition of metal films onto solid electrodes. These materials have been utilized to design advanced sensors and have also been integrated with conventional instrumental techniques such as flow injection analysis (FIA), liquid chromatography (LC), and gas chromatography (GC). The resulting nanostructured sensors have been tested for the detection and identification of bacteria based on antibiotic susceptibility, multiarray electrochemical sensor with pattern recognition techniques, as well as for metal-enhanced detection of DNA–DNA, DNA–toxin, and DNA–drug interactions. The detection of cyanobacteria microcystis (M) spp and other toxins have also been reported. These sensors coupled with pattern recognition have provided reliable detection, classification, and differentiation of bacteria at subspecies and strain levels. Most of these sensors have shown good-to-excellent pathogen recovery efficiencies as well as a reasonable efficacy for sensing contaminants from water in controlled laboratory experiments.
This chapter focuses on some of these novel approaches as well as the fundamental scientific questions involved in this emerging area of research. Advanced nanosensors will be described with respect to the mechanism of molecular recognition, material design, and characterization, sensing procedure and efficiency as well as potential application for improving water quality. The specific evaluation of these sensors will be described in terms of speed, simplicity, and cost for rapid and reliable measurements of some environmental contaminants such as pathogenic bacteria, endocrine disruption chemicals, cyanobacteria, and organophosphate pesticides.
Keywords
Metal-enhanced detection of DNA; environmental contaminants; pathogenic bacteria; endocrine disruption chemicals; cyanobacteria; organophosphate pesticides; photopolymerized organic conducting polymers; novel electrode materials; chemical sensor arrays; pattern recognition; support vector machines; chromatographic systems; chlorinated organics; endocrine disrupting chemicals
2.1 Introduction
Rapid and precise sensors capable of detecting pollutants at the molecular level could greatly enhance our ability to protect human health and the environment. Of particular interests are remote, in situ, and continuous monitoring devices capable of yielding real-time information, and also those that can detect pollutants at very low levels. The ability to functionalize metal particles, control the size, shape, and stability at the nanoscale level is important for fundamental and industrial applications [1,2]. This can be achieved by using polymeric membranes that provide an excellent dispersion and sequestering environment for the metal particles, thus generating polymer metal nanocomposites with the desired morphological, electrochemical, and structural properties.
Recently, an emerging area of research is the implementation of nanomaterials for the development of a novel class of biosensors, biochips, nanosensors, and other transducers. In this respect, metal nanoparticles have been used for the development of chemical and biological sensors because of their unique optical, magnetic, and electrical properties [3]. By adding complementary molecules to a bioconjugate probe, a systematic interaction of the target molecules with the probe creates aggregation of the nanoparticles and this can be monitored by extinction or scattering. Alternatively, the binding of target molecules results in the formation of a secondary polymer shell, as is commonly employed for the interaction between the conjugates of nanoparticles with monoclonal antibodies and the corresponding target antigen, or between the conjugates of nanoparticles with polyclonal antibodies and low molecular antigens (haptens) [4]. Moreover, the application of gold nanoparticles for signal amplification has attracted a lot of interest in the biosensor development field. This is not only attributed to their unique properties in the conjugation with biological recognition elements but also in the signal transduction with optical, electrical microgravimetric, and electrochemical methods [5]. The sensitive detection with these metal–nanoparticle-based bio-tags is attributed to the signal amplification with silver deposition also known as silver enhancement. Techniques that incorporate silver enhancement have been able to achieve 2- to 30-fold amplifications [6].
Many efforts are currently focused on finding the ideal material to ensure the greatest characteristics of the nanostructured sensor system. Basically, in the construction of biosensors there are several requirements for selecting an electrode material [7]: (i) biocompatibility with the biological element, (ii) absence of diffusion barriers, (iii) stability with changes in temperature, pH, ionic strength, or macroenvironment, (iv) sufficient sensitivity and selectivity for the analyte of interest, as well as (v) low cost and ease of mass production. Moreover, these materials should either possess the necessary functional groups needed for the attachment of biomolecules or be able to be easily functionalized. Some sensors require operation in harsh conditions and, in this case, materials with special mechanical and chemical resistance, almost inert, are needed [7]. Additionally, an ideal electrode material must be characterized by a good conductivity to ensure a rapid electron transfer.
Environmental monitoring typically involves several steps such as sampling, sample handling, and sample transportation to a specialized laboratory to determine the chemical composition and to establish the toxic effects. These conventional approaches are expensive, time consuming, and require highly trained personnel. Thus the multiple steps involved frequently prevent rapid information about the composition and/or the toxicity of the sample to be obtained in an efficient manner. Thus risk assessment and remediation efforts could take months or years. Consequently, the need for fast, sensitive, selective, and cheap alarm systems is becoming more apparent. In this chapter, we will focus on some of these novel approaches as well as the fundamental scientific questions involved in this emerging area of research. Advanced nanosensors will be described with respect to the mechanism of molecular recognition, material design and characterization, sensing procedure and efficiency, as well as potential application for improving water quality. We will describe specific evaluations of these sensors in terms of speed, simplicity, and cost, for rapid and reliable measurements of some environmental contaminants such as organophosphates (OPs), volatile organic chemicals, pathogenic bacteria, and DNA.
2.2 Nanostructured sensing materials developed
We have explored the feasibility of designing advanced conducting polymeric materials for sensing and remediation applications [8–34]. These include the synthesis of (i) polyamic acid–silver nanoparticle composite membranes [10], (ii) polyoxy-dianiline films [7–9], and (iii) electrochemical deposition of gold nanoparticle films onto functionalized conducting polymer substrates [8,9]. These were characterized using electrochemical and surface morphology techniques including transmission electron micrograph (TEM), atomic force microscopy (AFM), cyclic voltammetry (CV), and Fourier transform infrared (FTIR) spectroscopy. We have utilized the unique reactivity of polyamic acid (PAA) to design polymer-assisted nanostructured materials. This approach works by preventing the cyclization of the reactive soluble intermediate into polyimides at low temperature [9]. The ability to prevent the cyclization process has enabled the design of a new class of electrode materials. Three different materials that incorporate nanoparticles of varying sizes have been successfully synthesized and characterized using this approach. We have explored these as precursors for environmental sensing and remediation.
