Genomic Approaches and Abiotic Stress Tolerance in Plants
Bushra Rashid, Tayyab Husnain and Sheikh Riazuddin
Environmental threats that comprise factors related to abiotic stresses are among the major challenges to crop plants, which limit the yield and productivity. Plants respond to this threat for their endurance at the cellular and molecular level. Understanding plant responses to these stresses is imperative and thought provoking in order to combat the challenges in agricultural research. Consequently, a complex interaction between signaling molecules and pathways has been triggered. Success in breeding for better adapted varieties to abiotic stresses depends upon the combined efforts of various research domains including plant and cell physiology, molecular biology, and genetics. Genomics approaches for considering plant responses to stress have provided potent tools for in-depth dissection of tolerance mechanisms, primarily with models and latterly with other plant species. As an alternative to conventional breeding, a more competent technology is marker-assisted breeding, which has identified a number of desirable genomic regions (quantitative trait loci, QTL) of different crops under stress conditions. A large number of datasets are available through expressed sequence tags (ESTs) for species with larger but unknown genome sequences. Microarray technology is expanding rapidly and gene expression has been measured for a number of plant species, which has advanced our knowledge of the complex interaction between signaling molecules and pathways. Proteomics and metabolomics studies provide a broad representation of data related to abiotic stress outcomes and it will be supportive to improve crop breeding in the near future. Identification and isolation of abiotic stress-tolerant genes and transformation technology has made real progress in understanding how plants cope with these stresses as well as the different constituents exploited in the signaling and response pathways.
Keywords
abiotic stress tolerance; plant biotechnology; molecular biology; plant genomics
1.1 Introduction
The main goals of agricultural plant science for many decades have been to increase the yield and improve the quality of agricultural products. To attain these goals, improvement in the protection of crops against different types of abiotic stresses is important. Since the plants are sessile and complete their life cycle in a single location, they are afflicted by environmental challenges such as abiotic stresses, which include light, cold, heat, nutrition, water, salinity, and toxic concentrations of metals. As much as 80% of the crop harvest can be destroyed by these stresses. Abiotic stresses in crop plants negatively influence the whole plant and ultimately reduce the yield, whether it is for domestic use or for industrial purposes (Munns, 2002; Ashraf, 2002). Another drawback is the restricted use and further extension of the land for crop cultivation, which limits farmers to grow enough food to cope with the increased demand of the growing population. Other environmental factors and agricultural practices, like poor drainage, restricted rainfall, and higher vapor transpiration rate in combination with poor water and soil quality, may also contribute to enhance the problem in arid and semi-arid areas (Ashraf, 2004; Bao et al., 2009; Bhattarai and Midmore, 2009), and once the level is beyond the threshold then it will be more challenging and expensive to recover (Pisinaras et al., 2010).
Many agronomically important crops are affected by the abiotic stresses at different developmental stages, such as germination, leaf area and size, shoot and root length and weight, stem thickness, plant height, fruit initiation, setting, and maturity (Zhu, 2001; Akram et al., 2009b; Rodriguez-Uribe et al., 2011). Primary processes in the plants affected by abiotic stresses are photosynthesis (Munns et al., 2006; Chaves et al., 2009), osmotic potential (Hasegawa et al., 2000; Bor et al., 2003), stomatal conductance (Xue et al., 2004; Bao et al., 2009), and/or a combination of all these dynamics. These effects may ultimately influence the morphological, physiological, biochemical, cellular, and molecular mechanisms of the whole plant. Alteration in these processes may reduce the plants’ fresh and dry biomass and reduce the yield (Azevedo-Neto et al., 2004; Higbie et al., 2010).
Plants have developed tolerance to abiotic stresses to some extent by evolving defense systems such as adjusting osmotic regulation and controlled uptake of ions (Senadheera and Maathuis, 2009), but this system is very complex and not completely understood. There is relative interaction of the mechanisms involved at the physiological, biochemical, morphological, and molecular levels. There are a number of means of coping with these problems such as land reclamation through hydrological and chemical means but these are expensive (Corbishley and Pearce, 2007). Conventional breeding technology has restricted success in developing stress-tolerant cultivars due to variant germplasm in order to exploit natural or artificially induced diversity and, subsequently, to select for desired properties. The problem with traditional plant breeding is that it is time consuming and laborious; it is difficult to modify single traits; and it relies on existing genetic variability (Yamaguchi and Blumwald, 2005; Ashraf et al., 2008; Zhang et al., 2008). Currently, it is well understood that these complex mechanisms generally involve interactions of a number of genes at the molecular level (Flowers, 2004). Classification of candidate genes for stress tolerance and their expression is required to understand the metabolic phenomena. Success in breeding for better adapted varieties to abiotic stresses depends upon the concerted efforts of various research domains including plant and cell physiology, molecular biology, and genetics.
Genome-based studies have the potential to endorse persistent and improved plant genetic development. The progress made since the last decade related to the studies of functional genomics is becoming increasingly important as the genome sequences of model crop species have been released. Therefore, alterations to the genes’ behavior, such as overexpression or silencing related to the fabrication of particular plant components, are a possibility. This would be helpful to reveal regulatory mechanisms linked with the biosynthesis and catabolism of metabolites in crop plants (Gambino and Gribaudo, 2012). Therefore, for future progress in this area, efforts are required to develop genomic resources and tools for basic and applied genetics, genomics, and breeding research. This will pave the way to understand the molecular and metabolic pathways involved in the adaptation of plants to environmental challenges.
Nevertheless, significant progress in genomics studies has made this more valuable as this technology is one among other tools that have been exploited to recognize stress responsive genes in several species of plants (Rabbani et al., 2003; Arpat et al., 2004; Micheletto et al., 2007). This chapter presents reviews related to genomics and molecular processes associated with the developments for abiotic stress responses and tolerance in plants with significant features of the effects of stress on different crop plants.
1.2 Physiological, cellular, and biochemical mechanisms of abiotic stress in plants
Crop production is severely affected ultimately reducing yield by abiotic stress. According to their performance in extreme environmental conditions, plants have been classified into two groups, i.e., glycophytes (susceptible) and halophytes (tolerant), and most crops are considered glycophytes. These plants are not able to survive in extreme environments and accumulation of excessive metabolites in the growing medium hampers the different plants’ developmental stages (Hasegawa et al., 2000). By and large, stressful environments affect metabolism, which ultimately disturbs the physiological, biochemical, cellular, and morphological processes in plants. High salt accumulation or dehydration reduces plant growth by disturbing the osmosis or the ion toxicity. Higher concentrations of salts are toxic to plants, preventing the uptake of ions leading to ionic homeostasis and disrupting osmoregulation (Senadheera and Maathuis, 2009). This will reduce the potassium and calcium ions, and sodium and chloride will be increased with an increased ionic effect. Reactive oxygen species (ROS) are accumulated at cellular level by the induced salt stress, which may produce damaging or toxic reactions like lipid peroxidation, degraded proteins, and mutations in the nucleic acids (Pitman and Läuchli, 2002; Mittler, 2002; Bor et al., 2003). These factors negatively affect development, and change the physiological mechanism including deposition of inorganic ions and organic solutes, water relations, or photosynthesis of different plant species (Greenway and Munns, 1980; Niknam and McComb, 2000; Murphy and Durako, 2003).
1.2.1 Organic and inorganic solutes
Specific types of proteins work as the essential component of cell membranes, which is porous for the ions. The membranes act as the channels to bind the ions to those proteins through diffusion and form electrochemical potential gradient. This process requires energy, which is used in the form of stored adenosine triphosphate (ATP) or ATP and pyrophosphate. This gradient across the membrane will produce differences in pH and electric potential. Therefore, the modification in electric potential forces the inward movement of cations through channels and the variation in pH regulates the movement of ions through carriers, which leads to binding of the protons and ions (Flowers and Flowers, 2005). Therefore, the efficiency of cell membranes to control the rate of ion movement in and out of the plant cell is used as an indicator of damage to a great range of tissues (Farkhondeh et al., 2012).
Ion toxicity results after the increased accumulation of salts into the crop through growing medium. This mostly includes increased levels of Na+ and Cl−, which form ionic effects and whose level is different in various crop species depending upon the severity and duration of the stress (Abrol et al., 1988). This leads to surpassing the capability of the cell to compartmentalize ions into the vacuole, or they may pass onto the cell wall and dehydrate the cell, which may result in interruption of the water relations and effective use of essential nutrients (Lacerda et al., 2003; Munns, 2005). The inclusion of Na+ into the plant cell taken up through growing medium will result in cytoplasmic toxicity. Therefore, uptake, accumulation, and long-distance transport of Na+ throughout the plant system is a critical step for plants to be affected by ion toxicity (Munns et al., 2000; Ashraf, 2004). There are reports that higher accumulation of Cl− causes leaf injury in different plant species (Greenway and Munns, 1980; Flowers and Hajibagheri, 2001; Mansour et al., 2005). The toxicity of Cl− causes necrosis and finally leaf senescence (Nawaz et al., 2010). The roots absorb the Cl− and move through the xylem to shoots and leaves where its accumulation beyond the toxic level causes cell injury. Higher concentrations of salt increase the deposition of Na+ in the growing region of the root and hence decrease the selectivity for K+ (Ashraf et al., 2012). Therefore, proper uptake through roots and justified cellular movement, translocation, and compartmentalization in the cell are necessary for proper functioning of the plant metabolism in saline, dehydrated, and higher temperature environments (Akram et al., 2007).
Higher accumulation of Na+ causes a higher proportion of Na+/K+ and Na+/Ca+ in stress growth medium/conditions. The concentration of K+ is indirectly proportional to the Na+ in the cell, i.e., if Na+ is increasing then K+ and Ca2+ are decreasing in maize and Limonium perezii as reported by Suarez and Grieve (1988) and Carter et al. (2005), respectively, while K+ and Ca2+ both take part in the maintenance for integration and proper functioning of cell membranes, i.e., cell wall stabilization, triggering enzymes, and directing ion translocation (Carden et al., 2003; Wenxue et al., 2003).
