Silicon and Selenium
Two Vital Trace Elements that Confer Abiotic Stress Tolerance to Plants
Mirza Hasanuzzaman, Kamrun Nahar and Masayuki Fujita
Plants are sessile organisms and therefore must constantly adapt their growth and architecture to an ever-changing environment. The damages in growth and development of economic plants caused due to abiotic stresses like salinity, drought, extreme temperature, flooding, toxic metals, ozone, and UV radiation are quite alarming due to shrinking agricultural land area, recurrently expanding population, and rapid climate change throughout the world. To cope with adverse growing conditions, plant scientists are searching for ways to make plants adaptive. They are trying to understand the effect of environmental stresses on plants and to modify plants’ outer growing conditions and their internal cellular environment by applying different exogenous protectants. Silicon (Si) and selenium (Se) are widely studied trace elements and their roles in plant growth and physiology are well documented. Recently, these trace elements have been found to be protective under abiotic stress conditions. Silicon is the second most abundant element after oxygen in soils and its presence in the form of silicic acid allows its uptake by plants, so by nature plants have a great scope to uptake Si in their tissues. However, those plants not supplied with sufficient natural sources of Si may benefit from its exogenous application. Selenium, an essential element for animals and humans, has also been found to be beneficial to plants. Like Si, Se also plays a protective role in conferring tolerance to certain abiotic stresses when applied at lower concentrations, while higher concentrations show phytotoxicity. However, plant species differ strongly in Se uptake and accumulation as well as their tolerance capacity. Both Si and Se were reported to play roles in conferring oxidative stress tolerance by enhancement of the antioxidant defense system in plants. Although much research has been published on the effect of Si and Se on plants under abiotic stress, clear-cut underlying physiological mechanisms of the mode by which Si could protect plants from stressful conditions are elusive. In this chapter, we attempt to summarize the uptake and accumulation pattern of Si and Se in plants. Later, we discuss the recent reports regarding the role of Si and Se in conferring abiotic stress tolerance to plants.
Keywords
abiotic stress; antioxidant defense; climate change; oxidative stress; plant adaptation; plant stress responses; selenium; silicon; trace elements
16.1 Introduction
Doubtless, we all are in some way or other affected by the damage to economic plants caused by environmental stresses; this is particularly alarming in view of the shrinking agricultural land area and the continuously expanding population on Earth. Rapid climate change throughout the world has exposed plants to various environmental adversities that prevent them from reaching their full genetic potential and limit their productivity (Hasanuzzaman et al., 2012a). Abiotic stresses are the greatest restriction for crop production worldwide and account for yield reductions of as much as 50% (Rodríguez et al., 2005; Acquaah, 2007). Crop plants, as sessile organisms, encounter unavoidable abiotic stresses during their life cycles, including salinity, drought, extreme temperatures, heavy metal (HM) toxicity, flooding, UV-B radiation, ozone, etc., which all pose a serious challenge to plant growth, metabolism, and productivity (Ahmad and Prasad, 2012a,b; Hasanuzzaman et al., 2012a). Now is the right time to be strategic: first by understanding the reasons—fundamental to complex—for yield reductions so that precise research planning can be brought about to cope with ever-changing environmental conditions or stresses. With that view, plant scientists are now sacrificing their time searching for ways to make the plants adaptive even under adverse growing conditions. Researchers are trying to understand the effects of environmental stresses on plants so that they can modify the plant’s external growing condition by applying different exogenous protectants including trace elements and even by molecular mechanisms.
Many reports have appeared on silicon (Si) research in the last few decades, but this element continues to be an anomaly—an underappreciated element and in most cases one not considered as essential for plant function. However, in certain plant species like rice (Oryza spp.), Si is absorbed from soil in amounts that are even higher than those of the essential macronutrients (Datnoff et al., 2001). The beneficial effects of Si on growth have been reported in many crop plants in addition to rice, such as wheat (Triticum aestivum), barley (Hordeum vulgare), and cucumber (Cucumis sativus). However, plant species differ greatly in their ability to accumulate Si (Epstein, 1999; Ma et al., 2002; Richmond and Sussman, 2003). Numerous research reports have provided evidence for the notion that Si may also play a vital role in conferring plants with tolerance to adverse environmental factors. Hence, Si is considered as a beneficial element for plants growing under stressful conditions (Sacaa, 2009). Silicon-induced alleviation of abiotic stresses such as drought, salinity, high temperature (HT), chilling, UV radiation, nutrient imbalance, and metal toxicity have been observed by many researchers (Raven, 2001; Ma and Takahashi, 2002; Liang et al., 2007; Ma and Yamaji, 2008; Hasanuzzaman and Fujita, 2011a; Ahmed et al., 2012).
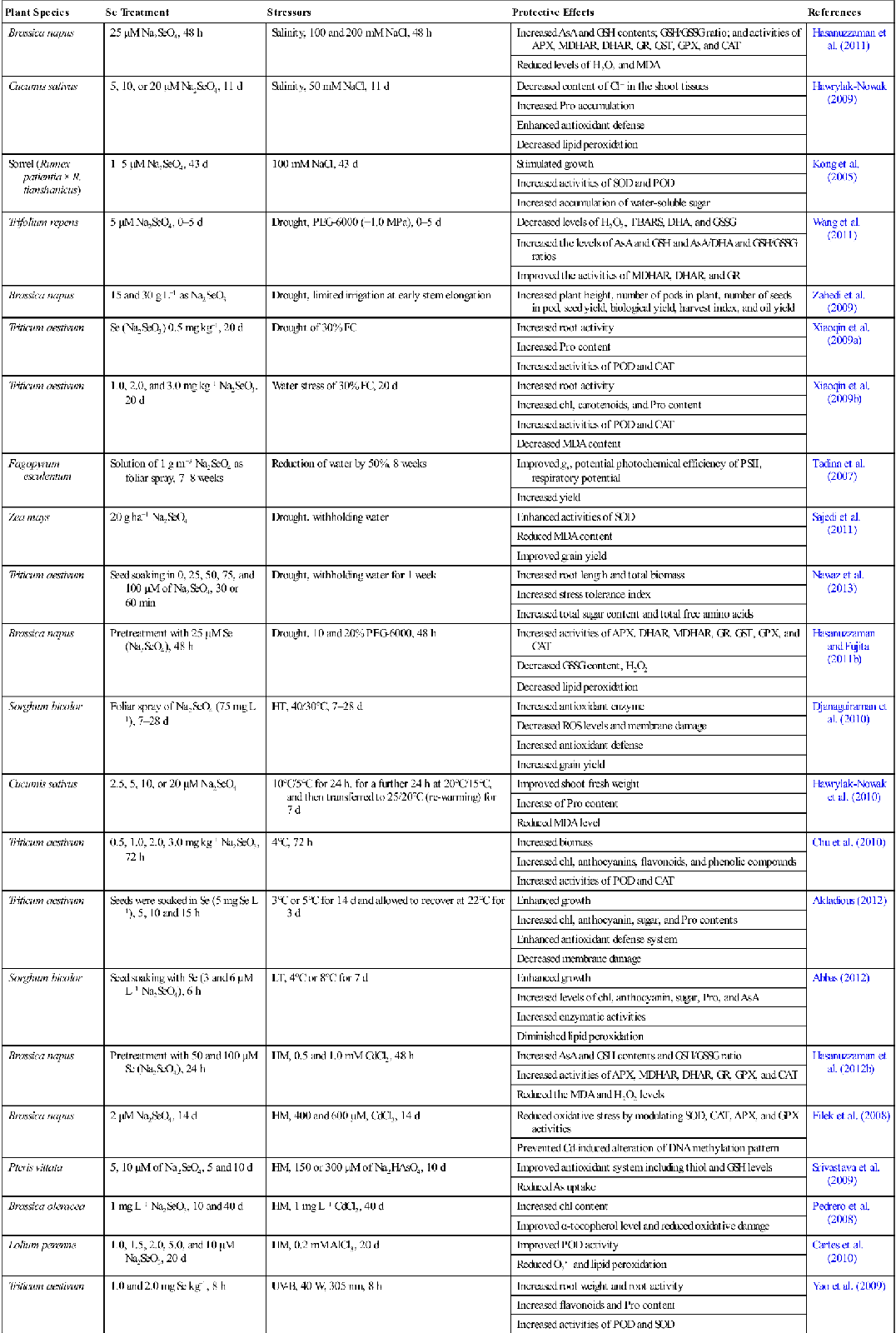
Selenium (Se) is a widely studied trace element in humans and animals due to its role in the antioxidant defense system, which is needed for the maintenance of health and hormone balance. For the past few decades, the beneficial role of Se in plants has been investigated by several groups of researchers but the question is still unresolved whether Se is an essential micronutrient for plants (Terry et al., 2000). Although Se has not been defined as essential, its role as a beneficial element in plants has been revealed in many plant studies (Xue et al., 2001; Hasanuzzaman et al., 2010). In many plant species, Se exerts a positive effect on plant growth and productivity at low concentrations (Terry et al., 2000; Xue et al., 2001; Turakainen et al., 2004; Hasanuzzaman et al., 2010). However, the precise mechanisms underlying the beneficial role of Se in plants have not been clearly elucidated yet (Turakainen, 2007). Some plant species supplemented with Se have shown enhanced resistance to certain abiotic stresses like salinity (Djanaguiraman et al., 2005; Hawrylak-Nowak, 2009; Hasanuzzaman et al., 2011), drought (Yao et al., 2009; Hasanuzzaman et al., 2010; Hasanuzzaman and Fujita, 2011b), extreme temperature (Chu et al., 2010; Hawrylak-Nowak et al., 2010; Djanaguiraman et al., 2010), metal toxicity (Hawrylak et al., 2007; Pedrero et al., 2008; Filek et al., 2008; Srivastava et al., 2009; Cartes et al., 2010), and UV radiation (Valkama et al., 2003; Yao et al., 2010a,b). Different plant studies have shown that Se could help in detoxification of reactive oxygen species (ROS) and thus enhanced plant tolerance to oxidative stress (Djanaguiraman et al., 2005; Hasanuzzaman et al., 2010; Hasanuzzaman and Fujita, 2011b).