2.2.1 Incorporation of metal nanoparticles in photopolymerized organic conducting polymers
Electroactive conducting polymers (ECPs) can be patterned by inexpensive methods (e.g., chemical, electrochemical, hot embossing, and imprinting techniques). Imprint method, which can allow submicrometer patterning with dimensions smaller than 100 nm, has been demonstrated using hard imprint materials [32–37]. Electrochemical methods can enable the preparation of thin films on electrodes, but are limited by the conducting substrates to be used during deposition. Moreover, the polymers suffer from poor stoichiometry, insufficient conductivity, and reproducibility. However, photopolymerization technique allows the use of insulating substrates such that several metals and alloys can be deposited from an electrolyte onto the nonconducting surface. Photochemical preparation of ECP films utilizes a mixture of monomer, electron acceptor, and photoactive species exposed to ultraviolet (UV)/visible light [38–41]. We have found that regularly arranged metal nanoparticles could be spontaneously prepared using photochemical polymerization of ECPs [7,8]. The oxidation of a Π-conjugated ECP with gold trichloride, silver nitrate, palladium ions, and copper sulfate following photochemical reaction was found to produce conducting films having metal clusters in 5–100 nm range [7,8]. The incorporated metal clusters enhanced the conductivity of the resulting polymers. We also found that the temperature dependence of ECP materials upon exposure to different compounds produced changes in surface conductivity, and this can be used to monitor the compounds. These metal clusters could also serve as novel catalysts for the removal of heavy metals in aqueous media [42,43].
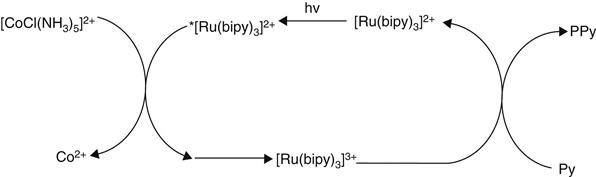
Alternative photochemical routes to polypyrrole (PPy or Ppy) that have advantages in some applications over the conventional chemical and electrochemical syntheses have been described. Segawa and coworkers reported that visible light irradiation of an aqueous pyrrole solution in the presence of [Ru(bipy)3]2+ (bipy=2,2′-bipyridine) as the photosensitizer and [CoCl(NH3)5]2+ as a sacrificial oxidant led to the deposition [41]. This powdery product exhibited a relatively low conductivity (3×10−4 S/cm2) compared with the polypyrrole chloride [PpyCl] prepared via standard chemical or electrochemical methods. The photochemically initiated polymerization is believed to proceed via the mechanism shown in Figure 2.1, where the oxidation of the pyrrole monomer is performed by the strong oxidant [Ru(bipy)3]3+ generated by oxidative quenching of the photo-excited *[Ru(bipy)3]2+ species by the sacrificial Co(III) complex.
In our laboratories, novel conducting polymers were prepared through a systematic photopolymerization of PPy with the incorporation of Group 1B metal particles [7,8]. This was achieved through a systematic photopolymerization of PPy with the incorporation of Group 1B metal particles (Eqs. (2.1) and (2.2)). The structural and morphological properties of the incorporated metal nanoparticles were examined with respect to the role of the metals ions, nature of substrates, exposure time, and monomer counterion ratios. Elemental analysis confirmed the identity of the particles with sizes of 100 nm diameter.
(2.1)
(2.2)
2.2.1.1 Polymerization mechanism
A mechanism is proposed for the formation of metal nanostructure during photopolymerization [7,8]. The reaction schemes in Eqs. (2.1) and (2.2) are offered to describe our findings for the photoreaction where gold trichloride is incorporated into the film as AuCl4− (Eq. (2.1)) and the condition where the additional anionic dopant, dodecylsulfate (DS−), excludes the AuCl4− (Eq. (2.2)). The reaction starts with the absorption of a photon and spontaneous reduction of AuCl3 salt from solution to form gold clusters. This leads to the oxidation of pyrrole and the formation of pyrrole radical cation through excitation of electrons in the π-orbital. Next is the coupling of two radical cations to form a dimer and a deprotonation process where two protons are lost per coupling. Upon excitation of the monomer, the dimers or higher oligomers are also oxidized and this can further react with the radical cation of the monomer to build up the chain. Coupling of oxidized oligomers may also occur.
When each pyrrole monomer loses just two protons, the polymer is not conductive. The polymer becomes conductive when it is further oxidized by an anion in the electrolyte. For each proton lost, an anion must associate with the polymer for charge neutrality. A necessary condition for the reaction is that the polymerization steps should proceed with 100% efficiency. In this case, all electrons produced during the reduction of AuCl3 ions must result in the oxidation of the pyrrole monomer. If, indeed, the metal ion is an active participant in the initiation step by facilitating pyrrole oxidation, then it is likely that the same effect will be observed using copper or silver solutions, given the similarities in electronic configuration and electron affinity. Thus the incorporation of other Gp 1B metal ions was investigated.
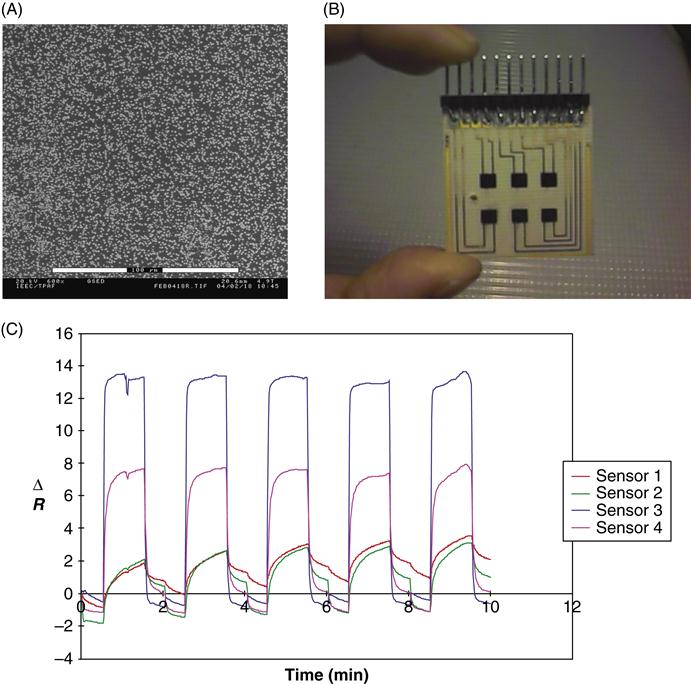
The rate of film formation was found to be dependant upon the metal salt utilized. Subsequent analysis of the ECP films shows the incorporation of zero valent metal particles and the resulting film is conductive and electroactive. By varying the metal salt to monomer ratios, stoichiometric relationships were determined. Addition of anionic dopant sources, such as sodium dodecylsulfate, are shown to alter the reaction stoichiometry, indicating that competition between the metal salt anion and surfactant ion for inclusion in the p-doped film may be a crucial factor in the mechanism of photonic polymerization. The photopolymerization techniques do not require a conductive substrate to deposit the films, and may be used in conjunction with silicon technology, the integration of which could lead to hybrid modules or integrated sensors, where signal processing can be carried out on the same chip (Figure 2.2).