Altered or lower levels of calcium sodium ratios in a saline environment will inhibit plant development and uptake of various nutrients in Atriplex griffithii (Khan et al., 2000), cotton (Higbie et al., 2010), and Agrostitis stolonifera (Majeed et al., 2010), and significantly change the morphological and anatomical structure (Cramer, 1992). Various nutrient deficiency with higher NaCl stress has also been reported in different crops, as can be found with K+ in spinach (Chow et al., 1990), artichoke (Graifenberg et al., 1995), and tomato (Lopez and Satti, 1996), and with nitrogen deficiency in cucumber (Cerda and Martinez, 1988) and lettuce and cabbage (Feigin et al., 1991), whereas phosphorus and potassium are deficient in tomato (Adams, 1991) and cucumber (Sonneveld and de Kreiji, 1999). However, artificial or foliar application of potassium in the form of KH2PO4, KCl, KOH, K2CO3, KNO3, and K2SO4 improved the plant growth in wheat, sunflower, and cotton for various physiological parameters (Sherchand and Paulsen, 1985; Akram et al., 2007, 2009a,b; Jabeen and Ahmad, 2009).
The organic solutes protect the plant metabolism and play an important role in adjusting the osmotic potential. Osmotic adjustment in a plant is altered due to inorganic ion or organic solute accumulation in the cell. Net increase in the organic solutes in a cell is due to the decrease in the water potential. Besides inorganic ion accumulation, the role of the accumulation of organic solutes is also important. Cell membrane is important in maintaining the proper concentration of these solutes in the cell. Proline, trehalose, sucrose, polyols, glycinebetaine, prolinebetaine, alaninebetaine, hydroxylprolinebetaine, pipecolatebetaine, and choline O-sulfate are among the common organic solutes that play an important role for osmotic adjustment in plants under salt stress, dehydration, and some other abiotic stresses (Rhodes and Hanson, 1993; Hasegawa et al., 2000). Therefore, higher concentrations of these solutes under salt stress are favorable to plants as they participate in ROS scavenging activity, act as a 1O2 quencher, and maintain the OP (Marcelo-Pedrosa and Queila-Souza, 2013).
1.2.2 Role of abscisic acid under abiotic stress
Under salinity, dehydration, extreme temperatures, and other abiotic stresses, plants respond in relation to metabolic and progressive variations. Different tissues exposed to variant stresses behave in a coordinated manner to implement these responses. Salts would not be accumulated in higher concentrations in actively dividing and growing cells. Plants’ responses under stress are initiated by some indicators such as primary osmotic stress and/or secondary metabolites (Chaves et al., 2003). Secondary metabolites signals comprise growth hormones like abscisic acid, ethylene, and cytokinins, and reactive oxygen species and intracellular phospholipids or sugars. It was observed that the ability of isopentenyl transferase (IPT) to increase cytokinin biosynthesis, hence delaying senescence and the ability of CBL-interacting protein kinase to sense and respond to the calcium concentrations, has been used to make cotton plants more robust under drought conditions (Kuppu et al., 2013; He et al., 2013). Abscisic acid (ABA) is initiated to produce in roots, translocates to shoots through xylem, and signals the stomata to close, which in the long run limits the cellular division and expansion and reduction in stomatal conductance in response to stress (Aldesuquy and Ibrahim, 2001). Munns et al. (2000) conducted experiments on shoot water relations and verified that the osmotic effects under salt stress induce the hormonal signals outside the roots to regulate cell expansion. Artificial application of ABA to common bean plants induced higher K+/Na+ ratios and so restricted the translocation of sodium to shoots (Khadri et al., 2007; Chaves et al., 2009). It is evident that along with root cells, ABA may also be synthesized in leaf cells and distributed from there throughout the whole plant (Wilkinson and Davies, 2002). Flexas et al. (2006) also proved that stomatal conductance is reduced when the leaves are cut off the plants, but the same has been recovered very quickly after the foliar application of ABA to the plants. They also reported the response of stomatal conductance to alterations in photoperiod, temperature, and availability of CO2 (Flexas et al., 2008).
The role of other growth hormones like GA3 has been reported to compensate for the adverse effect of stress on different plant tissues (Naqvi, 1999; Chakraborti and Mukherji, 2003). Shah (2007) reported on the foliar application of GA3 under salt-stressed plants and observed the improvements in the dry mass, leaf area expansion, photosynthetic rate, and stomatal conductance. The accumulation of salts under dehydrated conditions induces the production of ABA to close the stomata and regulate the plants’ growth under stress conditions which accounted for the restricted production of ABA during the process of conjugation. The reduced stomatal conductance and rescuing of productivity was aggravated by the foliar application of GA3 as these results were inconsistent with those of Afroz et al. (2005). Similarly, Guo et al. (2011) guessed that GhWRKY3—a transcription factor from Gossypium sp.—will possibly play a role towards ABA- and GA3-intermediated pathways that signal and regulate plant growth and development and generate defense responses against pathogens.
Late embryogenesis abundant (LEA) genes have been identified that are responsive to ABA, and a number of ABA responsive elements (ABREs) are present on the promoters that work together with other nuclear protein factors in different crops. These LEA genes are mostly present in mature embryos and some other vegetative tissues under abiotic stresses (Luo et al., 2008). LEA proteins are known for their role in protecting cells under osmotic stress due to hydrophilic characteristics and as they accumulate under the abiotic stress in plants. Advances in this research indicate that the pH of xylem and leaf apoplast affects ABA production and subsequently translocates to the stomata. Under salt stress or more alkaline pH, exclusion of ABA from xylem and leaf apoplast may be decreased and hence more ABA will reach the guard cells, which will facilitate the variation of stomatal aperture (Jia and Davies, 2007).
1.2.3 Reactive oxygen species
Higher amounts of salts taken up from the soil by plants at higher temperatures restrict the availability of water and cause drought stress as well. Consequently, plants close the stomata to retain water and this will also limit the entrance of carbon dioxide into the leaves. This will decrease the process of photosynthesis, or if the salts are at too high a concentration or at toxic levels, then photosynthesis will be inhibited directly. In this situation, plants suffer from oxidative stress, which is an additional significant feature of this stressful condition.
The formation of reactive oxygen species (ROS) is the outcome of sunlight absorption by plants. This mainly takes place in the chloroplast through superoxide anion, hydrogen peroxide, hydroxyl radical, and singlet excited oxygen as a routine function of plants’ aerobic respiratory system. ROS are the reason to why cell membranes, nucleic acids degradation, and proteins in the cell are damaged because they are extremely reactive. Photorespiration, oxidation of fatty acids, and mitochondrial and chloroplast electron transport systems (PSI and PSII) are the various categories of functions performed in the cell that generate ROS (Hernàndez et al., 2001; Foyer and Noctor, 2003). A number of reports are available for the effects of abiotic stress comprising ROS (Bor et al., 2003; Stepien and Klobus, 2005), but the limit of these processes stimulated under specific conditions as well as the magnitude of the damage or the differences in their capability acquired by the plants under stress has not been well understood. Irregular concentrations of antioxidants in chloroplast and changes to enzymatic activities may regulate the ROS to damage the plant systems (Asada, 2000), but transgenic plant development is another way to control the damage (Apse et al., 1999; Kumar et al., 2012).
1.3 Effects of abiotic stresses on physiological, cellular, and biochemical processes in plants
Flexibility of the cell wall regulates the cell growth rate and maintains the turgor pressure, which actually determines the cell growth and elongation (Peters et al., 2001). Therefore, cell wall elasticity is considered to be the prerequisite for cell growth and expansion. Cell growth is affected by the osmotic adjustment regulated by the contribution of roots and leaves to maintain the water absorption, uptake, transportation, and turgor pressure. This will lead to maintaining the primary physiological processes such as stomatal opening and photosynthesis, and cell expansion, up-regulation of antioxidants, accumulation of organic solutes like amino acids, polyamines and carbohydrates (Lockhart, 1965; Serraj and Sinclair, 2002; Munns et al., 2006; Stepien and Klobus, 2006; Yue et al., 2012). This is caused by the decrease in availability of CO2 due to decrease in the stomatal conductance (Flexas et al., 2004).
Water relations or maintenance of relative water contents is also an important physiological criterion for stress tolerance in plants. Relative water contents are the estimation of water uptake and the leaf turgidity, whereas water potential is the maintenance of water–soil–plant–atmosphere under continuous status. Therefore, osmotic adjustment is related to the leaf relative water contents as it maintains the leaf water potential, but the water potential is not related to the osmotic adjustments (Suriya-Arunroj et al., 2004). Seed germination is also one of the physiological processes affected by the inhibitory effects of temperature, salts, and dehydration stress and this may be relieved by plant growth hormones including gibberellic acid, ethylene, cytokinin, etc. (Xu et al., 2011).
Photosynthesis is one of the main contributing factors in abiotic stresses that induce reduction of plant growth and yield (Mi et al., 2012). It is one of the major physiological processes during plant development and reduction under abiotic stresses—as water uptake and CO2 availability are reduced. Photosynthesis will reduce the intercellular carbon dioxide availability and other non-stomatal functions. During photorespiration in C3 plants, ribulose-1,5-bisphosphate carboxylase/ oxygenase (RUBISCO) catalyzes the absorbed carbon dioxide. The same step in C4 plants is regulated by phosphoenol pyruvate carboxylase (PEPC). The higher accumulation of salts increases the oxygenase activity of RUBISCO as the CO2 availability is reduced and therefore carboxylation is reduced or ceased (Sivakumar et al., 2000). Transpiration efficiency and stomatal conductance in combination with water use efficiency are the essential components of photosynthetic machinery in plants. Stressful environmental conditions decrease the stomatal conductance, which limits the transpiration rate and leads to restriction of water use efficiency, and this whole cycle will reduce the photosynthesis (Gamma et al., 2007; Akram et al., 2009b).