In this chapter, we review the recent findings on the diverse roles of Si and Se on plant growth and development. Special emphasis is given to their roles in conferring abiotic stress tolerance to plants. We also shed light on the mechanisms of uptake and transport of these elements in plants.
16.2 Silicon uptake and transport in plants
Silicon is the second most abundant element after oxygen (O2) in soils, with a value as high as 26%. It has strong affinity with O2 and thus it always exists as silica (SiO2) or silicate (SiO44−). In nature, Si does not occur as an elemental form but is found as a compound within many minerals which form rocks. Minerals containing Si are resistant to weathering processes and decomposition; hence, the amount of Si in the soil solution is low (Brogowski, 2000). The Si content of soils can vary considerably, from <1 to 45% of dry weight (Sommer et al., 2006), and its presence in the form of silicic acid [Si(OH)4] (or its ionized form, Si(OH)3O2, which predominates at pH 9) allows its uptake by plants. Therefore, by nature, plants have a great scope to uptake Si into their tissues. Although there is extensive literature on the uptake, transport, and distribution of Si in plants, the mechanisms by which these processes occur is still a matter of research because the nature of uptake and transport and the concentration of Si in plant tissues varies greatly between species (i.e., 0.1–10% of shoot dry weight). These differences are mainly due to the differential characteristics and capacities of Si uptake and transport among various species (Epstein, 1994; Liang et al., 2007; Ma et al., 2007).
In their review, Sacaa (2009) summarized three types of plants based on their capacity for Si accumulation (Table 16.1). In general, monocotyledonous plants, especially the plants in the Poaceae family, are able to take up more Si than are other plant families. Among the crop plants of this family, rice has the highest capability to uptake Si (Tamai and Ma, 2003) so that rice contains much higher Si levels than do barley, maize, rye, sorghum, and wheat. Some other plant families (namely, Equisetaceae, Cyperaceae, and Balsaminaceae) also show high Si accumulation. Table 16.2 presents the differences of Si concentration in some plant species (Broadley et al., 2012). At the same levels of soil Si, rice was shown to accumulate 39.1 mg g−1 dry weight of Si, whereas chickpea (Cicer arietinum) accumulated only 3.0 mg g−1 dry weight. According to Takahashi et al. (1990), plants possess three methods of Si uptake: (1) active, (2) passive, and (3) rejective. For instance, rice, which is an Si accumulator, takes up Si through an active process (Ma et al., 2006), while some dicots such as cucumber (Cucumis sativus), muskmelon (Cucumis melo), strawberry (Fragaria × Ananassa), and soybean (Glycine max) take up Si passively (Takahashi et al., 1990; Ma et al., 2001a; Mitani and Ma, 2005; Liang et al., 2007). Most of the dicotyledonous plants absorb Si passively and some cannot take up Si into their tissues (Liang et al., 2007). Some crops like tomato (Solanum lycopersicum) exclude Si from uptake (Mitani and Ma, 2005). According to Ma and Takahashi (2002), the Si content in the rice tops was 20-fold higher than that in the roots, while the Si content in tomato tops was one-tenth that in the roots. Their explanation was that rice roots can take up large amounts of Si, which could be translocated from the roots to the tops in rice, whereas tomato could not take up and translocate Si. This difference was due to the dissimilar ability of the roots to take up Si (Ma and Takahashi, 2002). Among the crops, rice is the most potent accumulator of Si, while other cereal crops such as wheat (Casey et al., 2004) and barley (Barber and Shone, 1966) and some cyperaceous plants take up Si actively. However, not all cereal crops take up Si actively; for instance, certain cereals like oats take it up passively (Jones and Handreck, 1965). In their report, Ma et al. (2001b) claimed that the lateral roots of rice play an important role in Si uptake but the root hairs do not. Apart from these species differences, different parts of the same plant may show significant differences in Si accumulation. For instance, in rice, Si content was measured as 0.5, 50, 130, 230, and 350 g kg−1 in polished endosperm, bran, straw, hulls, and nodes (joints), respectively (Van Hoest, 2006).
Table 16.1
Types of Plant on the Basis of Si Accumulating Capacity
| Types | Accumulation Capacity | Example |
| Si accumulators | Higher than 1% Si on dry weight | Rice and some wetland grasses |
| Si non-accumulators | Less than 1% Si on dry weight | Most dicotyledonous plants like chickpea, tomato, etc. |
| Intermediate | Intermediate (1–3%) Si on dry weight | Rye, oat, etc. |
Table 16.2
Variations in Si Concentration in Some Plants Species under Same Levels of Soil Si
| Plant Species | Family | Si Concentration (mg g−1 dry Weight) |
| Oryza sativa | Poaceae | 39.1 |
| Triticum aestivum | Poaceae | 15.4 |
| Cucurbita maxima | Cucurbitaceae | 13.4 |
| Cucurbita pepo | Cucurbitaceae | 19.8 |
| Cicer arietinum | Fabaceae | 3.0 |
| Cucumis sativus | Cucurbitaceae | 22.9 |
| Zea mays | Poaceae | 21.0 |
Ma et al. (2002) compared two rice varieties, Nipponbare (japonica) and Kasalath (indica), under different doses of Si. When the plants were grown in a nutrient solution containing 0.15 mM Si(OH)4, the shoot Si content was higher in Nipponbare than in Kasalath. In contrast, when grown in a solution containing 1.5 mM Si(OH)4, the Si content was nearly the same in both varieties. These variations were due to different mechanisms involved in accumulation of Si in the two varieties (Ma and Takahashi, 2002). In their advanced study, Ma et al. (2007) investigated the genotypic differences in Si uptake and expression of Si transporter genes in these same two rice varieties. Both Si transporter genes (Lsi1 and Lsi2) were more highly expressed in Nipponbare than in Kasalath, so that Nipponbare was a higher Si accumulator. In addition, Nipponbare had a more extensive root system and the genes Lsi1 and Lsi2 were localized at the distal and proximal sides, respectively, of both exodermis and endodermis of the roots; hence, this variety was capable of greater Si uptake. These results confirmed that the genotypic differences in the Si uptake were due to the differences in abundance of Si transporters in roots of different rice varieties.
Ma et al. (2003) found great variations in Si content, ranging from 1240 to 3600 mg kg−1, among 400 barley cultivars grown in the same soil. Mitani and Ma (2005) investigated the comparative uptake potential of three different crop species (rice, cucumber, and tomato) and found that shoots of these plants showed variations in Si accumulation. Their explanation was that this was due to the differences in radial transport of Si from the rhizophere to the root cortical cells and subsequent transport to the xylem. Among the species tested, rice accumulated the largest amount of Si in the root–cell symplasm, at levels three- and five-fold higher than in cucumber and tomato. As a result, the Si transport in the xylem sap in rice was also the highest, and was 20- and 100-fold higher than in cucumber and tomato, respectively. The lower Si accumulation in cucumber and tomato was explained by the lower densities of the Si transporter that transports Si from the external solution to the cortical cells. In addition, the absence or defects in the Si transporter that moves Si from the cortical cells to the xylem was also considered a causal factor (Mitani and Ma, 2005). In a recent review, Ma et al. (2011) described the process of Si transport from roots to panicles in plants and showed that this transport is mediated by different transporters such as Lsi1, Lsi2, etc. In rice, transport of Si by these transporters is driven by a proton gradient (Figure 16.1). However, the expression patterns and cellular localization of these transporters differs depending on the plant species.