2.2.1.2 Polypyrrole films for vapor sensing
Additionally, we tested the possibility of photopolymerized [PPy] for vapor sensing (Figure 2.2). When the PP arrays are presented with pulses of organic vapors, they generate characteristic temporal response for each vapor. The resistance change was reversible upon the removal of the organic vapor and sensor signals returned to baseline. The reproducible signals obtained could be attributed to that caused by defects contained in the conjugated structure as well as the energy of sorption and desorption processes, and solubility consideration [8,38–42].
2.2.2 Nanostructured PAA membranes as novel electrode materials
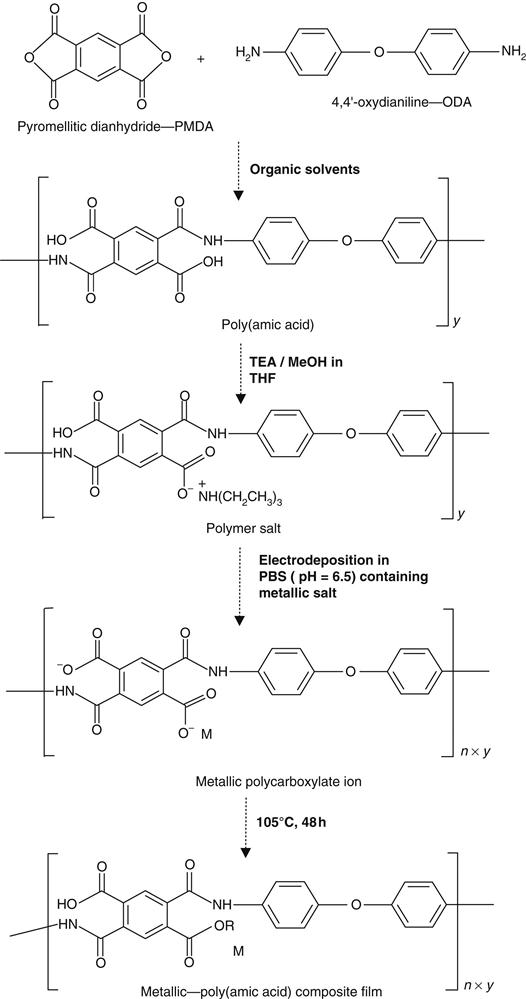
We have also reported a new approach for the preparation of PAA composites containing Ag and Au nanoparticles. The composite film of PAA and metal particles were obtained upon electrodeposition of a PAA solution containing gold or silver salts with subsequent thermal treatment, whereas imidization to polyimide is prevented [9]. The composites of poly (amic acid)–metallic gold and silver nanoparticles were prepared using pyromellitic dianhydride (PMDA) and 4,4′-oxydianiline (ODA) solutions containing the salts of the metal ions [9]. The resulting PAA composites were thermally cured between 40°C and 105°C and then electrochemically reduced by galvanostatic deposition. Thermal curing at relatively low temperature was sufficient to eliminate all residual solvents after 2 h with concomitant reduction of the metal ions into metallic nanoparticles. FTIR spectroscopy, scanning electron microscopy (SEM), and CV and NMR analysis were used for structural and morphological characterization. The molecular weight of the solid PAA was estimated to be 10,000 dalton using gel permeation chromatography. Elemental analysis and TEM confirmed that electrochemical or thermal reduction of PAA-metal composites produced homogeneous metallic nanoparticles (Figure 2.3). In our system, we prepared novel PAA metal nano-composites onto RVC electrodes in which the polymer will retain the carboxyl groups and exhibit high stability. In this approach, salts of the desired metal particles were introduced into the PAA solution so that subsequent electrochemical reduction or thermal curing at low temperature would produce monodispersed nanoparticles. For this purpose, we used two solvent systems: first was in tetrahydrofuran with triethylamine (TEA) as surfactant and methanol as precipitant agent. The other was developed in acetonitrile using similar surfactant and precipitating agent. PAA was obtained as a viscous solution in the first solvent system, whereas it was obtained as solid using acetonitrile as solvent. The uniqueness of this approach lies in the incorporation of metal nanoparticles within the electropolymerized PAA at low temperature and the ability to prevent the cyclization process at low temperature. The low temperature ensured that the thermal reduction occurred and imidization process was avoided [9].