Chlorophyll is the green pigment essential for photosynthesis, having absorbed the spectra of visible light. Estimation of chlorophyll contents is the essential criterion to measure the pigments in the leaves and in turn the estimation of nutrient status from time to time (Gao et al., 2008). The older leaves are affected earlier and leaf dropping starts more quickly as compared to the new and actively growing leaves (Parida and Das, 2005). This is interconnected with the prolonged stress period. The accumulation of ions is increased in the chloroplast, which affects the electron transport system during photosynthesis (Sudhir and Murthy, 2004). This will affect the chlorophyll contents, i.e., an increase in susceptible plants (Hamada and El-Enany, 1994) and a decrease in tolerant plants (Singh et al., 1990). Mg+ has been reported for its important role in the structure of chlorophyll, enzyme co-factor, and distribution of photosynthesis. Increased degradation of chlorophyll in Mg+-deficient plants has been observed, which leads to the enhancement of oxidation of RUBISCO (Ramoliya et al., 2004; Saleh, 2011). It has been reported that the tolerant species of plants may not be affected by the saline medium, but the salt-sensitive species has great negative effects on the chlorophyll contents. This was proven when Arabidopsis showed sensitivity to 150 mM NaCl, in comparison to Thellungiella, which tolerated the same concentration of NaCl very satisfactorily and chlorophyll contents had not been altered (Stepien and Johnson, 2009).
1.4 Conventional breeding technology to induce abiotic stress tolerance in plants
Traditional breeding techniques have been the routine practice to study the genetic variability among crop species. Genetic variation has been identified among sexually compatible species and desirable agronomic traits have been introduced to the agriculturally important crops. Many of the crop cultivars such as wheat (Villareal et al., 1994; Valkoun, 2001; Zaharieva et al., 2001), rice (Mackill et al., 1993; Mishra et al., 1996), soybean (VanToai et al., 1994), and maize (Bänziger et al., 2004) have been introduced through different procedures of conventional breeding programs. Therefore, conventional breeding technology has yielded improvements to the quality of crops at morphological, biochemical, physiological, and cellular levels against various abiotic stresses. But the limitations related to this technology are worth mentioning as it is laborious, time consuming, inefficient, and costly. Hence, it takes several years to develop a new cultivar with improved traits through this technology. Moreover, some of the genetic material with irrelevant or unwanted characteristics may also be introduced, which might be difficult to remove. Another important factor for selection may be the availability of germplasm or the low genetic variation (Ashraf, 2010).
Therefore, breeding is difficult due to the complexity of the plant’s adaptation under stress. So, this suggests that a number of traits may combine and contribute to adapt the plants for tolerance against a range of abiotic stresses. Thus, there is a need to seek more efficient approaches for genetically tailoring crops for enhanced drought tolerance.
1.5 Functional genomics approaches to induce abiotic stress tolerance in plants
Fundamental processes involved in abiotic stress tolerance mechanisms are water transporters and relevant practices, cell signaling modules (heat shock proteins, specific transcription factors, molecular chaperones, and late embryogenesis proteins), reactive oxygen species, osmolyte adjustment, and ion accumulation, all of which are common within plant species. These processes are regulated by a number of genes simultaneously and offer goals to conduct research in crop plants. Therefore, currently, genomic tools are suggested to use resources to generate the comprehensive datasets making contribution to adapt the genes’ expression, protein synthesis, and modifications to secondary metabolites after exposure to abiotic stresses (Figure 1.1).

1.5.1 Advances in phenology assist the genomic studies of abiotic stress tolerance
An abiotic stress mechanism is complex to understand at the molecular level, but the advances in phenotypic studies have helped to quantify the components contributing to tolerate the abiotic stress at the genetic level. Therefore, phenomic developments along with genomic methods in the crops with more complex genomes are becoming increasingly amenable because the genomic evidences in non-model crops are now available (Roy et al., 2011).
Plant morphological or phenotypic studies are commonly difficult, time consuming, and destructive as most require removing the whole plant biomass. But now there are imaging tools that are non-destructive and are used to take several images of the same plant at different time intervals and wavelengths. These advances have made it possible to offer non-destructive approaches to obtain computable data in a number of crops at different growth stages under different types of abiotic stresses like drought, salt, cold, and heat (Morison et al., 2008; Rajendran et al., 2009; Sirault et al., 2009; Berger et al., 2010). Most of the phenotypic studies have concentrated upon the shoots or traits related to the stems or leaves and have ignored the roots. But in spite of the contribution of the roots to tolerate abiotic stress, the studies related to the roots are fewer. This might be because it is difficult to phenotype the roots without damaging or donating the whole plant (Richards et al., 2010; Fleury et al., 2010; Zhu et al., 2011b). Some of these reports are known for the important root traits that tolerate abiotic stresses, like metal toxicity (Jefferies et al., 1999; Ma et al., 2005) and nutrient deficiency (Laperche et al., 2006; Walk et al., 2006; Zhu et al., 2011a). Therefore, for further explanations of abiotic stress tolerance through roots, it is desirable to make improvements to the methods developed for phenotyping of roots, and environmental factors affecting plants growth may also be considered (Berger et al., 2010). Comparison of the crops growing under controlled conditions, i.e., in a greenhouse and in the field conditions, is important to elucidate the complexity of the crops’ tolerance mechanism for superimposed environmental variations or the naturally occurring climatic disorders.
With the introduction of high-throughput studies, a number of phenomics assays are now accessible online to speed up the gene identification and molecular marker-assisted genomics studies related through phenomics. One of these assays is rapid elemental analysis of plant tissue, which is helpful to estimate the ionic accumulation in different plant tissues under particular abiotic stress conditions (Baxter et al., 2007). A number of developed countries have plant growth chambers/facilities furnished with mechanized systems that bring the plants for experimental analyses to, for example, irrigation, imaging, and/or weighing stations. Data obtained are analyzed with computational software that includes: The Plant Accelerator1 (http://www.plantaccelerator.org.au) in Australia, Crop Design (http://www.cropdesign.com) in Belgium, and the Leibniz Institute of Plant Genetics and Crop Plant Research in Germany (http://www.ipk-gatersleben.de/Internet). Results of these analyses in combination with the genomics approaches will help to discover the genes contributing to the known abiotic stress tolerance mechanism in crop plants. To minimize the experimental errors, the plant populations tested in greenhouses are required to be compared/tested under different field conditions several times and the abiotic stress-related components must be studied carefully.
The latest advances in phenomics for measurement of shoot-related parameters without damaging the plant aerial parts are digital RGB images and infrared thermography (Jones et al., 2009; Berger et al., 2010; Munns et al., 2010). Similarly, minirhizotrons, ground penetrating radar, and electrical resistivity imaging have been introduced to analyze the roots non-invasively (Zhu et al., 2011b). Genomics studies for plants grown in field conditions became more accurate with high-resolution EM38 mapping for characterization of defined growth conditions (Furbank, 2009; Robinson et al., 2009). Thus, genetic approaches in combination with phenotypic studies to expose the molecular mechanism of abiotic stress tolerance in crops are becoming more easily attainable.
1.5.2 Molecular mapping
Correlation of molecular markers with phenotypic studies of plants is important to know the exact point of qualitative and quantitative genetic variation harboring the dynamics manipulating the plant’s response under abiotic stress. Yield or associated secondary traits are main components for crop improvement, and identification of quantitative trait loci (QTLs) for these traits is very strategic to improve stress tolerance in plants through marker-assisted selection. The loci for variation may be identified at structural or expression level and further tested in other germplasms, or alleles may be discovered to be used for breeding and selection into high yielding and elite cultivars of agriculturally important crops (Langridge et al., 2006). To date, QTL mapping is an exciting option, rather than using previously listed DNA markers such as RFLP, AFLP, RAPD, SSR, and SNP (Ashraf et al., 2008). So, gene pyramiding at two or more loci is possible through molecular marker-based selection (Asins, 2002). The selection procedure is mainly dependent on specific environmental conditions, which are major limitations encountered in the conventional breeding of the traits affected by abiotic stresses. Once a marker-related featured link has been marked undoubtedly, then selection could be minimized to a great extent (Humphreys and Humphreys, 2005; Tuberosa and Salvi, 2006).
Several mapping studies have identified numerous QTLs associated with salinity, drought, extreme temperatures, and other abiotic stress tolerance loci relevant to performance in less yielding areas in a number of crop species (such as that found at http://www.gramene.org/qtl/). The major crops mapped for different traits through QTL-related abiotic stresses are for cold, heat, salinity, mineral toxicities, nutrient deficiencies, and drought in cotton (Saranga et al., 2001), wheat (Quarrie et al., 1994), maize (Feng-Ling et al., 2008), soybean (Cornelious et al., 2005), barley (Teulat et al., 1997), sorghum (Sanchez et al., 2002), and rice (Lafitte et al., 2004; Mizoi and Yamaguchi-Shinozaki, 2013), among others (Table 1.1). Although the application and achievements through marker-assisted selection seems to be simple and easy-to-induce stress tolerance, the constraint is the precise and accurate identification of QTL and the proficient capability relevant to this research application. Broad diversity has been identified in the complexity of stress tolerance through the mapping program, and its association with breeding has expanded the mapping studies. Most of these studies are based on the field evaluation of crops for abiotic stress tolerance (Ahmed et al., 2013). This identified diversity in the stress tolerance mechanism may prove a key source to validate the candidate genes and will be a helpful mechanism to communicate the findings of genomics to an efficient/practical plant breeding program (Ismail et al., 2007).
Table 1.1
QTLs Identified in Different Crop Species for Abiotic Stress-related Parameters
| Crop | Relevant Stress | QTL Trait Identified | Trait Improved | References |
| Cotton | Pathogens, water potential. Osmotic potential | Fiber related, cell membrane stability | Seed cotton, panicles, disease and insect resistance, plant biomass, fiber quality and yield | Wright et al. (1998); Jiang et al. (2000); Ulloa et al. (2006) |
| Wheat | Drought, cold, salinity, metal toxicity, nutrient deficiency, heat | Osmotic potential | Plant biomass, early flower and maturity, yield | Yang et al. (2007); Baga et al. (2007); Balint et al. (2009); Laperche et al. (2008); Ma et al. (2007) |
| Barley | Drought, salinity, water logging, metal toxicity | Water potential, osmotic potential | Grain | Teulat et al. (1997); Xue et al. (2009); Li et al. (2008); Navakode et al. (2009) |
| Rice | Drought | Osmotic potential, water potential, cell membrane stability | Yield, grain, plant biomass | Lafitte et al. (2004) |
| Pearl millet | Drought | Osmotic potential, water-related attributes, cell membrane stability | Grain and yield | Serraj et al. (2004) |
| Maize | Drought | Ionic balance, osmotic adjustment | Yield and grain | Feng-Ling et al. (2008) |
| Sorghum | Drought | Water-related attributes, ionic balance, osmotic adjustment | Delayed leaf senescence, yield and grain | Sanchez et al. (2002) |

Since the abiotic stress tolerance mechanism has a multi-gene trait, then wide mapping of some specific traits in different populations of the same plant species may identify the common loci (Langridge et al., 2006). Large segregating populations are needed for positional cloning through high-resolution linkage maps, and combining tasks such as identification of loci and positional cloning may be the alternative easier way in the future for plant breeding to induce abiotic stress tolerance (Roy et al., 2011).