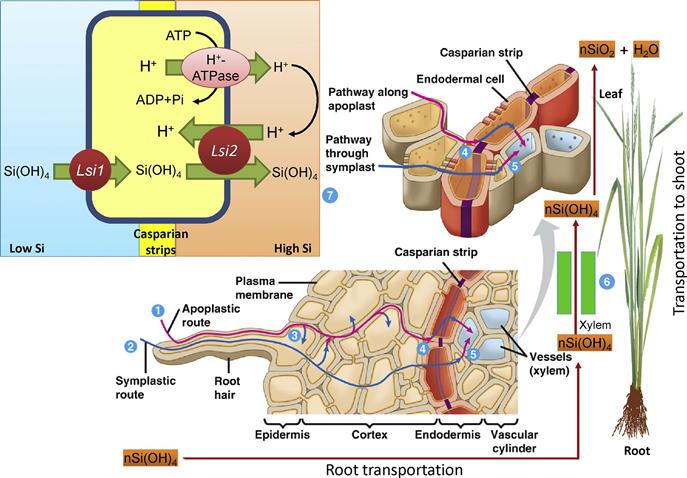
Ma et al. (2011) also reported that the differences in Si uptake were also partly mediated by root anatomy. Rice roots, for example, possess two Casparian strips at the exodermis and endodermis (Figure 16.1; Ma et al., 2011). However, in maize and barley, only one Casparian strip is present at the endodermis. Rice roots also contain well-differentiated anatomical structures, namely, highly developed aerenchyma and destroyed cortical cells between the exodermis and endodermis. Maize and barley, on the other hand, do not possess such structures. These differences in anatomical structures and localizations of transporters are predicted to explain at least part of the variation in Si uptake capacity among different species (Ma et al., 2011).When Si is uptaken by roots through transporters, it is translocated to the shoots by transpirational volume flow through xylem; more than 90% of the Si uptaken by roots is translocated to the shoots (Ma et al., 2002). Because it follows the transpiration stream, Si is largely accumulated and distributed in the leaves (Dagmar et al., 2003). In most cases, Si uptake occurs in the mature regions of the roots, rather than the root tips (Yamaji and Ma, 2007). The transport of Si from the xylem to the leaf cells involves another transporter Lsi6 in rice (Yamaji et al., 2008). The transportation of Si sometimes depends on its concentration in the sap. When the concentration of Si(OH)4 exceeds 2 mM, Si(OH)4 was polymerized to form silica gel (SiO2•nH2O) (Ma and Yamaji, 2006; Figure 16.1). High concentrations of Si are very common in some grasses. For instance, in rice and wheat, the concentration of Si in the xylem sap can exceed 2 mM and in such cases monomeric silicic acid (H4SiO4) was also identified as the major form of Si in the xylem (Figure 16.1; Mitani and Ma, 2005; Mitani et al., 2005; Ma and Yamaji, 2006). Silicon is further concentrated in the shoots through loss of water and polymerization, which converts Si(OH)4 to colloidal Si(OH)4 and finally to SiO2•nH2O with increasing Si(OH)4 concentration (Ma and Takahashi, 2002). In some species, like rice, Si is sometimes deposited as a thin layer in the space immediately beneath the thin cuticle layer, to form a cuticle Si double layer in the leaf blades. This is one of the ways that rice plants are protected from multiple abiotic and biotic stresses (Ma and Yamaji, 2006). Silicon distribution in the aerial parts of plants depends on the intensity of transpiration. The xylem transpiration stream transports Si(OH)4 to the leaves, where it accumulated in older tissues (Sacaa, 2009).
At the cell level, Si is transported through two different processes: (1) passive transport of Si(OH)4 across membranes and (2) active transport of Si(OH)4 across membranes (Figure 16.1). The default condition of Si transport across the cell membranes is that the solute crosses the membrane by diffusion across the lipid components of the membrane. The active transport of Si(OH)4 can be recognized at the cell level by the demonstration of Si(OH)4 movement from a lower to a higher concentration. In some cases, the occurrence of active Si transport is deduced from the occurrence of intercellular SiO2 deposition in cells growing in a medium with Si(OH)4 at lower than saturated concentrations (Raven, 2001). At the tissue level, the movement of Si(OH)4 through plant tissues can involve apoplasmic and symplasmic pathways. During apoplasmic movement, Si(OH)4 is transported through cell walls and water-filled intercellular spaces, etc., while during symplasmic movement it is transported through the cytosol, including the cytosolic sleeve of the plasmodesmata and the conducting cells of phloem (Raven, 2001; Figure 16.1). Both of these processes involve diffusion and mass flow.
Different physiological processes also affect Si transport among the plant parts. For instance, in rice, transpiration plays a certain role in translocation and accumulation of Si to the tops. The Si concentration is high in leaf blades and husks where the transpiration rate is high. Although rice roots play an important role in active uptake of Si, the Si content in the roots is much lower than that found in the tops. Therefore, Si taken up by the roots is supposedly translocated to the shoot along with the transpiration steam and then concentrated and finally physically gelled in rapidly transpiring organs. Nutrient ions present in the soil also sometimes affect Si uptake. For example, excess nitrogen is reported to decrease the Si content and number of silica bodies in rice (Ishizuka and Tanaka, 1950). Different metabolic inhibitors like pyruvate and acetate were found to disturb Si uptake (Ma and Takahashi, 2002). Some environmental factors like low temperature (LT) sometimes inhibit transportation (Mitani and Ma, 2005).
16.3 Selenium uptake and metabolism in plants
Selenium has a chemical nature similar to sulfur (S). In nature, Se can exist in different oxidative states, namely, in the −2 (selenide, Se2−), 0 (elemental Se), −4 (selenite, SeO32−), and −6 (selenate, SeO42−) oxidation states (Brown and Shrift, 1982). Selenium may also exist as organic complexes. The forms of Se present in soil mostly depend on pH and redox potential (Eh); the predominant form is SeO42− in alkaline and well-oxidized soils (pe+pH >15), SeO32− in well-drained mineral soils with pH from acidic to neutral (7.5 <pe+pH <15), and selenide under reduced soil conditions (pe+pH <7.5) (Elrashidi et al., 1987). In soil, Se is found in small amounts (ranging from 0.01 to 2 mg kg−1). However, in seleniferous areas, its content may be as high as 1200 mg kg−1 total Se and 38 mg kg−1 soluble Se (Mayland et al., 1989; Pezzarossa and Petruzzelli, 2001).
Plant species differ strongly in Se uptake and accumulation as well as in their tolerance capacity (Table 16.3; Broadley et al., 2012). Their differences in the capacities to accumulate and tolerate Se place plants into different classifications: (1) non-accumulators, (2) indicators, and (3) accumulators (Terry et al., 2000; Dhillon and Dhillon, 2003; White et al., 2004). Shrift (1973) hypothesized that Se is an essential microelement in accumulator plants, based on the following evidence: accumulator plants grow only on seleniferous soils and accumulate higher quantities of Se than do non-accumulator plants; the growth of accumulator plants is stimulated by adding small amounts of Se to the growth solution, whereas the growth of non-accumulator plants is inhibited; the assimilation path of Se in the accumulator plants differs substantially from non-accumulator plants. Apart from these categories, some plant species are called Se hyperaccumulators, which are further divided into two groups: the primary Se accumulators are capable of accumulating thousands of milligrams of Se kg−1 (>4000 mg kg−1), and the secondary accumulators hundreds of milligrams Se kg−1 (Terry et al., 2000). One of the promising Se hyperaccumulator plant families is the Brassicaceae, which includes mustard (Brassica juncea L.), broccoli (B. oleracea botrytis L.), and canola (B. napus spp. oleifera L.), which have been classified as primary accumulators (Whanger, 2002). However, most cultivated crop plants have a low tolerance to high Se levels. Generally, they contain less than 25 μg Se g−1 dry weight (DW) and are considered to be non-accumulators. Potato (Solanum tuberosum) is classified as an Se non-accumulator (White et al., 2004) and cannot tolerate high concentrations of Se. Plant species greatly vary in their potential to uptake Se into their tissues. The critical tissue Se concentrations that decreased the yield were 105 μg g−1 DW in B. juncea, 77 μg g−1 DW in Z. mays, 42 μg g−1 DW in O. sativa, and 19 μg g−1 DW in T. aestivum. These levels were attained by Se addition as SeO32−at 5 μg g−1 soil for B. juncea and Z. mays, 4 μg g−1 soil for T. aestivum, and 10 μg g−1 soil for O. sativa (Rani et al., 2005).