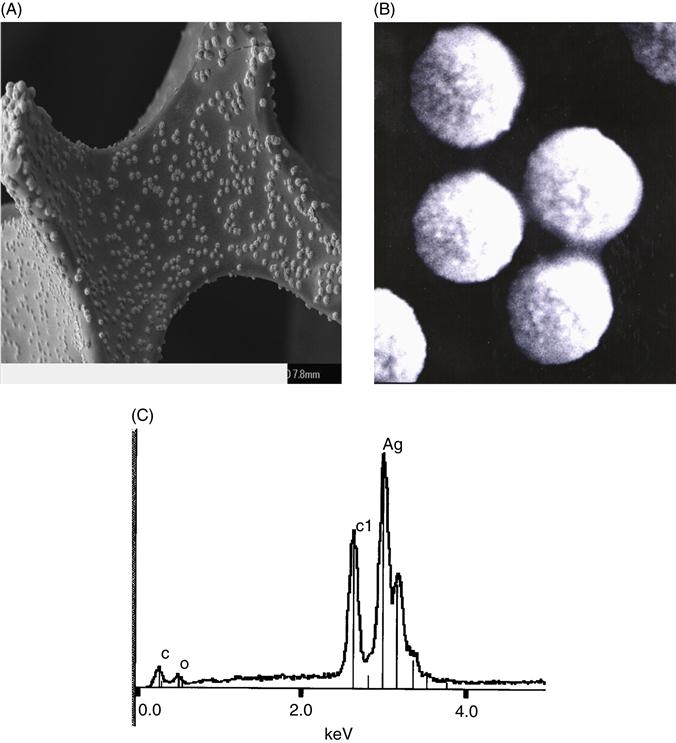
Figure 2.4 shows the chemistry of the synthetic process including the electrodeposition of silver–composite PAA film from PMDA and ODA. The rationale is that when constant current is applied, the PAA salt is dissociated. Under the influence of electric field, the negatively charged poly- or monocarboxylates migrate to the anode, accept protons, and precipitate as continuous polymer film [9]. The presence of free carboxylic groups in the PAA–silver-modified electrode will allow the utilization of this material as immobilization matrices in sensors and biosensor devices [9]. Structural analysis of PAA and Ag–PAA were achieved by using FTIR spectroscopy to confirm the presence of specific functional groups [9]. The strong peak around 1225 cm−1 that appeared in both spectra was associated to stretching vibration of the ether group [33]. All these bands were still present in the Ag–PAA spectrum after thermal treatment. The absence of typical imide functional bands in the cured PAA and Ag–PAA films at 1778 cm−1 (for symmetric C=O) and 1374 cm−1 (C–N–C axial) [9] confirmed that the imidization process had been avoided at 105°C even after 5 days of thermal treatment. Moreover, the similarity in both spectra (spectra not shown) suggests that the characteristic functional groups present in PAA basic molecule retained their integrity after electrodeposition and drying.
2.3 Chemical sensor arrays and pattern recognition
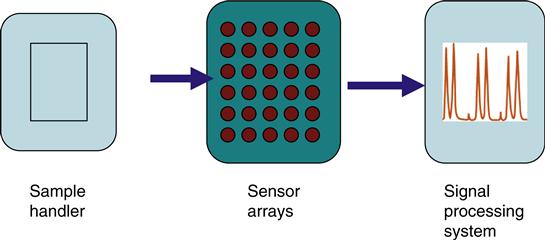
The nanostructured materials synthesized were subsequently incorporated as sensing elements in an array system. A sensor array system (SAS) consists of three functional components that operate serially on a sample containing the analyte, a sample handler, an array of polymer sensors, and a signal processing system (Figure 2.5). The output of the SAS can identify an environmental toxin or biological molecule, estimate the concentration, or determine the characteristic properties of the compound present in air or other samples.
Fundamental to the SAS is the idea that each sensor in the array has different sensitivity. For example, Compound No. 1 may produce a high response in one sensor and lower responses in the others, whereas Compound No. 2 may produce high readings for sensors other than the one that exhibit significant response to Compound No. 1. What is important is that the pattern of response across the sensors is distinct for different compound. This allows the system to identify an unknown agent from the pattern of sensor responses or database. Thus, each sensor in the array has a unique response profile to the spectrum of chemicals under test. The pattern of response across all sensors in the array is used to identify and/or characterize the analyte.
We have further demonstrated the effective use of our nanostructured polymer arrays coupled with machine learning for the detection and classification of OP nerve agents’ stimulants. For OPs and volatile organics, we showed a significant 168% specificity improvement and a 40.5% positive predictive value improvement using the s2000 kernels at 100% and 98% sensitivities when compared to commercial system [30,31]. The OP molecules that are dissociated at the surface of the polymer electrode cause the change in sensor resistance. This chemisorption-driven process can change the electrical resistance considerably, and can make the sensor array sensitive to OP concentrations over a wide range of concentrations. Since the sensor completely regains its original resistance with OP cycling, it appears that the OP diffusion is confined to the surface layer.
2.3.1 Data processing, pattern recognition, and support vector machines
The task of a sensor array system is to identify the presence of toxic environmental chemicals (TECs) in the sample and perhaps to estimate its concentration. This is achieved by means of signal processing and pattern recognition (Figure 2.5). These two steps may be subdivided into four sequential stages: preprocessing, feature extraction, classification, and decision-making. Preprocessing compensates for sensor drift, compresses the transient response of the sensor array, and reduces sample-to-sample variations. Typical techniques include manipulation of the sensor baseline; normalization of sensor response ranges for all sensors in an array and compression of sensor transients. Feature extraction has two purposes: to reduce the dimensionality of the measurement space and to extract information relevant for pattern recognition. Feature extraction is generally performed with classical principal component analysis (PCA) and linear discriminant analysis (LDA). PCA finds projections of maximum variance and is the most widely used linear feature extraction techniques. However, it is not optimal for classification since it ignores the identity (class label) of the analyte examples in the database. LDA on the other hand looks at the class label of each example. Its goal is to find projections and maximize the distance between examples of the same TEC agent. As an example, PCA may do better with a projection that contains high variance random noise whereas LDA may do better with a projection that contains subtle, but maybe crucial, agent-discriminatory information. LDA is more appropriate for classification purposes.
Once the sensor signal is projected on an appropriate low-dimensional space, the classification stage can be trained to identify the patterns that are representative of each class of compound. The classical methods of performing the classification task are k-nearest neighbors (k-NN), Bayesian classifiers, and artificial neural networks (ANN). k-NN classifiers find examples in the TEC database that are closest to the unidentified agents and will assign the nature of the agent represented by a majority of those examples. Bayesian classifiers first build a probability density function of each agent class on the low-dimensional space. When presented with an identified compound, the Bayesian classifier will pick the class that maximizes the precompiled probability distribution. The classifier produces an estimate of the class for an unknown sample along with an estimate of the confidence placed on the class assignment. However, the inability to present error-free detection, requirement for large training data set, as well as the inability to minimize true risk has led to the development of other processing approaches such as the support vector machines (SVMs).