1.5.3 Expression sequence tags
Although the genetic sequencing was found to be a good source of genome information, the large size of the genome of some plant species or the non-coding part of the genome sequence was still a limitation related to the genetic information. Plants with larger genomes are unpopular or are difficult to sequence because they contain copies of large repeats of genes, which do not provide information of coding genes; the purpose of sequencing is to get the information for discovery and characterization of the genes for proteomics. This has led to the progression to mass mode to anticipate the actual regions of protein coding constituents in a genome. Therefore, an expression sequence tags (EST)-based study was developed as the genomics approach to overcome the problems associated with the large genome sequence or noncoding part of the genetic sequence (Rudd, 2003).
The early 1980s saw the initial use of cDNA as a method to explore gene discovery (Putney et al., 1983), which in 1990 was further extended by supporting the implication of a high-throughput approach for transcript profiling as characterization of the coding region of human genome would involve messengers from the expressed genes (Brenner, 1990). Continuing this study, Adams et al. (1991) described the term EST, correlated with gene discovery and the human genome project. Since these reports, more than 10 million ESTs have been sequenced from more than 500 distinctly annotated species (fungi, plants, animals) (Rudd, 2003).
Langridge et al. (2006) considered ESTs and cDNA libraries as “Electronic Northern” and John and Spangenberg, (2005) considered them as “in silico Northern analysis,” producing a large number of sequences appraising gene expression and being a useful method for prevalence of transcript abundance at the primary level. Analysis of EST data shows that the homologous gene shows variation among the genes and also that they are tissue specific (Mochida et al., 2004). There are a large number of data produced by the ESTs and there is a report of 449,101 ESTs for drought, 312,353 ESTs for salt, 103,898 ESTs for low temperature, 252,595 ESTs for high temperature, 19,384 ESTs for nutrient deficiency, and 135,578 ESTs for light stress on the National Center for Biotechnology Information browser (see http://www.ncbi.nlm.nih.gov/).
EST is a simple and easy way to obtain the sequencing of complicated and targeted plant species under abiotic stress conditions. It provides the mRNA with abundant expression profile of specific sequences from cDNA under specific conditions. To observe the expression of clones differentially expressing certain genes, libraries are constructed with cDNA that contain 10,000 clones acquired from targeted plant species under specific abiotic stress compared with the control (Pariset et al., 2009). Ubiquitously expressing housekeeping genes within the cells may also be considered for validation studies. It depends upon sampling of the plant tissues or organs taken from roots, leaves, etc., under salt, drought, frost, or other abiotic stresses.
Complete analysis of the core gene assembly is a complicated issue and difficult to resolve. Sampling of all transcribing genes is only possible if mRNA is gathered from all available types of cells at each and every growing stage of the plant under as many combinations of biological and environmental trials as possible. This is very complex and can only be possible through extensive experimental planning and comprehensive collection of cell types under a wider variety of environmental threats. These assemblies of cDNA and ESTs are expected to comprise the differentially expressed genes, which are stress induced and expected to play key a role in plants’ tolerance mechanisms.
Demonstration of the host gene within a library and the quality of sequence are the limiting factors associated with EST technology and this is difficult to overcome due to the difficulty in observing any transcript from a tissue under some specific stress, and the sequencing may contain a background of incorrect (partially or incomplete) sequences. Initially, the EST sequencing was preferred for the 5′ end due to the coding or conserved regions, but with the advancements in sequencing technology, EST sequencing at both ends is becoming prevalent due to high-throughput sequencing of plants’ ESTs. It may also provide more distinctive sequences and hence be the possible solution to sequencing problems within ESTs. Hence, this is the link to the genome if the complete sequence of the genome is lacking.
1.5.4 Microarray
Limitations for improvements or development of salt-tolerant crops are the partial understanding of physiological criterion that reveals the genetic prospective and/or hereditary restrictions in combination with conventional breeding under abiotic stress-growing medium. But the turning point in understanding the key traits responsible for reducing productivity under abiotic stress is the meaningful approach to assimilate the plant’s responses at the physiological as well as molecular level. Combining this approach with traditional breeding will boost the improvements in productivity (Araus et al., 2002). This will also help to produce crops suitable for our environment and for sustainable agriculture. “Omics” (metabolomics, transcriptomics, proteomics, and genomics) is involved throughout stress responses in plants (Saeed et al., 2012). A number of abiotic stress responsive genes with known structure and functions have been identified after the advent of extensive projects on genomics and helped to unravel the complexity of many associated genes (Hamel et al., 2006). In addition to identifying genes, high-throughput studies are aimed at the regulation of genes at the transcriptional level (Yanik et al., 2013). Proteome analyses reveal several proteins as showing peculiarity in their expression patterns under drought conditions (Zadraznik et al., 2013). Careful interpretation of these analyses has brought to light various mechanisms that operate during drought and other environmental challenges at both the molecular and cellular level. Recent advances in genomics research have progressively contributed to enhance the genetic improvements of the agriculturally important crops and a number of other promising plant species.
Microarray technology has been used over the past 20 years to identify the expression of high-throughput genes or to measure the expression of thousands of genes in a single experiment. These studies may be of cDNA inserts, fragments of short 20-25mers, or long oligonucleotides of 60-80mers (Lockhart and Winzeler, 2000). The model plant Arabidopsis played a key role in the identification of many stress-induced genes. But now this technology is being used to identify other abiotic stress-related genes in a number of plant species such as cotton (Arpat et al., 2004; Udall et al., 2006; Zhang et al., 2008; Payton et al., 2011; Barozai and Husnain, 2012), model plant Arabidopsis (Bray, 2002), rice (Gorantla et al., 2007; Hadiarto and Tran, 2011), beans (Micheletto et al., 2007; Sanchez et al., 2011), alfalfa (Kersey, 2004), and many others (Oh et al., 2010).
A variety of microarray platforms is available, i.e., oligonucleotide and cDNA. Oligonucleotide microarray consists of an abundance of mRNA transcripts in some specific tissue or cell for differential expression of several genes at one time. The “array or grid” of linearized complementary DNA from genes under investigation is plotted onto the glass slides. A single slide may have an array of thousands of spots. Private companies (Affymetrix, Agilent, and NimbleGen) are also available to design and spot the arrays. The in-house printing may be cheaper than from the private companies. Spotted slides are hybridized with the mRNA extracted from the sample to be investigated. After hybridization, the expression of the differentially expressed genes will be investigated by correlating the spots of complementary DNA and abundance of mRNA transcript from the labeled sample (Lipshutz et al., 1999). Complex and extensive computational and statistical analysis is compulsory for the exploration of data from such experiments.
Several batches of cDNA- or EST-based microarray data have developed in cotton for fiber, plant growth and development, abiotic stresses, and pathogen-related genes (Zhang et al., 2008; Rodriguez-Uribe et al., 2011). Cotton fiber genes have been studied at different developmental stages such as 10 and 24 days post-anthesis and it was found that gene expression was altered progressively from initiation to elongation. Microarray results showed the developmental differences at primary to secondary cell wall metabolism. The number of genes up- or down-regulated confirmed that this process is associated with the plant developmental stage (Arpat et al., 2004). A number of ESTs have been collected to generate cDNA microarray from different plant species subjected to a number of abiotic stresses such as freezing, salt, water deficit, and metal toxicity. Microarray was developed by spotting unigene, and a transcript profile was done to classify the genes expressing under subjected abiotic stresses. Functional categorization of a number of candidate genes expressing have been performed and the method was found to be a useful tool for sequence annotation and transcriptome analysis (John and Spangenberg, 2005).
Identification and characterization of the genes involved in different metabolic processes have facilitated understanding of the basic molecular mechanism involved and thus breeders can improve the genetic capability of the crops (Parida and Das, 2005; Yokotani et al., 2013). Certain regulatory pathways are common and shared across the species, such as a microarray of certain species of wheat showing expression of enzymes and osmolytes under water deficit, which suggests that these mechanisms are genotype, duration, severity, and type of stress exposure dependent (Mohammadi et al., 2007). Insufficient identification of transcripts sometimes means that there is scarce selection of a cDNA array based on EST selection. For this solution, large-scale EST selection and sequencing from different tissues at different developmental growth stages are required (Cushman and Bohnert, 2000). There is evidence that many of the genes identified through microarray have confirmed the tolerance of abiotic stress in transgenic plants (Bar et al., 2013). For those species where representative array is not available for analysis or comparison, cross-species hybridization and analysis are now available on the publicly available repositories. Model species such as Arabidopsis, rice, etc., play an important role in this limitation (Pariset et al., 2009), as whole genome of Arabidopsis was complete in 2000, so all the predicted genes could be monitored for expression profiles in a single microarray experiment at one time (Hirayama and Shinozaki, 2010). In a broader sense, the abiotic stress-induced genes identified through microarray can be classified as the first group of proteins (heat shock proteins and late embryogenesis abundant proteins) transcribed directly under abiotic stress. The second group consists of the regulatory proteins related to signal transduction pathways (MAPK, enzymes, and transcription factors). These functional groups of genes competently stimulate the cellular machinery of plants to respond to abiotic stress.