Table 16.3
Se Concentration in Shoots of Different Plant Species Grown in Soils with the Same Se Level (2–4 mg Se kg−1)
| Plant Species | Se Concentration (mg Se kg−1) |
| Astragalus pectinalus | 4000 |
| Stanleya pinnnata | 330 |
| Gutierrezia fremontii | 70 |
| Zea mays | 10 |
| Helianthus annuus | 2 |
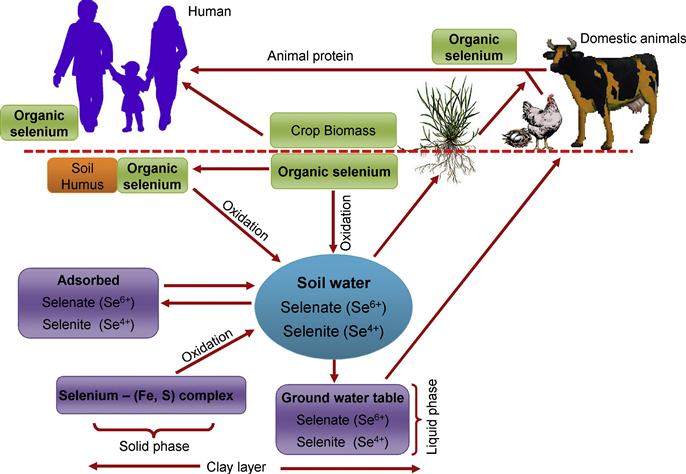
Accumulation of Se also differs greatly among the plant organs in the same plant species. With some exceptions, most of the plants accumulate more Se in upper parts (stem and leaf) than in roots (Zayed et al., 1998). Studies on potato (Solanum tuberosum) plants by Turakainen (2007) indicated that, at early stages, Se concentration was higher in young upper leaves. However, at maturity, Se content declined in the aerial parts, but a rigorous accumulation took place in the tubers (Turakainen et al., 2006; Turakainen, 2007). Selenium accumulation was also influenced by the application methods. Foliar spray of SeO42− on tea leaves considerably increased Se content in the cells (Hu et al., 2003). Smrkolj et al. (2006) observed a linear relationship between exogenous Se spraying and the accumulation of Se in Pisum sativum seeds. Other factors, like presence of ions (Cl−, SO42−, PO43−), salinity, and trace elements, also affect the uptake and metabolism of Se in plants (Gupta et al., 1982). The interactions between Se and other ions may be due to chemical reactions either in the soil or in the plant, or to the dilution effect due to increased plant growth.
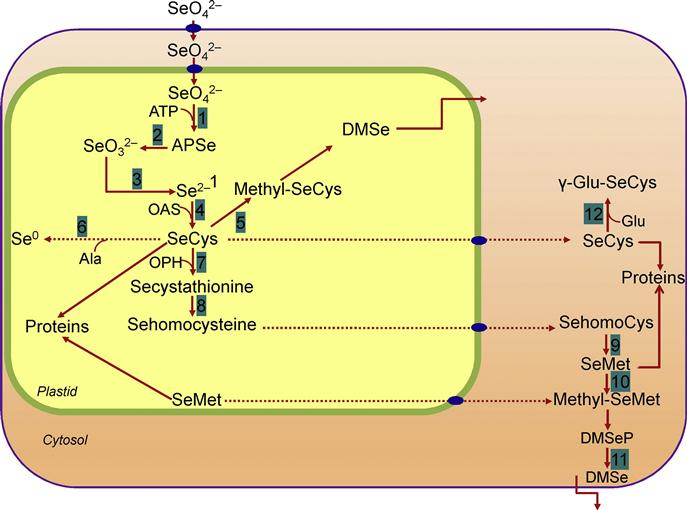
Selenium is taken up from soil by plant roots mainly as SeO42−, SeO32−, or organoselenium compounds. The biosynthesis of most Se compounds in plants follows the same pathways that lead to isologous S compounds (Çakır et al., 2012). However, SeO42− is taken up faster than SeO32− but acquires organoselenium compounds, namely, selenocysteine (SeCys) and selenomethionine (SeMet) (White et al., 2007). Thereafter, it is metabolized (via the S assimilation pathway) into SeCys, SeMet, and other Se analogues of S-based metabolites (Ellis and Salt, 2003). The most abundant bioavailable form of Se in soils is SeO42−, which is taken up via sulfate transporters. It then forms SeO32− and Se2− through reduction and SeCys through combination with O-acetylserine. SeCys is further metabolized to form Se-cystathionine, Se-homocysteine, and SeMet. Glutathione (GHS) plays a role in Se metabolism, serving as a reductant for the reduction of SeO32− to Se2− (Sors et al., 2005).
The mechanisms of SeO32− uptake by plants are not well understood. Early studies suggested that SeO32− may enter root cells passively by diffusion (Terry et al., 2000). However, SeO32− uptake is at least partly active (Li et al., 2008). Both SeCys and SeMet can be non-specifically incorporated into proteins, replacing Met and Cys (Figure 16.2; Valdez Barillas et al., 2011). Further, SeCys can also be converted into Se2− and alanine (Ala) through breakdown, or to methyl-SeCys through methylation. However, the methylation reaction is generally observed in Se-hyperaccumulator plants, where it acts as one of the mechanisms of Se tolerance since methyl-SeCys cannot be incorporated into proteins (Figure 16.2; Freeman et al., 2006; Valdez Barillas et al., 2011). Later, this methyl-SeCys is metabolized to a volatile compound, dimethyldiselenide (DMDSe). Non-hyperaccumulators also volatilize Se as DMSe using SeMet as a starter (Figure 16.2; Terry et al., 2000). The cellular metabolism of Se and its sequestration patterns in plant tissues are dissimilar in non-hyperaccumulators and hyperaccumulators. In non-hyperaccumulators, Se is mainly sequestered in the vascular tissues (Freeman et al., 2006), while hyperaccumulators accumulate Se mainly in the leaf hairs and the vacuoles of epidermal cells (Freeman et al., 2006).
Through plants, Se is easily transported from the soil to the human food chain (Figure 16.3). It is mixed with various agricultural products and fodders to a variable extent, depending on the concentration and bioavailability of Se in soil (Koivistoinen, 1980; Yläranta, 1983a, 1985). In fact, the availability of Se in soils is restricted due to reduced weathering status and acidity (Sippola, 1979; Yläranta, 1983b). Quantities of Se in the soil solution are also governed by the solubility of its adsorbed forms and by the biological transformation to other forms (Figure 16.3).
16.4 Involvement of silicon and selenium in plant growth, development, and physiology
Although Si is considered a non-essential element, exogenous application promotes the growth of many plant species (Epstein, 1999). Increases in different growth parameters following Si application is dose and plant specific, and also depends on different edaphic and environmental conditions (Agurie et al., 1992; Hossain et al., 2002; Ali et al., 2009).
Application of low concentrations of Si (50–100 μg g−1) to cowpea (Vigna unguiculata) increased the relative yield of root and shoot, nitrogen (N), phosphorus (P), and calcium (Ca) concentrations (Mali and Aery, 2008). It also significantly enhanced the growth, number, and fresh weight of nodules (Mali and Aery, 2008). Silicon is also reported to increase biomass production of wheat plants (Tahir et al., 2012), Prosopis juliflora (Bradbury and Ahmad, 1990), and Zinnia elegans (Kamenidou et al., 2009). Silicon (12 ml L−1 OryMax) application increased the internode diameter in rice by 17%, increased the internode wall thickness by 13%, and resulted in an Si content on dry weight basis of 13.7% in stems and 13.1% in husks (Nhan et al., 2012). The effects of Si (0.31–80 mg L−1) on growth and somatic embryogenesis were studied in Phragmites australis. Silicon had positive effects in terms of callus production, number of plants regenerated per callus, number of differentiated roots per callus, number of somatic embryos, increase in fresh weight, and the obtained results were clearly Si dose and genotype dependent (Máthé et al., 2012). Silicon fertilizer (SiO2) improved the N, P, Ca, magnesium (Mg), and S levels in soybean (Glycine max) plants. It also increased dry matter and number of plants per unit area. The same fertilizer increased white oat dry matter by 18% (Castro and Crusciol, 2013). Fresh weight and diameter of flower of Paeonia lactiflora Pall. were increased by Si (Zhao et al., 2013). Again, Si application increased the layer number of thickened sclerenchyma cells, thickness of cell walls, lignin content of inflorescence stems, cortex and xylem Si content, and the strength of stalks, and improved the quality of the cut flowers (Zhao et al., 2013).