SVMs are a new and radically different type of classifiers or “learning machines” that use a hypothesis space of linear functions in a high-dimensional feature/space. SVMs are generally trained with learning algorithms originating from optimization theory that implements a learning bias derived from statistical learning theory. The use of SVMs for computational intelligence is a recent development, and certainly unknown for analytical monitoring of TECs. Several reviews provide extensive backgrounds to develop the mathematical foundation of SVMs ([30,31] and cited references). In the context of classifying TEC, the objective of SVMs is to construct an “optimal hyperplane” as the decision surface such that the margin of separation between two different chemical substances is maximized. SVMs are based on the fundamental ideas of: (i) structural/empirical risk minimization (SRM/ERM); (ii) the Vapnik–Chervonenkis (VC) dimension; (iii) the constrained optimization problem; and (iv) the SVM decision rule. Properly designed SVMs should have a good performance on untested data because of their ability to generalize and scale up to problems that are more complex. The fact that the margin does not depend on input dimensionality means it is immune to the curse of dimensionality. SVMs have been successfully applied to a variety of classification problems including text categorization, handwritten digit recognition, gene expression analysis, and simple chemical and mixtures recognition.
We have studied the integration of our sensor network with SVM for the detection of TEC. This approach reduced the number of false negative errors by 173%, while making no false positive errors when compared to the baseline performance [30,31]. The reader may recall that obtaining larger and larger sets of valid training data would sometimes produce (with a great deal of training experience) a better performing neural network (NN), which resulted from classical training methods. This restriction is not incumbent on the SRM principles and is the fundamental difference between training NNs and training SVMs. Finally, because SVMs minimize the true risk, they provide a global minimum.
2.3.2 Integration of sensor array with chromatographic systems
We have previously shown that combining ECP with conventional instrumental techniques (such as chromatographic and flow injection analysis (FIA)), provides a suitable approach to the development of sensitive and reproducible analytical signals [44,45]. Results obtained by the use of integrated conducting polymer sensors with chromatographic analysis and FIA revealed a significant improvement in sensor performance and the overall analysis with respect to time and selectivity [44–47]. In Section 2.3, we saw how sensor arrays and SVMs were utilized in the detection and classification of OP nerve agent simulants. However, sensor arrays cannot quantitatively detect mixtures of compounds. This is because SAS systems analyze mixtures of compounds as a one-component solution. To overcome this limitation, we have coupled sensor arrays to a gas chromatograph. In this scenario, the analyte mixture is first separated by the gas chromatography (GC) column based on their relative retention times and detected using the multiarray polymer sensors. This new system showed significant improvement over the existing multisensor array systems in the analysis of mixtures [46]. We have also demonstrated how the hyphenated combination of GC and conducting polymer sensor arrays could effectively be used in the separation and detection of mixtures of volatile organic compounds [47].
Other workers have also reported their research findings on GC–sensor arrays. A good example is a prototype portable GC that combines a multiadsorbent preconcentrator, a tandem-column separation stage, and a detection consisting of an integrated array of polymer-coated surface acoustic wave (SAW) devices [48–52]. In that report the determination of vapor mixtures of common indoor air contaminants including 2-propanol, 3-methyl butanol, and 1 octen-3-ol was demonstrated. The advantages of sensor arrays include the fact that they do not require auxiliary gases and hazardous materials commonly required in classical GC detectors (e.g., flammable hydrogen gas in FID) and hazardous radioactive material (Ni-63 in ECD) for operation. Most sensor arrays are small thus leading to small dead volumes and increased sensitivity. The small size also has inherent advantages in miniaturization of chromatography–sensor array systems. Furthermore, the sensor arrays can also be used in conjunction with machine learning programs.
2.4 Biosensing applications of nanostructured materials
Our laboratories and others have utilized the uniqueness of nanoparticles to develop biosensors for different environmental, clinical, and security applications [15,16]. Some of these are discussed in the following sections.
2.4.1 Biosensors for polychlorinated biphenyls
The persistence of polychlorinated biphenyls (PCBs) in the environment and their extensive usage in numerous industrial and commercial applications are currently of great concern. The limited information on the effect of PCBs indicated that these compounds might produce immunological abnormalities, reproductive dysfunction or an increased thyroid volume, increased prevalence of thyroid and liver disorders [53,54]. Nowadays, there is an increased interest to detect these environmental chemicals from both scientific and regulatory communities. Consequently, significant efforts are focused on developing a fast and reliable method for their determination.
Immunosensors have been reported to exhibit considerable potential for PCB detection [54–57]. For instance, we have developed a PCB immunosensor, constructed by immobilizing anti-PCB antibody (Ab) into a conducting PPy membrane. Pulsed-accelerated immunoassay for signal generation in stationary cell or FIA is obtained by applying a pulsed waveform between +0.60 and −0.60 V and a pulse frequency of 120 and 480 ms. With the optimized sensor, a linearity of 0.3–100 µg/L and a detection limit of 3.3, 1.56, 0.39, and 1.66 ng/mL, respectively, were obtained for Aroclors 1242, 1248, 1245, and 1016 [54,57].
Most studies with immunosensors are carried out in aqueous solutions in which large molecules as Ab function ideally. However, PCBs, as well as other important environmental analytes are poorly soluble in this medium. In addition, extraction and concentration of the sample are commonly carried out in organic solvents. In this context, a detection method for these compounds in the presence of organic medium is also required. In most cases, the immunological activity of immobilized Ab is generally lower in organic solvents compared to water. Significant improvement in this direction was achieved when Ab was encapsulated in reversed micelles [58]. Detection of PCBs was also achieved using DNA biosensors designed for environmental monitoring. In a recent work developed in our group we reported a detection limit of 10 nM PCB using supramolecular dsDNA sensor on Ag–Au-coated quartz crystal electrode with impedance spectroscopy [59]. Using an electrochemical system, Marrazza et al. detected as low as 0.2 mg/L PCBs using screen-printed disposable DNA biosensor [60]. In this case, determination was achieved by measuring changes of the electrochemical signal of guanine in calf thymus DNA extract immobilized onto the electrode surface.