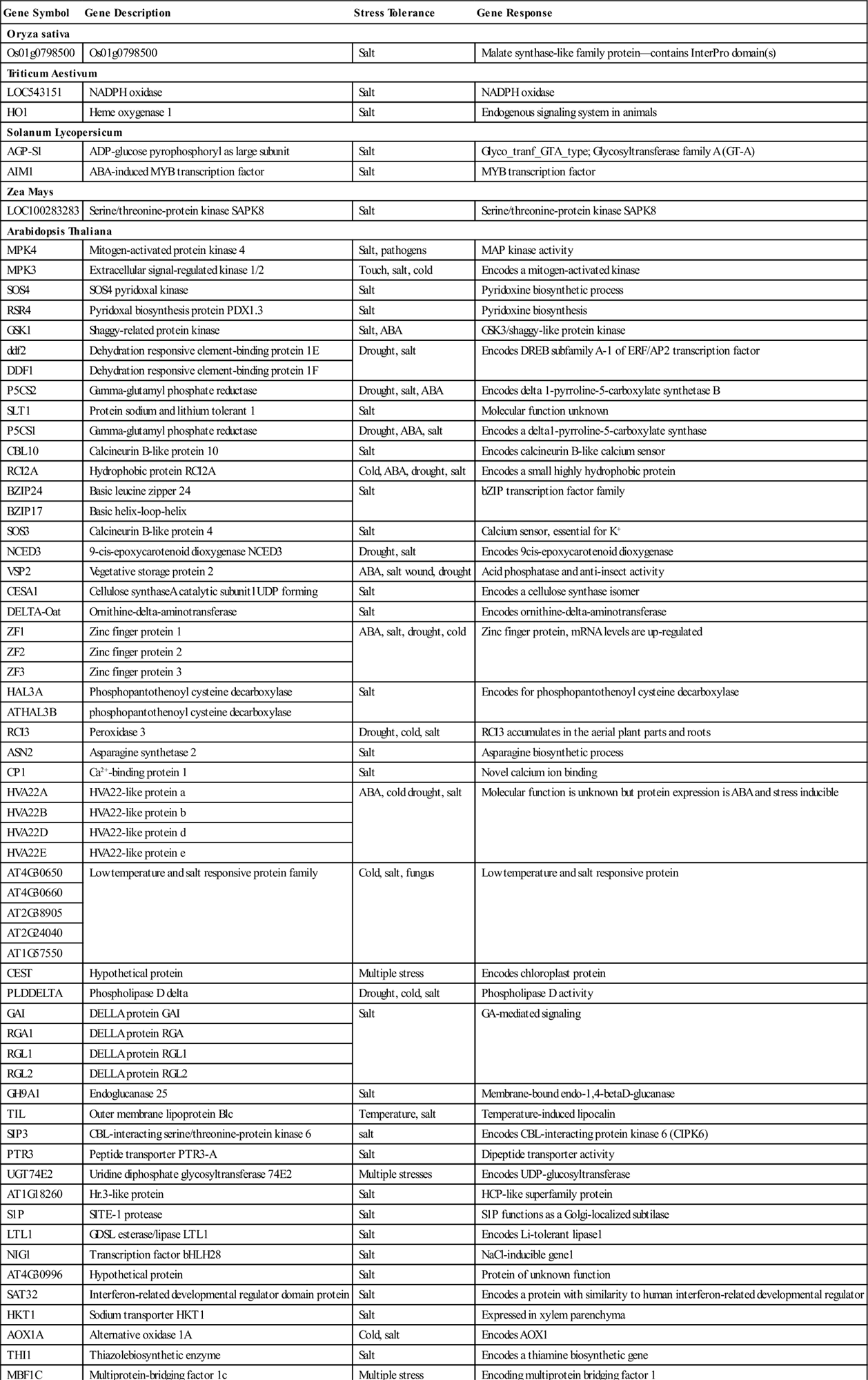
There are public repositories, like GEO (Gene Expression Omnibus) and Array Express, where microarray data can be deposited. These databases are easily available and accessible to the public, which is helpful for further hypothesis and research findings that may lead to major conclusions (Mah et al., 2004; Sanchez et al., 2011; Tseng et al., 2012). Table 1.2 summarizes the different plant species under salt and/or in combination with multiple abiotic stresses as reported on the GEO Data Set record (http://www.ncbi.nlm.nih.gov/gds). There are certain standards for data submission like experimental design, data input, normalization, and data annotation, so that scientists from different backgrounds can access the data in the same pattern and genes have been identified related to responsiveness to abiotic stresses alone or in combination with different plant species. The significance of microarray technology can be further strengthened due to the publication and availability of 88,696 free full text articles over the last 5 years (http://www.ncbi.nlm.nih.gov/gquery/?term=microarray) compared with only 4800 articles in 2000–2003 (Mah et al., 2004).
Table 1.2
Summary of the Record of GEO Datasets for Different Crops under Abiotic Stresses
| GDS record | Organism | Tissue Type | Stress |
| GDS1914 | Hordeum vulgare | shoot tissue | salinity |
| GSE3097 | Barley | shoot tissue | salinity |
| GSE13921 | Medicago truncatula | seedling | salt stress |
| GSE14029 | seedling | salt stress | |
| GSE13907 | NM* | salt stress | |
| GSE10942 | Bruguiera gymnorhiza | roots | salt stress |
| GSE7228 | NM | salt/osmotic/ionic | |
| GSE16401 | Tomato | NM | salt stress |
| GSE8883 | cotyledon/shoot | salt stress | |
| GSE4961 | seedling | salt stress | |
| GSE31594 | Vitis vinifera | NM | salt stress |
| GSE2981 | Thellungiella | NM | drought/cold/high salinity |
| GSE4681 | Chlamydomonas reinhardtii | NM | salt stress |
| GSE671 | Maize | NM | UV-B |
| GSE33494 | NM | location change | |
| GSE28209 | Rice | NM | salinity |
| GSE2415 | NM | salt/drought | |
| GSE32065 | leaves | abiotic | |
| GSE31974 | NM | drought | |
| GSE11175 | NM | salt stress | |
| GSE14403 | roots | salt stress | |
| GSE8380 | NM | pathogen | |
| GSE7530 | shoot/seed/panicle | physiological | |
| GSE6600 | leaves | salt stress | |
| GSE6901 | seedling | drought/salt/temperature | |
| GSE6533 | leaf, shoot, panicle | drought/salt stress | |
| GSE8064 | Wheat | seedlings | salt stress |
| GSE8060 | NM | salt stress | |
| GSE8158 | Solanaceous spp. | NM | salt stress |
| GSE10338 | Potato | tuber | heat necrosis |
| GSE18053 | leaves | salt stress | |
| GSE8205 | root/leaves | cold/heat/salt | |
| GSE2416 | NM | heat/cold and stress | |
| GSE8554 | Chickpea | leaf/root/shoot | drought/cold/salinity |
| GSE7504 | NM | drought/cold/salinity | |
| GSE7418 | leaf/root/shoot | salt stress | |
| GSE9748 | Poplar | NM | salt stress |
| GSE10874 | NM | ozone | |
| GSE10932 | NM | ozone | |
| GSE27861 | Arabidopsis thaliana/rice | NM | drought/salt stress |
| GSE13165 | Rice/Arabidopsis | NM | multiple (salt/heat/light/UV) |
| GSE29941 | Arabidopsis thaliana | seeds/seedlings | hypoxia |
| GSE26983 | NM | salt stress | |
| GSE16765 | NM | salt stress | |
| GSE21553 | roots | iron | |
| GSE13803 | NM | wound | |
| GSE18217 | NM | salt stress | |
| GSE19893 | NM | salt stress | |
| GSE15063 | NM | osmotic | |
| GSE10349 | NM | drought/salt | |
| GSE10384 | NM | drought/salt stress | |
| GSE10670 | NM | drought | |
| GSE8556 | seedling | salt stress | |
| GSE8787 | roots | salt stress | |
| GSE10420 | seedling | salt stress | |
| GSE9459 | NM | salt stress | |
| GSE8936 | NM | drought | |
| GSE7942 | NM | xenobiotic | |
| GSE7743 | NM | irradiance | |
| GSE5623 | NM | salt stress |
The use of microarrays in future functional genomics tools will probably lead to gene regulation at the transcriptional level for different tissues in cotton and other agriculturally important crops. However, these microarray-based methods are expected to be innovative when they become an essential part of the research/experimental activities of a routine molecular biology laboratory.
1.5.5 Proteomics and metabolomics
Plants will always be open to environmental threats as they are exposed to various abiotic stresses. Adaptation to these stresses is a complex process at the cellular and physiological level, and the acquired modifications in gene expression monitor the alterations in protein profiles (Parker et al., 2006). Proteomics and metabolomics are comparatively new research areas to emerge from the post-genomic era and there are only a few published reports on these applications to abiotic stress tolerance (Bahrman et al., 2004). Hence, these innovative “omics” perspectives have provoked curiosity among genomics scientists and opened up new horizons in plant stress biology (Khan et al., 2007). While transcriptomic studies are in progress to unravel gene transcription and regulation, gene functions and expression profiles can certainly be accomplished by proteomic study, as the primary protein sequences undergo proteolysis after post-translational modification. Accordingly, protein-level quantification of expressed genes is essentially done to measure the plant’s response to abiotic stress.
Proteomics techniques such as GC-MS and 2D gel electrophoresis are well known for protein profiling. A number of key proteins could be identified and quantified in a single gel for a specific subject area. For example, Salekdeh et al. (2002) identified 3000 proteins from rice drought and salt-stressed plants from a single gel: over 1000 were quantified among those and 42 were found worthy of further study related to their abundance or position in response to the subjected stress. Defined tissues of wheat resolved significant proteins through proteomic studies. Those proteins identified by proteomic methods have proven their functional involvement in abiotic stress tolerance mechanisms (Skylas et al., 2005; Woo et al., 2003).
Abiotic stresses impact the plant’s physiological processes such as photosynthesis—the effects on the Calvin cycle and results in reduction of plant growth and development. To adapt or to cope with these conditions, protein profiles regulating the mechanism of glycolysis and amino acid biosynthesis are up-regulated for ATP synthesis and osmotic adjustments. Molecular chaperones and C4 photosynthesis encoding proteins are up-regulated as defense mechanisms against salt in maize, and such genes may be the valuable source for genetic transformation to induce salt tolerance to other agronomically important crops. Soybean and potatoes are relatively salt sensitive and up- or down-regulation of certain protein profiles suggests that these crops are sensitive to photosynthesis and protein biosynthesis-related proteins, but tolerant to mechanisms like water potential, osmotic adjustments, and antioxidants (Sobhanian et al., 2011).