The effects of Si on the reproductive development of plants are well recognized. In G. max, Si-deficient plants showed anomalies in pod development when compared to Si-supplemented plants (Miyake and Takahashi, 1985). Silicon application ameliorated abnormal flower development in tomato (Miyake and Takahashi, 1985). Silicon influenced inflorescence production in Gerbera jamesonii (Savvas et al., 2002). Ma and Yamaji (2008) reported that silicate stimulated the growth and development of flower and inflorescences of Gramineaceous plants. In O. sativa, Si influenced panicle development and grain fertility (Ma and Yamaji, 2008). Silicon suppressed anther dehiscence in rice by 85%. It also improved diameter and number of pollen grains in stigma and subsequent fertilization (Li et al., 2005). Silicon also improves photosynthesis and other physiological processes. Silicon-induced increases in water use efficiency were reported in rice (Nwugo and Huerta, 2008) and cucumber (Feng et al., 2010). Addition of Si increased the chlorophyll (chl) content and ribulose bisphosphate carboxylase (Rubisco) activity in cucumber (Adatia and Besford, 1986). Significant increases in growth and photosynthetic parameters, including SPAD value, net photosynthetic rate, cellular CO2 concentration, stomatal conductance (gs), transpiration rate, and chl fluorescence efficiency, were recorded in barley following Si (1 and 2 mM) application (Ali et al., 2013). Stomatal movement is variously affected by Si. Besides promoting rapid and elevated stomatal movement under normal growth conditions, under stress conditions, Si is supposedly incorporated into epidermal tissue, where it may reduce the diameter of stomatal pores and thus control stomatal movement, which might be beneficial under certain stress conditions (Efimova and Dokynchan, 1986), possibly by reinforcement of cell walls (Epstein, 1999). Thus, Si treatment has been confirmed to reduce stomatal resistance in some crops like Vitis vinifera L. (Soylemezoglu et al., 2009), rice (Yeo et al., 1999), and tomato (Romero-Aranda et al., 2006) under salinity stress. When Z. mays plants were treated with nine levels of Si (0, 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, and 3.2 mM) improved gas exchange and photosynthetic attributes were noted, along with improved growth relative to the control. Si could improve transpiration, gs, net CO2 assimilation rate, and leaf substomatal CO2 concentration quite remarkably. Silicon levels of 0.8, 1.6, and 2.8 mM showed better results overall (Parveen and Ashraf, 2010). In rice leaves, Si at 12 ml L−1 supplied as OryMax increased the chl a content by 43%, chl b content by 47%, and total carotenoid content by 47%; whereas, Si at 6 g L−1supplied as SilySol increased these parameters by 45, 54, and 15%, respectively (Nhan et al., 2012). Si promoted the development of an erect plant and improved photosynthetic efficiency (Pulz et al., 2008). Silicon deposition decreased evapotranspiration water loss (Ma and Yamaji, 2006). Silicon increases a plant’s capacity for transpiration, thereby allowing cooling and aiding bulk flow of soluble nutrients from the soil (Soylemezoglu et al., 2009).
16.5 Effect of silicon and selenium in improving yield of crop plants
The improvement in yields of different crop plants due to exogenous application of Si has been reported by many authors (Rafi and Epstein, 1999; Kim et al., 2012; Castro and Crusciol, 2013). Silicon deficiency reduced pod yield in G. max (Miyake and Takahashi, 1985), while Si application increased the yield of tomato in terms of fruit number and fruit weight (Miyake and Takahashi, 1985). Effects of SiO2 fertilizer were studied on different growth and yield attributes of G. max, T. aestivum, A. sativa, and Z. mays. When compared to control G. Max plants, the pods per plant increased by 8%, grains per pod by 19%, mass of 100 grains by 21%, and total grain yield by 49% in response to Si. In white oat, Si increased the yield attributes and yield as shown by a 15% increase in panicle number m−2, an 11% increase in grain-filled spikelets panicle−1, a 6% increase in the mass of 1000 grains, and a 70% increase in grain yield. The yield attributes of maize were increased by Si, with increases in grains ear−1 and grain yield of 23 and 18%, respectively (Castro and Crusciol, 2013). Silicon also increased yields in T. aestivum (Rafi and Epstein, 1999), S. officinirum (Savant et al., 1999), and H. vulgare (Liang, 1999). In rice, Si (12 mL L−1 OryMax) increased the number of panicles pot−1 (7%), the number of filled grains panicle−1 (22%), the filled grain ratio (4%), the weight of 1000 grains, and the yield pot−1 (15%) (Nhan et al., 2012). Kim et al. (2012) also found positive effects of Si supplementation in rice; Si application reduced the lodging index (13.7%) and increased plant height (12.2–≈16.7%), pushing resistance (10.5–≈13.8%), and yield up to 15.1%. Addition of Si to the nutrient solution increased the shelf-life, and improved the quality and quantitative yield of Valerianella locusta (Gottardi et al., 2012).
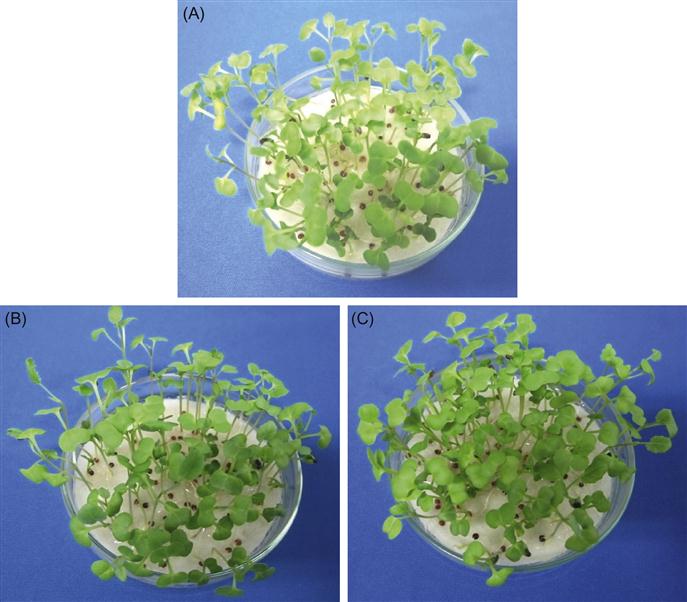
A beneficial role of Se has been observed in plants that are capable of accumulating large amounts of this element (Shanker, 2006). However, the role of Se is mostly dose dependent (Hasanuzzaman et al., 2010, 2012b). Hamilton (2004) reported three levels of biological activity of Se: (1) trace concentrations required for normal growth and development; (2) moderate concentrations that can be stored to maintain homeostatic functions; and (3) elevated concentrations that can result in toxic effects. In ryegrass (Lolium perenne) and lettuce (Lactuca sativa), Se exerted beneficial effects at low concentrations (0.1 mg kg−1 soil), while it showed toxic effects at high concentrations (>10 mg kg−1 and 1.0 mg kg−1, for ryegrass and lettuce, respectively) (Hartikainen et al., 2000; Xue et al., 2001). The positive growth responses of plants to Se added at small concentrations have been attributed to the antioxidative effect of Se, which counteracts oxidative stress (Seppänen et al., 2003). Selenium also has a demonstrated effect on seed germination. Carvalho et al. (2003) reported that elevated concentration of Se (>29 mg kg−1 soil) inhibited the growth and germination of S. lycopersicum and Raphanus sativus seeds. In contrast, priming of seeds with SeO32− increased the germination of Momordica charantia seeds (Chen and Sung, 2001). Turakainen (2007) demonstrated that Se supplementation increased the carbohydrate accumulation in the young upper leaves and in stolons of potato plants, although this was not correlated with increased production of photoassimilates as net photosynthesis did not differ among Se treatments (Turakainen, 2007). Bekheta et al. (2008) observed marked increases in growth and synthesis of photosynthetic pigments (chl a, b and carotenoids) in Gerbera jamesonii following Se supplementation (5–20 mg L−1). In our laboratory, we observed better phenotypic appearance of B. napus seedlings supplemented with exogenous Se (Figure 16.4).