2.4.2 Endocrine disrupting chemicals, chlorinated organics, and other analytes
2.4.2.1 Biosensors for polyphenols and other analytes
A novel label-free electrochemical scheme for probing the electronic properties of DNA binding with small molecules was recently described by our group [61]. The strategy, called metal-enhanced electrochemical detection (MED) involved the electrooxidation of silver ions, deposited as a monolayer onto a gold electrode surface in the presence of dsDNA molecules. The results indicated that by oxidizing the silver monolayer, highly reactive oxides of silver ions are generated, causing a change in the electronic properties of the immobilized dsDNA. Thus, in the presence of low molecular weight organic DNA-binding molecules, structural change in the DNA occurs, generating corresponding change in the redox properties of the silver monolayer. These variations are proportional to the concentration of DNA-binding molecules and can be easily quantified by voltammetric techniques. The approach offers an alternative route for the detection of DNA hybridization reactions, DNA–protein interactions, and gene detection. It was demonstrated that the performance of the DNA biosensors are strongly dependent on hybridization reactions at the transducer–solution interface. Thus, control of the hybridization conditions such as temperature and hybridization time can considerably extend the dynamic range and lower the sensitivity [61].
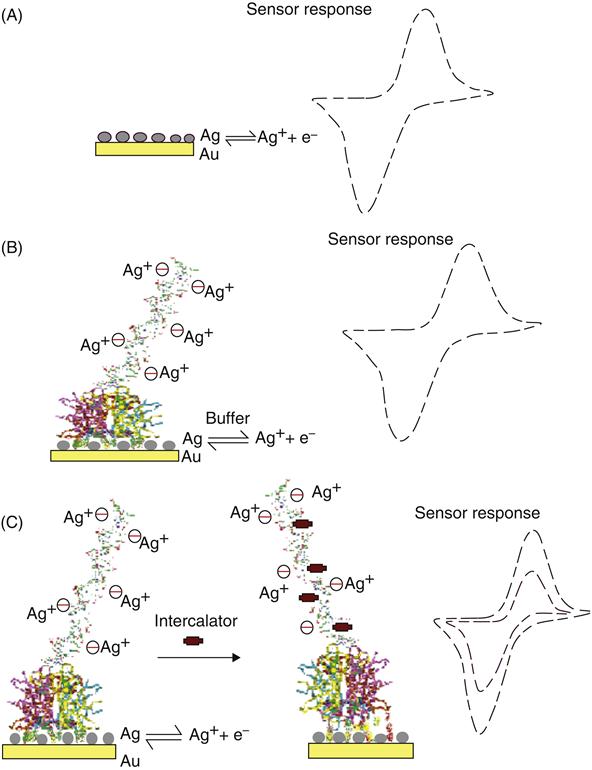
The strategy for MED using the reactivity of silver ions is illustrated in Figure 2.6. A monolayer of silver is deposited on a gold electrode or other conducting substrates (e.g., platinum or glassy carbon). The silver deposition can also be achieved by incubating silver compounds for approximately 5 min in the dark at room temperature. The electrochemical oxidation of silver produces silver ions and electrons accompanied by a reversible redox signal (Figure 2.6A). If dsDNA is present at the surface, the silver ions are dispersed and are held electrostatically. Upon reduction, the silver ions return to the surface and a reduction current is measured (Figure 2.6B). If a DNA-binding low molecular weight organic molecule is introduced into the solution, structural change in the DNA molecule occurs, which is signified by a simultaneous change in the redox currents from silver, and a decrease in current is measured (Figure 2.6C). This decrease is proportional to the concentration of the DNA-binding molecule. The underlying signal transformations produced here could result from the DNA conformational or structural changes in the presence of the analyte molecules, which ultimately hinder the flow of electrons. Typical results for MED are shown in Figure 2.7 using differential pulse voltammetry.
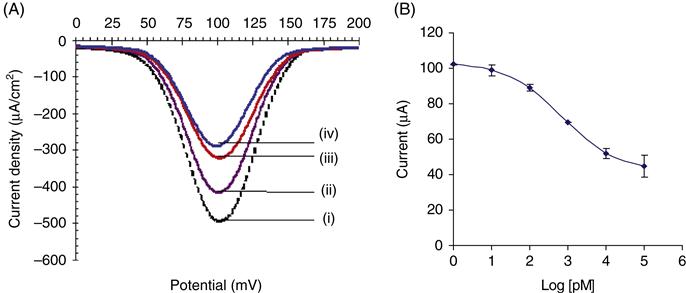
Using the proposed MED concept and un-optimized assay format, a detection limit of 4 ppt has been recorded for Bisphenol A (BPA), an endocrine disrupting chemical (EDC) (Table 2.1), whereas the limit of detection for the same analyte using ELISA was 228 ppb. This represents a 1000-fold improvement over the gold standard. In addition, the time required for the MED biosensor was about 20 min, compared to between 24 h and 3 days necessary for the ELISA techniques. Apart from small organics, we have tested the utility of the MED concept for other molecules with molar masses ranging from 200 to 150,000 amu. The range of analytes tested include nucleic acids, PCBs, nonylphenols, cisplatin, and other naturally occurring isoflavonoids [58,59].
Table 2.1
Comparison of MED-Based Biosensor Performance with Conventional Techniques
| Analytes | Limit of Detectiona,b,c | ||
| ELISA | Electrochemical Fe2+/Fe3+ | Detection Ag0/Ag+ Couple | |
| BPA | 228 ppb | 10 nM | 4 ppt |
| NP | 37.5 ppb | ND | 14 ppt |
| Cisplatin | 1 nM | 10 pM | |
| PCBs | ND | 5 ppt | |
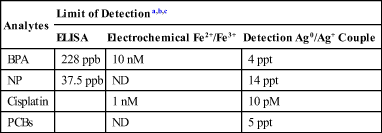
BPA: Bisphenol A; ND: None Detected; nM: Nanomolar; PCB: Polychlorinated biphenyls; pM: Picomolar; ppt: Parts per trillion.
aSadik et al, JACS, 123, 2001, 11335
bNgundi M., PhD Binghamton University, 2003
cKowino I., PhD Binghamton University 2006.