Plasma membrane plays a key role in regulating several cellular processes; therefore, it is considered to be a prime location of damage after abiotic stress such as cold. Freezing or cold is also a serious threat to crops especially to the cold-sensitive/susceptible species that do not adapt to severe cold temperatures due to limited functions to modify the cellular machinery. Tolerant species may have the adaptation mechanism as they may alter the plasma membrane, and hence many key proteins may be involved in the pathway. So, proteomic skills are useful to identify the regulatory and functional protein in the freezing mechanism, and identification of those candidate genes may help to alter the plasma membrane functions under low temperature stress. Hence, this research application is of key importance for plant molecular breeding strategies to induce the low temperature tolerance mechanism in crop plants (Takahashi et al., 2013). To induce abiotic stress tolerance to crops, different plant species should be identified for different types of stresses, and the approaches for tolerance levels should be listed. This would be accomplished with rigorous research on each plant species’ response to specific stresses. The evaluation of susceptible and tolerant cultivars/species should be done for proteome profiling in order to reveal the distinctive mechanisms involved in this challenge.
Plant growth and development is affected by exposure to salts and plants may experience various symbiotic restraints; these include water deficit, ionic toxicity, and oxidation stress, which lead to metabolic and nutrient discrepancy that culminates in a complex physiological disorder. Metabolomics is another area in the genomics approach that deals with the metabolic changes in plant responses to abiotic stresses, which suggests that thorough profiling of metabolites may offer enormous understanding for stress tolerance pathways. This is a relatively innovative area and there are not too many reports on its application. However, one of the available reports found 88 main metabolites from the extract of rice leaves, most of them covered pathways of sugar and amino acid metabolism (Sato et al., 2004). This was further confirmed by Sanchez et al. (2010), in that polyols, sugars, and specific amino acids are accumulated and usually termed as compatible solutes or osmolytes for osmotic adjustments. Further, these metabolites are also involved to regulate physiological responses such as ROS, cell membrane stability, protein protection from degradation, and cell signaling. Accumulation or decreased metabolites is also specific for distinct species, so it may help to identify the stress-susceptible or -tolerant species (Szabados and Savouré, 2010).
Carbohydrates and secondary metabolites play key roles during the post-harvest of fruit crops. Understanding the molecular pathways involved in the shelf-life of fruit can help to improve fruit quality (softening and ripening). Sorbitol concentration was reduced due to down-regulation of sorbitol dehydrogenase, and sugar was increased due to up-regulation of sucrose synthase in the transgenic apples harboring the aldose-6-phosphate reductase (A6PR) gene with antisense orientation, which led to improvement in photosynthesis and CO2 that produced good vegetative growth (Zhou et al., 2006). Sucrose contents were reduced with increased polymerization of pro-anthocyanidins with improved volatile compounds, such as carotenoid and shikimate-derived volatiles in grapevines-overexpressed Adh alcohol dehydrogenase, known to be regulated progressively under environmental threats. Strawberry, due to its small genome and faster growth, is a preferred model among fruit crops for functional genomics. A small subunit of ADP-glucose pyrophosphorylase (AGPase) was regulated under the promoter ascorbate peroxidase (APX), which overexpressed the pyrophosphate mechanism. Assessing the metabolic pathway reveals that fructose-6-phosphate 1-phosphotransferase (PFP) is a cytosolic enzyme that catalyzes the glycolysis, which leads to the accumulation of sugars and degrades the starch contents in transgenic plants (Basson et al., 2011).
The flavanoid compound family contains one of the water soluble pigments—anthocyanin—which are responsible for the color trait in fruits and flowers. These flavonoids are regulated and expressed through MYB well-known transcription factors. Strawberry transgenic plants harboring FaMYB10 showed enhanced levels of anthocyanins in different plant parts, but the silencing of anthocyanidin synthase (MdANS) in red-leaved apple produced necrotic leaves, and flavonoids/anthocyanins-altered metabolic pathway was observed (Szankowski et al., 2009b; Lin-Wang et al., 2010). Transgenic plants have been used to analyze the flavonoids and other metabolic profiling, up- or down-regulated genes, and associated metabolic pathways, and this study might be helpful to unravel the unknown genes and metabolites involved during the plants’ stress challenges. Hence, the genome sequences of many plant species are available and some of them are important as they contain genes involved for the production of particular components completely or partially known or unknown. Therefore this may likely to expose regulatory or functional mechanisms relevant to functions of metabolites in whole or plant parts.
1.5.6 Transgenic plants for improved salinity tolerance
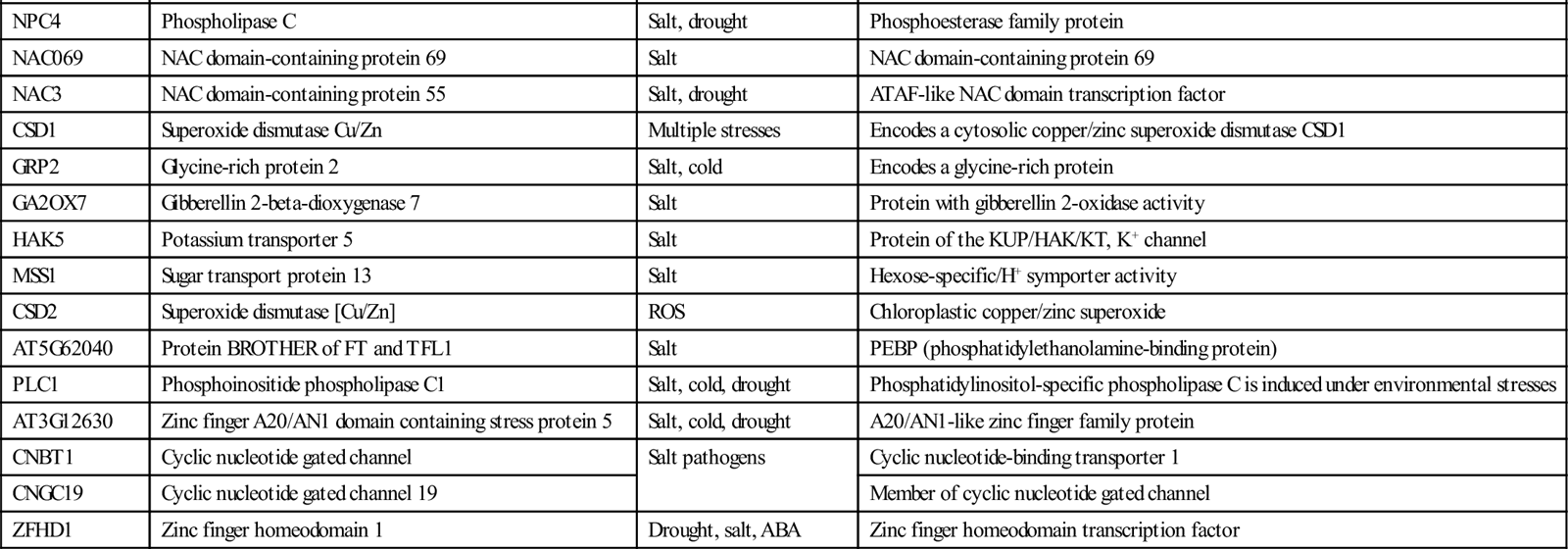
Abiotic stress causes significant plant yield losses and hence lowers crop production (Orsini et al., 2010). In addition to desiccation, ionic toxicity and ROS stress plants also face metabolic and nutrient mismanagement and all these lead to different physiological responses (Ashraf, 2004; Sanchez et al., 2011). Therefore, production of crops tolerant to stress is the ultimate and important solution for sustainable agriculture to meet the demands of the growing population (Aquino et al., 2011). Molecular biology, genomics, or biotechnology applications in combination with plant breeding are being successively used to produce transgenic crops/plants to overcome this crop productivity threat. Genomics approaches have provided insights into the functional regulation of the key genes participating in the stress tolerance mechanism of plants. New attempts have been reported by using genomics approaches such as marker-free selection of plants, tissue-specific promoters, and induction of disease resistance (fungal, bacterial, and viral) (Gambino and Gribaudo, 2012). Table 1.3 summarizes a number of genes identified individually or in combination with abiotic and biotic stress tolerance in different plants.