16.6 Protective roles of silicon and selenium under abiotic stress
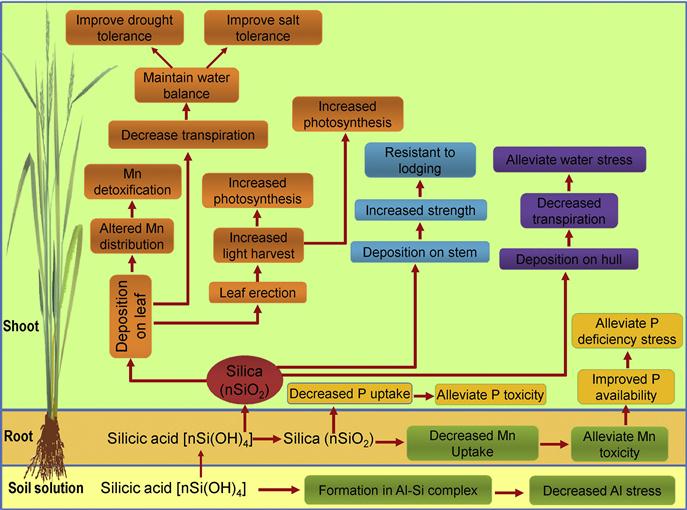
Silicon not only exerts beneficial effects on plant growth and productivity, it also plays a vital role in alleviating the damage caused by several abiotic stresses including salinity, drought, HT, chilling, UV radiation, HM toxicity, and nutrient imbalance (Liang et al., 2007; Ma and Yamaji, 2008; Table 16.4). The beneficial effects of Si on crops are depicted in Figure 16.5. These effects are achieved through the deposition of Si as SiO2•nH2O in leaves and stems of plants. Some of the effects of Si are also mediated by the interaction between Si(OH)4 and other elements such as Al. However, unlike other essential plant nutrients, the function of Si in plants might involve mechanical defense rather than physiological changes (Ma et al., 2001a). Like Si, Se has also been reported to play important protective roles in conferring tolerance to certain abiotic stresses like salinity, drought, HT, chilling, HMs, and UV irradiation (Hasanuzzaman et al., 2010, 2011b, 2012b; Table 16.5). One of the roles of Se in exerting beneficial effects on plant growth and stress tolerance is the enhancement of the antioxidant capacity (Hasanuzzaman et al., 2010, 2011, 2012b).
Table 16.4
Beneficial Effects of Exogenous Application of Si in Plants Grown under Various Abiotic Stresses
| Plant Species | Si Treatment | Stressors | Protective Effects | References |
| Saccharum officinarum | 2 mM Ca2SiO4 | Salinity, 100 mM NaCl | Reduced tissues Na+ concentration | Ashraf et al. (2010a) |
| Improved K+ uptake, K+/Na+ ratios, and Ca2+ content | ||||
| Increased shoot and root dry matter | ||||
| Vitis vinifera | 4 mM Si (Na2Si3O7) | Salinity, 20 mM NaCl | Reduced stomatal resistance | Soylemezoglu et al. (2009) |
| Reduced MDA and H2O2 contents | ||||
| Increased APX activity | ||||
| Brassica napus | 2 and 4 mM K2SiO3 | Salinity, 300 mM NaCl | Increased leaf area, leaf fresh weight, seed yield, and photosynthesis | Bybordi (2012) |
| Increased APX and NR activities | ||||
| Increased chl content | ||||
| Zea mays | 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8 and 3.2 mM Si(OH)4 | Salinity, 150 mM NaCl | Improved growth | Parveen and Ashraf (2010) |
| Increased net CO2 assimilation rate, gs, transpiration, and leaf substomatal CO2 concentration | ||||
| Spartina densiflora | 500 μM Na2SiO3 | Salinity, 171 and 680 mM NaCl | Improved growth associated with higher net photosynthetic rate, water-use efficiency, and gs | Mateos-Naranjo et al. (2013) |
| Reduced tissue Na+ content | ||||
| Triticum aestivum | Si 50 mg kg−1 and 150 mg kg−1 | Drought, 50%, 75%, and 100% of field capacity (FC) | Increased plant biomass, plant height, and spike weight | Ahmad et al. (2007) |
| Increased tissue Si concentration and uptake | ||||
| Zea mays | 0.8 mM Na2SiO3 | Drought, 50% water deficit | Increased dry mass, tissue nutrient content, and water-use efficiency | Janislampi (2012) |
| Reduced leaf wilting | ||||
| Glycine max | 1.70 mM Na2SiO3 | Drought, −0.5 MPa | Increased root and shoot dry matter and the ratio of root/shoot | Shen et al. (2010) |
| Increased leaf water potential and growth | ||||
| Increased net photosynthetic rate, gs, and chl content | ||||
| Decreased free Pro content, lipid peroxidation, and electrolyte leakage | ||||
| Sorghum bicolor | 200 mg L−1 Si | Drought, withholding irrigation | Increased net photosynthetic rate | Ahmed et al. (2011) |
| Decreased shoot to root ratio by increasing root growth | ||||
| Oryza sativa | 20, 40, 100 mg L−1 SiO2 | HT, 42°C | Reduced electrolyte leakage | Agarie et al. (1998) |
| Increased level of polysaccharide in cell wall with an increased ratio of polysaccharide in cell wall to total carbohydrate | ||||
| Oryza sativa | 1.5 mM Na2SiO3 | HT, 39°C | Increased number of pollen grains than those with higher diameter | Li et al. (2005) |
| Increased anther dehiscence percentage, pollination, and fertilization | ||||
| Cucumis sativus | 0.1 and 1 mM K2SiO3 | Chilling (15/8°C) | Reduced leaves withering | Liu et al. (2009) |
| Increased activities of SOD, GPX, APX, MDHAR, GR, and GSH | ||||
| Increased AsA content | ||||
| Decreased levels of H2O2, O2•−, and MDA | ||||
| Triticum aestivum | 0.1 and 1.0 mM K2SiO3 | Freezing, −5°C | Increased leaf water content | Liang et al. (2008) |
| Improved activities of antioxidant enzymes AsA, GSH, SOD, and CAT | ||||
| Reduced H2O2, free Pro and MDA content | ||||
| Citrus limon | 50, 150, and 250 mg L−1 K2SiO3 | Freezing, 0.5°C for 28 d | Increased phenolics and flavonoids concentration | Mditshwa et al. (2013) |
| Improved fruit quality | ||||
| Reduced chilling injury | ||||
| Oryza sativa | 1.25 and 2.5 mM Na2SiO3 | HM, 100 μM K2Cr2O7 | Increased seedling height, dry biomass, and soluble protein content | Zeng et al. (2011) |
| Reduced Cr uptake and translocation | ||||
| Improved antioxidant defense | ||||
| Brassica chinensis | 1.5 mM K2SiO3 | HM, 0.5, and 5 mg L−1 Cd | Increased shoot and root biomass | Song et al. (2009) |
| Decreased Cd uptake and root-to-shoot transport | ||||
| Increased SOD, CAT, APX, reduced MDA and H2O2 concentrations | ||||
| Cucumis sativus | 1 mM Na2SiO3 | HM, 100 μM CdCl2 | Reversed chlorosis, protected the chloroplast from disorganization | Feng et al. (2010) |
| Increased pigment contents, intercellular CO2 concentration, gs, and net photosynthetic rate | ||||
| Improved water-use efficiency | ||||
| Zea mays | 1 mM Si as Si(OH)4 | HM, 200, or 500 μM MnSO4 | Ameliorated chloroplast damage and photoinhibition | Doncheva et al. (2009) |
| Improved detoxification and compartmentation of Mn | ||||
| Picea abies | 0.2, 0.5, and 1.0 mM Si | HM, 0.2, 0.5, and 1.0 mM Al | Ameliorated adverse effects on cell wall thickening, degree of vacuolation, and the degeneration of mitochondria, Golgi bodies, endoplasmic reticulum, and nucleus | Prabagar et al. (2011) |
| Reduced cell death | ||||
| Vitis vinifera | 4 mM Na2Si3O7 | HM, 20 mg kg−1 H3BO3 | Reduced tissue B concentration | Soylemezoglu et al. (2009) |
| Increased activities of CAT and APX | ||||
| Reduced Pro, H2O2, and MDA content | ||||
| Oryza sativa | Si fertilizer (CaSiO3) @ 40 g m−2 | UV-B radiation 250 to 350 nm | Improved cell walls of sclerenchyma, vascular bundle sheath and metaxylem vessel cells, cellulose, non-cellulosic polysaccharides, lignin, and phenolic acids | Goto et al. (2003) |
| Glycine max | 1.70 mM Si | UV-B radiation 290–320 nm | Increased root and shoot dry weight and their ratio | Shen et al. (2010) |
| Increased net photosynthetic rate and gs | ||||
| Decreased H2O2 content |

Table 16.5
Beneficial Effects of Exogenous Application of Se in Plants Grown under Various Abiotic Stresses
| Plant Species | Se Treatment | Stressors | Protective Effects | References |