2.4.2.2 Biosensors for EDCs
In recent years, it has become evident that many environmental chemicals, including synthetic and endogenous estrogens, can mimic, block, or alter the action of endogenous steroid hormones and can interfere with hormone-regulated physiological processes [13,62–66]. These industrial and environmental chemicals are known as EDCs and structurally resemble endogenous estrogens. They have been analyzed for many decades in numerous biological and medical investigations. Screening and confirmatory strategies for these steroids involve chemical or immunochemical methods followed by the complete instrumental confirmation of steroids by mass spectrometry (MS). Until recently, the standard technique for analyses has been GC/MS. Moreover, the limits of detection were not sufficient to analyze steroids at low levels in urine and environmental samples. Also, these techniques typically require sample pretreatment, expensive apparatus, and skilled personnel.
Synthetic estrogens are characterized by the presence of phenolic functional groups, a common structural feature that is also found in natural estrogens. This structural feature could facilitate binding to estrogen receptor [62–66] and possibly generate receptor-induced transformations. Sadik et al. presented a summary of different approaches reported for EDCs [13] and also demonstrated the feasibility of in situ monitoring of the interaction between BPA and dsDNA [59]. In another report, Ngundi et al. demonstrated the comparative electrochemical behavior of β-estradiol and selected EDCs, specifically alkylphenols, and proposed a possible link between the structure and their estrogenic activity [66]. More recently, we have reported the isolation and complete structural characterization of quercetin (QRC) and other isoflavonoids [67]. We have also developed and optimized a fully autonomous electrochemical biosensor for studying the role of alkylphenols on A549 lung adenocarcinoma cell line. This advanced biosensor uses a prototype 96-electrode (DOX-96) well-type device that allows the measurement of cell respiratory activity via the consumption of dissolved oxygen. The system provides a continuous, real-time monitoring of cell activity upon exposure to naturally occurring polyphenols, specifically resveratrol (RES), genistein (GEN), and QRC. The system is equipped with a multipotentiostat, a 96-electrode well for measurements and cell culturing with three disposable electrodes fitted into each well. A comparison with classical “cell-culture” techniques indicates that the biosensor provides real-time measurement with no added reagents. A detection limit of 1×104 was recorded versus 200 and 6×103 cells/well for MTT and fluorescence assays, respectively. This method was optimized with respect to cell stability, reproducibility, applied potential, cell density per well, volume/composition of cell culture medium per well, and incubation. Others include total measuring time, temperature, and sterilization procedure. This study represents a basic research tool that may allow researchers to study the type, level, and specific influence of isoflavonoids on cells.
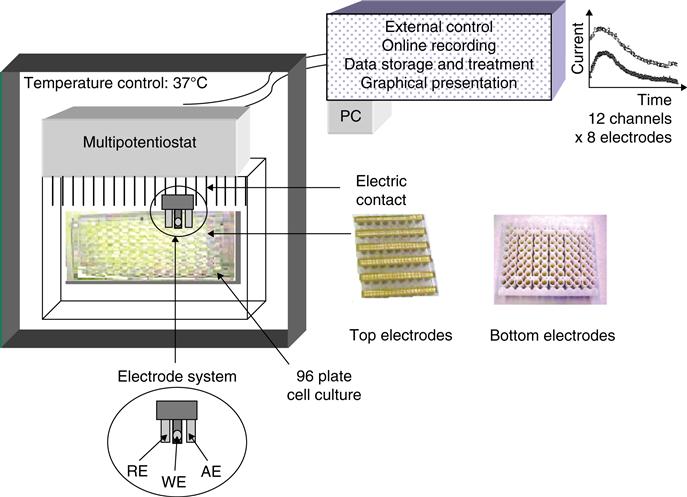
The DOX approach utilizes a prototype multichannel system that enables simultaneous, quantitative, and continuous measurement of dissolved oxygen using a 96-well electrode biosensor prototype shown in Figure 2.8 known as the DOX device. DOX is fully automated, portable, equipped with a multipotentiostat, and can be connected to a computer. The latter enables external control of the instrument, online recording of experimental parameters, graphical presentation and data storage. The instrument software plots current intensity versus time for each well while data are simultaneously processed for 12 channels, each corresponding to 8 sensors. Experimental setup involves the use of 96 disposable electrodes in a three-electrode format (reference, working, and auxiliary). These 96 sensors are placed in a conventional 96-well plate in which the cells are cultured. Respiration of cells generates a reduction in the concentration of dissolved oxygen, which is determined using electrical current produced [19,20] according to the following reaction:
(2.3)
Cytotoxicity measurements involve the application of cells into the optimum growth medium containing a selected isoflavonoid. This is followed by a continuous monitoring of the oxygen consumed by the cells with time. Basically, if an inhibition of cell proliferation occurred in the presence of PPh this will generate higher oxygen content, proportional with the inactivation level induced by PPh. This could be indirectly correlated to the concentration of PPh introduced in the medium.
2.4.3 Multiarray electrochemical sensors for monitoring pathogenic bacteria, cell viability, and antibiotic susceptibility
Bacterial pathogens are found not only in soil, food, marine, and estuarine waters but also in intestinal tracts of humans and animals. Microbial diseases constitute one of the major causes of death in many developing countries. For this reason, significant efforts are needed to develop new systems for rapid detection of pathogenic bacteria in a variety of fields. Among these, enzyme, DNA or immunosensors coupled with electrochemical, piezoelectric, optical, acoustic, and thermal detection represent viable alternatives. We reported the use of amperometric signals of the DOX coupled with PCA for continuous monitoring, identification, and differentiation of bacteria. Two types of differentiation mechanisms were tested: (1) direct monitoring of respiratory activity via oxygen consumption and (2) quantification of the effect of three broad-spectrum antibiotics on bacteria growth and respiration over time (Figure 2.8). Five species of bacteria were examined including: Escherichia coli, Escherichia adecarboxylata, Comamonas acidovorans, Corynebacterium glutamicum, and Staphylococcus epidermidis. The addition of small concentrations of antibiotics to the growth medium alters the oxygen consumption of the cells and a unique fingerprint is created for a specific cell. This fingerprint is shown to evolve over a specific concentration range that is dependant of instrumental constraints of the DOX system. The application PCA to classify the data was also examined (Figure 2.9). It was shown that bacteria could be classified simply by their oxygen consumption rates over a varying concentration range. Discrimination between species can also be increased by the effects of the antibiotics on the oxygen consumption of varying concentrations of cells (Table 2.2). The proposed DOX–PCA system illustrates a generic template that can be tailored to meet specific research goals by the selection of specific cell/antibiotic combinations and concentrations.