Table 1.3
Abiotic Stress-tolerant Genes Identified in Different Crops
| Gene Symbol | Gene Description | Stress Tolerance | Gene Response |
| Oryza sativa | |||
| Os01g0798500 | Os01g0798500 | Salt | Malate synthase-like family protein—contains InterPro domain(s) |
| Triticum Aestivum | |||
| LOC543151 | NADPH oxidase | Salt | NADPH oxidase |
| HO1 | Heme oxygenase 1 | Salt | Endogenous signaling system in animals |
| Solanum Lycopersicum | |||
| AGP-S1 | ADP-glucose pyrophosphoryl as large subunit | Salt | Glyco_tranf_GTA_type; Glycosyltransferase family A (GT-A) |
| AIM1 | ABA-induced MYB transcription factor | Salt | MYB transcription factor |
| Zea Mays | |||
| LOC100283283 | Serine/threonine-protein kinase SAPK8 | Salt | Serine/threonine-protein kinase SAPK8 |
| Arabidopsis Thaliana | |||
| MPK4 | Mitogen-activated protein kinase 4 | Salt, pathogens | MAP kinase activity |
| MPK3 | Extracellular signal-regulated kinase 1/2 | Touch, salt, cold | Encodes a mitogen-activated kinase |
| SOS4 | SOS4 pyridoxal kinase | Salt | Pyridoxine biosynthetic process |
| RSR4 | Pyridoxal biosynthesis protein PDX1.3 | Salt | Pyridoxine biosynthesis |
| GSK1 | Shaggy-related protein kinase | Salt, ABA | GSK3/shaggy-like protein kinase |
| ddf2 | Dehydration responsive element-binding protein 1E | Drought, salt | Encodes DREB subfamily A-1 of ERF/AP2 transcription factor |
| DDF1 | Dehydration responsive element-binding protein 1F | ||
| P5CS2 | Gamma-glutamyl phosphate reductase | Drought, salt, ABA | Encodes delta 1-pyrroline-5-carboxylate synthetase B |
| SLT1 | Protein sodium and lithium tolerant 1 | Salt | Molecular function unknown |
| P5CS1 | Gamma-glutamyl phosphate reductase | Drought, ABA, salt | Encodes a delta1-pyrroline-5-carboxylate synthase |
| CBL10 | Calcineurin B-like protein 10 | Salt | Encodes calcineurin B-like calcium sensor |
| RCI2A | Hydrophobic protein RCI2A | Cold, ABA, drought, salt | Encodes a small highly hydrophobic protein |
| BZIP24 | Basic leucine zipper 24 | Salt | bZIP transcription factor family |
| BZIP17 | Basic helix-loop-helix | ||
| SOS3 | Calcineurin B-like protein 4 | Salt | Calcium sensor, essential for K+ |
| NCED3 | 9-cis-epoxycarotenoid dioxygenase NCED3 | Drought, salt | Encodes 9cis-epoxycarotenoid dioxygenase |
| VSP2 | Vegetative storage protein 2 | ABA, salt wound, drought | Acid phosphatase and anti-insect activity |
| CESA1 | Cellulose synthaseA catalytic subunit1UDP forming | Salt | Encodes a cellulose synthase isomer |
| DELTA-Oat | Ornithine-delta-aminotransferase | Salt | Encodes ornithine-delta-aminotransferase |
| ZF1 | Zinc finger protein 1 | ABA, salt, drought, cold | Zinc finger protein, mRNA levels are up-regulated |
| ZF2 | Zinc finger protein 2 | ||
| ZF3 | Zinc finger protein 3 | ||
| HAL3A | Phosphopantothenoyl cysteine decarboxylase | Salt | Encodes for phosphopantothenoyl cysteine decarboxylase |
| ATHAL3B | phosphopantothenoyl cysteine decarboxylase | ||
| RCI3 | Peroxidase 3 | Drought, cold, salt | RCI3 accumulates in the aerial plant parts and roots |
| ASN2 | Asparagine synthetase 2 | Salt | Asparagine biosynthetic process |
| CP1 | Ca2+-binding protein 1 | Salt | Novel calcium ion binding |
| HVA22A | HVA22-like protein a | ABA, cold drought, salt | Molecular function is unknown but protein expression is ABA and stress inducible |
| HVA22B | HVA22-like protein b | ||
| HVA22D | HVA22-like protein d | ||
| HVA22E | HVA22-like protein e | ||
| AT4G30650 | Low temperature and salt responsive protein family | Cold, salt, fungus | Low temperature and salt responsive protein |
| AT4G30660 | |||
| AT2G38905 | |||
| AT2G24040 | |||
| AT1G57550 | |||
| CEST | Hypothetical protein | Multiple stress | Encodes chloroplast protein |
| PLDDELTA | Phospholipase D delta | Drought, cold, salt | Phospholipase D activity |
| GAI | DELLA protein GAI | Salt | GA-mediated signaling |
| RGA1 | DELLA protein RGA | ||
| RGL1 | DELLA protein RGL1 | ||
| RGL2 | DELLA protein RGL2 | ||
| GH9A1 | Endoglucanase 25 | Salt | Membrane-bound endo-1,4-betaD-glucanase |
| TIL | Outer membrane lipoprotein Blc | Temperature, salt | Temperature-induced lipocalin |
| SIP3 | CBL-interacting serine/threonine-protein kinase 6 | salt | Encodes CBL-interacting protein kinase 6 (CIPK6) |
| PTR3 | Peptide transporter PTR3-A | Salt | Dipeptide transporter activity |
| UGT74E2 | Uridine diphosphate glycosyltransferase 74E2 | Multiple stresses | Encodes UDP-glucosyltransferase |
| AT1G18260 | Hr.3-like protein | Salt | HCP-like superfamily protein |
| S1P | SITE-1 protease | Salt | S1P functions as a Golgi-localized subtilase |
| LTL1 | GDSL esterase/lipase LTL1 | Salt | Encodes Li-tolerant lipase1 |
| NIG1 | Transcription factor bHLH28 | Salt | NaCl-inducible gene1 |
| AT4G30996 | Hypothetical protein | Salt | Protein of unknown function |
| SAT32 | Interferon-related developmental regulator domain protein | Salt | Encodes a protein with similarity to human interferon-related developmental regulator |
| HKT1 | Sodium transporter HKT1 | Salt | Expressed in xylem parenchyma |
| AOX1A | Alternative oxidase 1A | Cold, salt | Encodes AOX1 |
| THI1 | Thiazolebiosynthetic enzyme | Salt | Encodes a thiamine biosynthetic gene |
| MBF1C | Multiprotein-bridging factor 1c | Multiple stress | Encoding multiprotein bridging factor 1 |
| NPC4 | Phospholipase C | Salt, drought | Phosphoesterase family protein |
| NAC069 | NAC domain-containing protein 69 | Salt | NAC domain-containing protein 69 |
| NAC3 | NAC domain-containing protein 55 | Salt, drought | ATAF-like NAC domain transcription factor |
| CSD1 | Superoxide dismutase Cu/Zn | Multiple stresses | Encodes a cytosolic copper/zinc superoxide dismutase CSD1 |
| GRP2 | Glycine-rich protein 2 | Salt, cold | Encodes a glycine-rich protein |
| GA2OX7 | Gibberellin 2-beta-dioxygenase 7 | Salt | Protein with gibberellin 2-oxidase activity |
| HAK5 | Potassium transporter 5 | Salt | Protein of the KUP/HAK/KT, K+ channel |
| MSS1 | Sugar transport protein 13 | Salt | Hexose-specific/H+ symporter activity |
| CSD2 | Superoxide dismutase [Cu/Zn] | ROS | Chloroplastic copper/zinc superoxide |
| AT5G62040 | Protein BROTHER of FT and TFL1 | Salt | PEBP (phosphatidylethanolamine-binding protein) |
| PLC1 | Phosphoinositide phospholipase C1 | Salt, cold, drought | Phosphatidylinositol-specific phospholipase C is induced under environmental stresses |
| AT3G12630 | Zinc finger A20/AN1 domain containing stress protein 5 | Salt, cold, drought | A20/AN1-like zinc finger family protein |
| CNBT1 | Cyclic nucleotide gated channel | Salt pathogens | Cyclic nucleotide-binding transporter 1 |
| CNGC19 | Cyclic nucleotide gated channel 19 | Member of cyclic nucleotide gated channel | |
| ZFHD1 | Zinc finger homeodomain 1 | Drought, salt, ABA | Zinc finger homeodomain transcription factor |
Plants are adapted to saline environmental conditions in a number of ways and among these is the compartmentalization of Na+ into the vacuole, which helps to maintain the osmotic balance. Transgenic legume forage (Medicago sativa) was developed with AVP1, a vacuolar H+-pyrophosphatase (H+-PPase) gene from Arabidopsis thaliana, to adapt to saline and arid soils (Bao et al., 2009). Transgenic plants were developed which expressed the AtNHX1 gene (a vacuolar Na+/H+ antiporter). This gene has been reported to reduce the cytosolic Na+ by sequestering Na+ in the vacuole (Asif et al., 2011; Jha et al., 2011). The transgenic plants were produced with better germination rates and showed improved fresh and dry biomass in severe saline conditions (Xue et al., 2004). Another plasma membrane Na+/H+ antiporter, i.e., the SOS1 gene from Arabidopsis thaliana, was tested into the model plant tobacco and showed improved germination, vegetative growth, and chlorophyll contents in transgenic plants as compared to the non-transgenic (Pons et al., 2011; Yue et al., 2012). By reducing the expression of the sodium/proton antiporter SOS1 through genetic engineering affects the numerous pathways, indicating a role for SOS1 that exceeds its known function as an antiporter (Oh et al., 2009, 2010). Tissue-specific expressions for the SOS genes may contribute a significant role to Na+ regulation (Kumar et al., 2009). The higher amount of Na+ and Cl− separately under NaCl treatment restricts the plants’ growth and productivity through different but simultaneous mechanisms. It has been documented that a higher level of Na+ hampers K+ and Ca2+ nutrients whereas a high concentration of Cl− degrades the chlorophyll and decreases the photosynthesis (Tavakkoli et al., 2010).
Cyclophilin (Cyp) genes belong to the ubiquitous proteins family and are capable of peptidyl-prolyl isomerase activity, which helps to catalyze the cis/trans isomerize, the peptide bond at proline residues and protein folding. These Cyps have been reported to regulate gene functions at cell division, signaling, transcriptional regulation, and pre-mRNA splicing under different environmental stresses including temperature, salt, and drought (Zhu et al., 2011a). There is the possibility that some of the genes perform vital roles to acclimatize and tolerate the stressful environment, but this is not likely to conclude the stress transcriptome of a genus on the basis of only one species (Sanchez et al., 2011). Proline and trehalose accumulation has been reported in response to plant adaptation, and related genes expression has been found increased and this could be applied to help the plants cope under abiotic stresses (Nounjan et al., 2012). The MtSAP1 gene isolated from Medicago truncatula has been found overexpressing in tobacco transgenic seedlings and showed tolerance to extreme temperature, osmotic, and salt stresses (Charrier et al., 2013).
Transcription factors are considered as the potent application for the plants to adapt under different abiotic stresses (Martin et al., 2012; Mizoi and Yamaguchi-Shinozaki, 2013; Naika et al., 2013). Transcription factors genes of the basic leucine zipper (bZIP) and zinc finger protein regulate the pathways responsible for plant growth under abiotic stress and metal toxicity. ZmbZIP72, a transcription factor from maize bZIP, has showed improved tolerance in Arabidopsis under different types of abiotic stresses (Ying et al., 2012). ABI5 is a transcription factor of the bZIP family, which binds with the ABA response element (ABRE) present in AtEm6 gene, hence regulating its expression. AtEm6 is reported to increase salt tolerance by manipulating calcium-dependent protein kinases (Tang and Page, 2013). The NAC is found to be one of the largest transcription factor families and its proteins are characterized by a highly conserved DNA-binding domain. These are abundant only in plants and perform key roles during different plant developmental stages. TaNAC2, an NAC transcription factor, was isolated from wheat and then overexpression was observed in the model plant Arabidopsis under different abiotic stresses including salt, drought, ABA, etc. (Mao et al., 2012). The DREB-1A transcription factor gene has enriched the drought tolerance in transgenic wheat (Shen et al., 2003). Another illustration is the overexpression of ornithine aminotransferase for salt- and water-deficit tolerance in Arabidopsis, which further has been transformed into wheat to induce the tolerance mechanism (Roosens et al., 1998). Another transcription factor gene from the ethylene responsive factor W6 gene from wheat showed overexpression in tobacco and improved the tolerance mechanism under saline stress condition.