| Brassica napus | 25 μM Na2SeO4, 48 h | Salinity, 100 and 200 mM NaCl, 48 h | Increased AsA and GSH contents; GSH/GSSG ratio; and activities of APX, MDHAR, DHAR, GR, GST, GPX, and CAT | Hasanuzzaman et al. (2011) |
| Reduced levels of H2O2 and MDA | ||||
| Cucumis sativus | 5, 10, or 20 μM Na2SeO4, 11 d | Salinity, 50 mM NaCl, 11 d | Decreased content of Cl− in the shoot tissues | Hawrylak-Nowak (2009) |
| Increased Pro accumulation | ||||
| Enhanced antioxidant defense | ||||
| Decreased lipid peroxidation | ||||
| Sorrel (Rumex patientia × R. tianshanicus) | 1–5 μM Na2SeO4, 43 d | 100 mM NaCl, 43 d | Stimulated growth | Kong et al. (2005) |
| Increased activities of SOD and POD | ||||
| Increased accumulation of water-soluble sugar | ||||
| Trifolium repens | 5 μM Na2SeO4, 0–5 d | Drought, PEG-6000 (−1.0 MPa), 0–5 d | Decreased levels of H2O2, TBARS, DHA, and GSSG | Wang et al. (2011) |
| Increased the levels of AsA and GSH and AsA/DHA and GSH/GSSG ratios | ||||
| Improved the activities of MDHAR, DHAR, and GR | ||||
| Brassica napus | 15 and 30 g L−1 as Na2SeO3 | Drought, limited irrigation at early stem elongation | Increased plant height, number of pods in plant, number of seeds in pod, seed yield, biological yield, harvest index, and oil yield | Zahedi et al. (2009) |
| Triticum aestivum | Se (Na2SeO3) 0.5 mg kg−1, 20 d | Drought of 30% FC | Increased root activity | Xiaoqin et al. (2009a) |
| Increased Pro content | ||||
| Increased activities of POD and CAT | ||||
| Triticum aestivum | 1.0, 2.0, and 3.0 mg kg−1 Na2SeO3, 20 d | Water stress of 30% FC, 20 d | Increased root activity | Xiaoqin et al. (2009b) |
| Increased chl, carotenoids, and Pro content | ||||
| Increased activities of POD and CAT | ||||
| Decreased MDA content | ||||
| Fagopyrum esculentum | Solution of 1 g m−3 Na2SeO4 as foliar spray, 7–8 weeks | Reduction of water by 50%, 8 weeks | Improved gs, potential photochemical efficiency of PSII, respiratory potential | Tadina et al. (2007) |
| Increased yield | ||||
| Zea mays | 20 g ha−1 Na2SeO4 | Drought, withholding water | Enhanced activities of SOD | Sajedi et al. (2011) |
| Reduced MDA content | ||||
| Improved grain yield | ||||
| Triticum aestivum | Seed soaking in 0, 25, 50, 75, and 100 μM of Na2SeO4, 30 or 60 min | Drought, withholding water for 1 week | Increased root length and total biomass | Nawaz et al. (2013) |
| Increased stress tolerance index | ||||
| Increased total sugar content and total free amino acids | ||||
| Brassica napus | Pretreatment with 25 μM Se (Na2SeO4), 48 h | Drought, 10 and 20% PEG-6000, 48 h | Increased activities of APX, DHAR, MDHAR, GR, GST, GPX, and CAT | Hasanuzzaman and Fujita (2011b) |
| Decreased GSSG content, H2O2 | ||||
| Decreased lipid peroxidation | ||||
| Sorghum bicolor | Foliar spray of Na2SeO4 (75 mg L−1), 7–28 d | HT, 40/30°C, 7–28 d | Increased antioxidant enzyme | Djanaguiraman et al. (2010) |
| Decreased ROS levels and membrane damage | ||||
| Increased antioxidant defense | ||||
| Increased grain yield | ||||
| Cucumis sativus | 2.5, 5, 10, or 20 μM Na2SeO4 | 10°C/5°C for 24 h, for a further 24 h at 20°C/15°C, and then transferred to 25/20°C (re-warming) for 7 d | Improved shoot fresh weight | Hawrylak-Nowak et al. (2010) |
| Increase of Pro content | ||||
| Reduced MDA level | ||||
| Triticum aestivum | 0.5, 1.0, 2.0, 3.0 mg kg−1 Na2SeO3, 72 h | 4°C, 72 h | Increased biomass | Chu et al. (2010) |
| Increased chl, anthocyanins, flavonoids, and phenolic compounds | ||||
| Increased activities of POD and CAT | ||||
| Triticum aestivum | Seeds were soaked in Se (5 mg Se L−1), 5, 10 and 15 h | 3°C or 5°C for 14 d and allowed to recover at 22°C for 3 d | Enhanced growth | Akladious (2012) |
| Increased chl, anthocyanin, sugar, and Pro contents | ||||
| Enhanced antioxidant defense system | ||||
| Decreased membrane damage | ||||
| Sorghum bicolor | Seed soaking with Se (3 and 6 μM L−1 Na2SeO4), 6 h | LT, 4°C or 8°C for 7 d | Enhanced growth | Abbas (2012) |
| Increased levels of chl, anthocyanin, sugar, Pro, and AsA | ||||
| Increased enzymatic activities | ||||
| Diminished lipid peroxidation | ||||
| Brassica napus | Pretreatment with 50 and 100 μM Se (Na2SeO4), 24 h | HM, 0.5 and 1.0 mM CdCl2, 48 h | Increased AsA and GSH contents and GSH/GSSG ratio | Hasanuzzaman et al. (2012b) |
| Increased activities of APX, MDHAR, DHAR, GR, GPX, and CAT | ||||
| Reduced the MDA and H2O2 levels | ||||
| Brassica napus | 2 μM Na2SeO4, 14 d | HM, 400 and 600 μM, CdCl2, 14 d | Reduced oxidative stress by modulating SOD, CAT, APX, and GPX activities | Filek et al. (2008) |
| Prevented Cd-induced alteration of DNA methylation pattern | ||||
| Pteris vittata | 5, 10 μM of Na2SeO4, 5 and 10 d | HM, 150 or 300 μM of Na2HAsO4, 10 d | Improved antioxidant system including thiol and GSH levels | Srivastava et al. (2009) |
| Reduced As uptake | ||||
| Brassica oleracea | 1 mg L−1 Na2SeO3, 10 and 40 d | HM, 1 mg L−1 CdCl2, 40 d | Increased chl content | Pedrero et al. (2008) |
| Improved α-tocopherol level and reduced oxidative damage | ||||
| Lolium perenne | 1.0, 1.5, 2.0, 5.0, and 10 μM Na2SeO3, 20 d | HM, 0.2 mM AlCl3, 20 d | Improved POD activity | Cartes et al. (2010) |
| Reduced O2•− and lipid peroxidation | ||||
| Triticum aestivum | 1.0 and 2.0 mg Se kg−1, 8 h | UV-B, 40 W, 305 nm, 8 h | Increased root weight and root activity | Yao et al. (2009) |
| Increased flavonoids and Pro content | ||||
| Increased activities of POD and SOD | ||||
| Reduced MDA and O2•− | ||||
| Euglena gracilis | 10−7, 10−8, 10−9, and 10−10 M, Na2SeO3·5H2O, 40 min | UV-A, 320–400 nm, 40 min | Improved light-enhanced dark respiration and photosynthesis | Ekelund and Danilov (2001) |

16.6.1 Salinity
The role of silicon in alleviating salt stress in plants has been studied widely in many plant species including T. aestivum (Ahmad et al., 1992; Saqib et al., 2008; Hashemi et al., 2010), Z. mays (Liang et al., 2003), L. esculentum (Al-Aghabary et al., 2004), O. sativa (Gong et al., 2006), C. sativus (Zhu et al., 2004), B. napus (Hashemi et al., 2010), S. officinarum (Ashraf et al., 2010a,b), and H. vulgare (Liang et al., 2003). In Spartina densiflora, exogenous Si (500 μM) improved growth parameters, relative growth rate, and leaf elongation rate under salinity (680 mM NaCl), which was associated with higher net photosynthetic rate, greater water-use efficiency, and balanced nutrient concentrations (Mateos-Naranjo et al., 2013). Wheat plants supplemented with 0.25 and 0.50 mM Na2SiO3 and subsequently exposed to 100 mM NaCl showed amelioration of the negative effects of salinity by improvements in dry matter, chl content, and Pro content (Tuna et al., 2008). Shi et al. (2013) showed that Si (3 mM) could modulate various physiological processes that improved the growth of O. sativa under saline conditions (NaCl 50 mM). In canola seedlings (cv. Okapi), Si (2 and 4 mM K2SiO3) application increased leaf area, leaf fresh weight, and seed yield during salt stress conditions (100, 200, and 300 mM NaCl) (Bybordi, 2012).