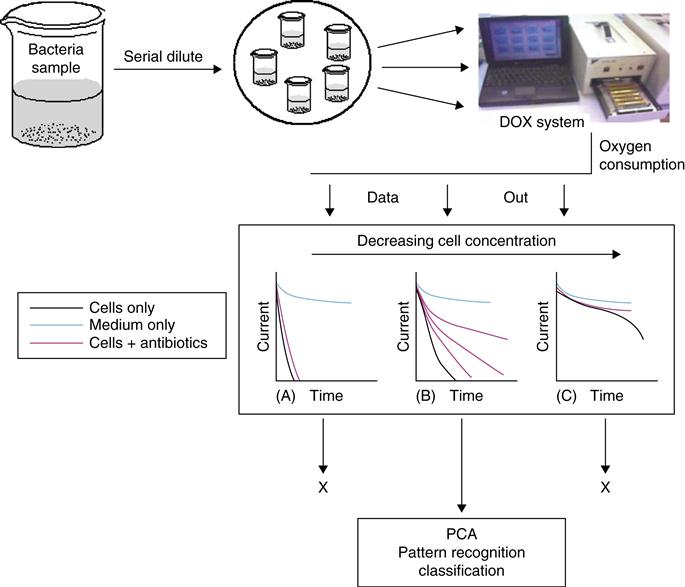
Table 2.2
Bacteria Concentrations Used in the DOX–PCA Experiments
| Species of Bacteria | Concentration×106 cfu/mL | ||||
| C1 | C2 | C3 | C4 | C5 | |
| C. acidovarans (ATCC# 51340) | 112 | 56a | 28 | 14 | 7.5 |
| C. glutamicum (ATCC# 13032) | 79.3 | 39.65 | 19.83a | 9.91 | 4.96 |
| S. epidermidis (ATCC# 12228) | 81.7 | 40.85 | 20.43a | 10.21 | 5.10 |
| E. adecarboxylata (ATCC# 23216) | 45 | 22.5 | 11.25a | 5.63 | 2.81 |
| E. coli (ATCC# 25922) | 150 | 75 | 37.5a | 18.8 | 9.4 |
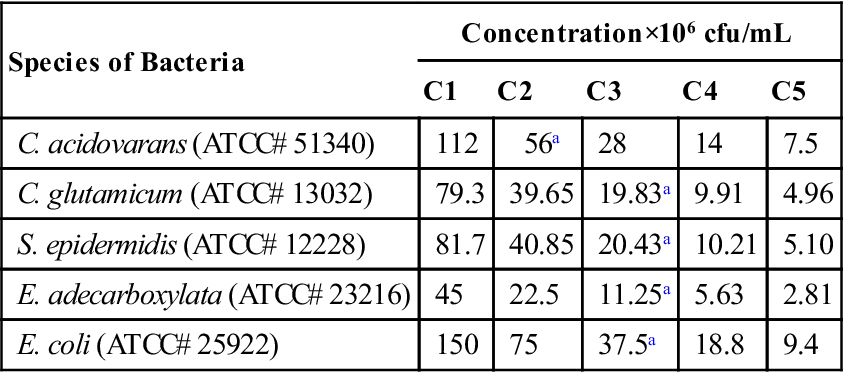
aMaximum cell concentration that can produce a DOX fingerprint.
Another prototype variation uses a bottom electrode design in which the electrodes are manufactured directly into the bottom of each well in the plate. All experiments in this project were performed with the top electrode system. Each element in the disposable electrode array is composed of a working, reference, and auxiliary electrode for individual wells in the plate. The gold working electrode has dimensions of 0.19 mm2×5 mm2×5 mm2. After the electrodes are placed into the sample, the plate is inserted into the DOX-96 instrument, which resembles a small optical microplate reader. The instrument contains a multipotentiostat, and the current is measured for each of the 96 wells automatically according to user-set parameters. Options include voltage (negative range only), total time, and measurement intervals (e.g., 1 measurement per minute). Data is processed by the software and represented graphically in a time versus current plot and simultaneously tabulated in Microsoft excel.
2.5 Conclusions and future perspectives
We have developed a number of nanostructured materials as candidates for assessing the occurrence of water contamination. These include PAA–metal nanoparticle composite membranes, polyoxy-dianiline membranes, sequestered gold, silver or palladium nanoparticles within electroconducting polymers and underpotential deposition of metal films, membranes, and colloids onto solid electrodes. We have also reported the application of these materials for the design of advanced sensors. In that respect, sensors have been developed for EDCs, volatile organic compounds, the detection and identification of bacteria based on antibiotic susceptibility, multiarray electrochemical sensor with pattern recognition techniques, as well as for metal-enhanced detection of cyanobacteria microcystis (M)75 spp based on DNA base-pair mismatches. Most of these sensors have shown good-to-excellent pathogen recovery efficiencies as well as a reasonable efficacy for sensing contaminants from water in controlled laboratory experiments.
Chemical and biosensors will continue to provide important monitoring, diagnostic and mechanistic solutions to many environmental, clinical, food, and security applications; and may open new areas of modern analysis. Despite important progress already achieved, the biosensor market is still relatively small, requiring important fundamental and mechanistic studies in order to fully explore their real potentials. Today, more than 90% of commercial biosensors are designated to glucose analysis and few analytes could still be detected. This is mainly a consequence of insufficient reliability associated with poor stability of the sensing material, multiple matrix effect, and also a dependence upon the physico/chemical parameters and interferences within the transducers. Nevertheless, with respect to rapid environmental analysis, sensitive and selective low-cost determination of a great variety of analytes, no suitable alternative exists for biosensors.
Acknowledgments
The author acknowledges the United States Environmental Protection Agency through the STAR program (RD-83090601 and R825323) and the National Science Foundation (CHE-0513470) for funding. Also acknowledged are the contributions of members of the author’s research group (past and present) at SUNY-Binghamton including Adam Wanekaya, Austin Aluoch, Isaac K’Owino, Samuel Kikandi, Jason Karasinski, Sharin Benda, Syeda Begum, Miriam Masila, Fei Yan, Marc Briemer, Hongwu Xu, Sydney Sheldon, Eugen Yevgheny, and Sean Falvey.