The changes in biochemical and physiological parameters observed in transgenic plants compared with non-transgenic plants found the up-regulation of transgene in transgenic plants (Yan et al., 2008). The Stress responsive Transcription Data Base (STIFDB) is a collection of identified stress-related signals, responsive genes, and transcription factors. It catalogues all the available information on putative binding sites of the stress responsive transcription factors in the model species Arabidopsis (Shameer et al., 2009). The updated version of this database is STIFDB2, which contains supplementary information related to other agriculturally important crop species, i.e., maize, sorghum and soybean, novel stress-related signals, newly identified transcription factors and their regulatory sites, and addition of the stress responsive genes from microarray-based experiments (Naika et al., 2013). All this information will be helpful for plant and computational biologists to further understand plants’ stress-related mechanisms.
Induction for production of certain enzymes like glutamate synthase, proline, or regulation of organic solute such as proline and glycinebetain and antioxidant enzymes under salt and other abiotic stresses is regulated by signal transduction pathway (Misra and Saxena, 2009; Yang et al., 2013). Salicylic acid is reported to be part of the signaling pathway and considered to take part in improving the functions related to photosynthesis, which ultimately would help to improve the contrary effects of salts and thus help to improve the plant defense mechanism by improving the physiological, metabolic, and biochemical pathway (Idrees et al., 2011). The combined effect of cassava Cu/Zn superoxide dismutase (MeCu/ZnSOD) and catalase (MeCAT1) improved cytosolic expression and maintained the ROS scavengers by showing improved shelf-life of cassava roots to combat abiotic stress. SOD, CAT, proline accumulation, and water-related attributes were improved and lowered the malendialdehyde in transgenic cassava under cold stress (Xu et al., 2013).
Ethylene production has a key function at post-harvest and affects the shelf-life of fruits. This induces several genes altogether, which transcribes and regulates the functional genes affecting the storage of fruits. ACS (ACC synthase; ACC-1-aminocyclopropane-1-carboxylic acid) and ACO (ACC oxidase) are the basic enzymes known for ethylene biosynthesis. Transgenic apple plants silenced with either of these enzymes produced less ethylene due to suppression of volatile ester synthesis, and fruit maturity features were observed (Dandekar et al., 2004; Johnston et al., 2009). ACO suppression was done by RNAi in kiwi and papaya fruits and volatile production was reduced, which led to reduced ethylene production and improved shelf-life of the fruits thereby extending and fruit storage (López-Gómez et al., 2009; Atkinson et al., 2011)
Plant growth and development under abiotic stresses is also regulated by the hormonal activation and in this regard brassinosteroid (BR) is reported to interact with ABA; various BR receptive genes are also responsive to ABA (Cui et al., 2012). These BRs are steroidal compounds, ubiquitous, distributed in free form, and conjugated to starch and lipids and thus help the plants to adapt under salt and other abiotic stresses (Hayat et al., 2012). ABA is considered a stress regulating plant hormone and plays a significant role in changing gene expression profile and cellular responses, as this is the most studied plant hormone under abiotic stress. NCEDs (9-cis-epoxycarotenoid di-oxygenases) and P450 CYP707As are the two enzymes involved in ABA biosynthetic and catabolic pathways, respectively, and their genes are activated after abiotic stresses. When the plants are growing under normal conditions, ABA exists within vacuoles and apoplasts as ABA glucosyl ester, which is an inactive form released by the action of β-glucosidase when the plants are dehydrated (Hirayama and Shinozaki, 2010).
There are other reports suggesting that some of the enzyme-related genes like betaine aldehyde dehydrogenase (BADH), pyrroline-5-carboxylate synthetase (P5CS), mannitol-1-phosphodehydrogenase (mtlD), 6-sorbitol dehydrogenase phosphatase (gutD), and late embryogenesis LEA, HVA1, and ME-leaN4 have been overexpressing among different crops under different stress condition (Swire-Clark and Marcotte, 1999; Prabhavathi et al., 2002; Sawahel and Hassan, 2004; Oraby et al., 2005; Park et al., 2005; Yan et al., 2008).
Even though inducing abiotic stress tolerance in plants through genetic engineering is quite successful in many agriculturally important crops, this approach will simply be authenticated after validation of the results of successive generations produced in field trials. When plants are grown as part of the plant community under field conditions and are subjected to multiple stresses, then various essential abiotic stress reactions induced in plants relate to their performance as a crop. Thus, the initial outcomes of the genes from model species are still to be studied to reach factual consideration.
1.6 Conclusion and future perspectives
Increased abiotic stresses are the major threats to agricultural crops, which limit plant growth and productivity. Plants respond to these stresses at molecular and cellular levels, which makes modifications to the morphological, physiological, biochemical, and molecular behavior of the plants. The complex nature of abiotic stress responses studied so far advocate that further expansion of discrete stresses at the individual level may not be the appropriate approach. Traditional breeding has introduced crop varieties with desirable characteristics, but the limitations associated with this restrict agricultural scientists to adopt this further. Genomics approaches now empower the study of plant responses to abiotic stresses.
The marker-assisted breeding approach is a potential substitute to conventional breeding with regard to time, labor, and cost effectiveness. A number of QTLs have successfully been identified for qualitative and quantitative characteristics in many crop species. This has improved the knowledge related the stress mechanisms and some crops have exhibited excellent performance for specific traits. However, the challenges related to this technology are the preciseness of the QTL identification, the genetic environment interaction, reproducibility of the results, numerous yield-related genes, and moreover germplasm restrictions, which may restrict scientists’ vigilance when using this approach. However, positional cloning may be the key challenge as QTL cloning can offer a more meaningful and reliable marker for breeding of superior allelic variation in crop species.
Plant species with longer genomes have not been sequenced due to the presence of copies of large repeats of genes lacking information of coding genes, while the purpose of sequencing is to obtain the information for discovery and characterization of the genes for proteomics. Expressed sequence tags (ESTs) helped to overcome this limitation and presently this is most abundantly sequenced nucleotide product from the plant genomes. A number of sequences from the nucleotides of models as well as several other plant species are accessible on publicly available databases—hence supporting the high-throughput technologies for transcriptome analysis. Therefore, at present, ESTs offer a high-throughput robust sequence resource to exploit gene discovery. ESTs are the key components for the cDNA microarray—the high-throughput experimentation for gene identification from certain tissues at specific developmental stages under abiotic stress. The limitations of this technology are the general demonstration of representative genes in the cDNA library and the specific features of different sequences within the collection of clones. The solution for real transcriptome analysis is the complete genome sequence, but ESTs with appropriate bioinformatics studies help to overcome the limitations.
Microarray is a high-throughput technology for gene expression analysis that consists of probes demonstrating several different genes organized on a glass slide in a systematic pattern. Several plant stress-related genes have been identified for expression profiles and functionally categorized under specific conditions. Probes are used as cDNA or oligonucleotides and the several thousands of array results are available on databases. The limitation associated with the microarray is its cost in terms of materials, design, expertise, and reproducibility of results; therefore, microarray experiments are not appropriate for small-scale funded projects. A great deal of expertise is required to design the experiment and for data analysis. Bioinformatics applications are required for in silico experimentation and conclusions. Gene expression analysis compared with other techniques such as real-time PCR would be more specific, which may be due to non-specific hybridization. However, the microarray is very sophisticated technology and the applications are vast, so the limitations may be overcome by considering the experimentation more accurately.
Succeeding transcriptome analysis or gene identification, significant efforts are now in progress to explore the gene expression process or their response under various abiotic stresses. So the post-genomic era in crop research now uses proteomics approaches to identify the proteins that are regulated in response to environmental threats. These studies found that proteins are expressing to change the physiological mechanisms, and plants from different species showed adaptive strategies under abiotic stresses including carbohydrate synthesis, protein synthesis, ATP formation, C4 photosynthesis, and heat shock protein profiles for ROS scavenging, etc. Proteomic studies should be comprised of assessments of susceptible and tolerant cultivars to reveal the specific mechanisms involved in stress tolerance. This will lead to the engineering of stress tolerance into agricultural crops.
Metabolic changes upon abiotic stress response adaptation involve accumulation of or increase in the levels of particular stress responsive metabolites such as amino acids, total soluble sugars, and polyols, which are commonly termed compatible solutes. The physiological role of stress responsive metabolites which are precisely altered remain to be examined. The extent of metabolic modifications within a genotype reveals the intensity of stress experienced by the plant. Several enzymes are key components for metabolic changes and further exploration of the genes expressing for these adaptations is under way.
Genetic transformation has made real progress to understand how plants cope with the stresses as well as alterations to the signaling and response pathways. Transgenic plants harboring genes related to organic solutes, antioxidant enzymes, molecular chaperones, and some of the transcription factors have the best specific stress tolerance under controlled conditions. Although some transgenic lines consist of the fully characterized genes and are performing best, most of them are transformed with a single stress-related gene whose function may not be properly understood. When the plants are grown in natural conditions, they have to combat a number of stress threats, so it is difficult for a transgenic plant transformed with a single gene and growing in controlled conditions to cope with multiple stresses for multiple exposures with specific severity. Therefore, a meaningful future approach for effective utilization of transgenic technology is to use gene pyramiding to stack multi-genic transformation technology. This should be further progressed with fully characterized genes’ known functions and the transgenic lines should be field tested. Thus, the complete system of complex cell signaling mechanisms and vital interrelating practices that hint at multi-defense responses might be elaborated.
Acknowledgments
We are thankful to Mr. Sajjad Saddique, Ms. Sameera Hassan, and Miss Fatima Batool for their help in the literature survey.