Al-Aghabary et al. (2004) suggested that Si enhanced the photochemical efficiency of tomato plants under salinity stress. Silicon could also improve plant defense systems and help to detoxify ROS induced by salt stress; this helps to increase chl and enhances the photochemical efficiency of photosystem II (PSII). At 150 mM NaCl, exogenously applied Si levels (0.8, 1.6, and 2.8 mM) in the rooting medium improved net CO2 assimilation rate, gs, transpiration, and leaf substomatal CO2 in Z. mays, which was positively correlated with plant photosynthetic and growth attributes (Parveen and Ashraf, 2010). Barley root tonoplasts maintained integrity, stability, and functions following Si mediation of salt stress (Liang et al., 2005). In Spartina densiflora, Si (500 μM) application improved photosynthesis under 680 mM NaCl stress, which was confirmed by higher net photosynthetic rate and greater water-use efficiency. Si also exerted beneficial effects on the photochemical (PSII) apparatus and chl concentrations. Moreover, when compared to salt stress alone, Si supplementation of plants exposed to NaCl resulted in higher gs that maintained higher intercellular CO2. Silicon addition alleviated the adverse effects of salinity on quantum efficiency of PSII (Mateos-Naranjo et al., 2013). Addition of 3 mM silicate under 50 mM NaCl stress improved net photosynthetic rate, gs, and transpiration in rice (Shi et al., 2013).
Motomura et al. (2002) indicated that the undesirable effects of Na during salt stress could be reduced by increasing the K/Na ratio in plants. In salt-stressed H. vulgare, Si was reported to activate the root plasma membrane H-ATPase pump, which helped to increase K conductivity and to improve the K/Na ratio (Liang et al., 2006a). Silicon also reduced the concentration of Na and Cl in H. vulgare (Inal et al., 2009) and grapevine (Soylemezoglu et al., 2009). One of the ways to maintain optimum cytosolic K+/Na+ ratio is via restriction of Na+ influx into root cells or into the xylem stream (Chinnusamy et al., 2005). Silicate crystals deposited in the epidermal cells create a barrier to ion movement and their balance is managed (Romero-Aranda et al., 2006; Ashraf et al., 2010a,b). In the endodermis and rhizodermis, deposition and polymerization of Si block Na+ influx through the apoplasmic pathway, as documented in the roots of O. sativa (Yeo et al., 1999). Silicate crystals deposited in the epidermal cells create a barrier to water loss through cuticles, which improves water relations in plant tissue in salt-stressed plants (Romero-Aranda et al., 2006). Supplementation of Si (1.0 mM) to the salt solution (120 mM NaCl) increased plasma membrane H+-ATPase activity and restored membrane fluidity levels in H. vulgare (Liang et al., 2006a). The activity of the plasma membrane H-ATPase was improved by Si, which significantly increased K+ uptake in barley under salt stress (Liang et al., 2003). Shoot K+/Na+ ratios in sugarcane were increased by 150 to 266% following Si treatment of saline growth medium, which boosted its tolerance to a great extent (Ashraf et al., 2010a). According to Mateos-Naranjo et al. (2013), Si may ameliorate nutrient imbalances under salinity stress conditions by maintaining higher concentrations of minerals in the tissues, specifically P. The K/Na ratio of leaves of Spartina densiflora was greater in Si-treated plants and these plants also had higher levels of essential nutrients (Si, Al, Cu, Fe, K, and P) in their tissues (Mateos-Naranjo et al., 2013).
Silicon was effective in improving the activity of antioxidative enzymes in different plants under salinity stress. In B. napus, Si (2 and 4 mM) increased growth and yield by improving photosynthesis, chl content, and enzyme activities including ascorbate peroxidase (APX) and nitrate reductase (NR) under salt stress (100, 200, and 300 mM NaCl) (Bybordi, 2012). Silicon improved Vitis vinifera L. shoot growth and reduced oxidative damage caused by salinity, as indicated by reduced membrane damage and H2O2 levels with associated elevation of catalase (CAT) and superoxide dismutase (SOD) activities. Silicon also decreased Pro content (Soylemezoglu et al., 2009). Addition of Si (1.0 mM Si) to the salt treatment (120 mM NaCl) increased the glutathione (GSH) concentration by 20% in a salt-tolerant (Jian 4) H. vulgare cultivar compared to 50% in a salt-sensitive (Kepin No. 7) cultivar, which supposedly maintained better membrane properties (Liang et al., 2006b). Grapevine (V. vinifera rootstocks: 41 B, 1103 P) treated with Si (4 mM Si) significantly reduced MDA: by 20% in 41 B, by 23% in 1103 P, due to increased modulation of CAT, SOD, and APX enzyme activities and Pro content (Soylemezoglu et al., 2009).
Several research results have shown that Se at low concentration provided protection to different plant species against salt stress. Kong et al. (2005) reported that low concentrations (1–5 μM) of Se stimulated growth and enhanced antioxidant enzyme (SOD and POD) activities in leaves of sorrel (R. patientia × R. tianshanicus) seedlings under salt stress. In contrast, at higher concentrations (10–30 μM), Se showed fewer beneficial effects. In C. sativus leaves, Se treatments (5–10 μM) increased the growth, synthesis of photosynthetic pigments, and Pro levels under salt stress (Hawrylak-Nowak, 2009). In our study, we observed beneficial effects of exogenous Se (25 μM Na2SeO4) in B. napus seedlings exposed to salt stress (100 and 200 mM NaCl; Hasanuzzaman et al., 2011; Tables 16.6 and 16.7). Selenium treatment had a synergistic effect: in salt-stressed seedlings, it increased the AsA and GSH contents; and the activities of CAT, APX, MDHAR, DHAR, GR, GST, and GPX, which in turn reduced levels of H2O2 and MDA when compared to plants exposed to salt stress alone (Tables 16.6 and 16.7). The phenotypic appearance was also better in the Se-treated seedlings (Figure 16.6). These results suggested that exogenous application at low concentration rendered the plants more tolerant to salt stress-induced oxidative damage by enhancing their antioxidant defense systems (Hasanuzzaman et al., 2011).
Table 16.6
Malondialdehyde (MDA), H2O2, Reduced Ascorbate (AsA), Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Contents in Rapeseed Seedlings in response to Se under Salt Stress Conditions
| Treatment | MDA Content (nmol g−1 Fresh Weight) | H2O2 Content (nmol g−1 Fresh Weight) | AsA Content (nmol g−1 Fresh Weight) | GSH Content (nmol g−1 Fresh Weight) | GSSG Content (nmol g−1 Fresh Weight) |
| Control | 25.58 d | 3.70 d | 5210.56 a | 251.45 e | 7.17 d |
| Na1 | 43.34 b | 6.50 ab | 4064.22 b | 432.56 c | 13.48 b |
| Na2 | 58.68 a | 7.02 a | 3141.51 c | 358.68 d | 16.95 a |
| Se | 24.22 d | 3.95 d | 4890.04 a | 261.28 e | 8.14 cd |
| Se + Na1 | 34.71 c | 5.25 b | 4973.40 a | 568.79 a | 9.43 c |
| Se + Na2 | 43.29 b | 6.06 c | 3980.97 b | 479.86 b | 12.06 b |

Na1, Na2, Se, Se + Na1, and Se + Na2 indicate 100 mM NaCl, 200 mM NaCl, 25 μM Na2SeO4, 100 mM NaCl + Na2SeO4, 200 mM NaCl + Na2SeO4 treatment, respectively. Values with different letters are significantly different at P < 0.05 applying LSD test.
Table 16.7
Activities of Antioxidant Enzymes in Rapeseed Seedlings in Response to Se under Salt Stress Conditions
| Treatment | CAT Activity (μmol min−1 mg−1 Protein) | APX Activity (μmol min−1 mg−1 Protein) | MDHAR Activity (nmol min−1 mg−1 Protein) | DHAR Activity (nmol min−1 mg−1 Protein) | GR Activity (nmol min−1 mg−1 Protein) | GST Activity (nmol min−1 mg−1 Protein) | GPX Activity (nmol min−1 mg−1 Protein) |
| Control | 28.52 a | 0.387 d | 47.08 b | 153.36 b | 26.41 b | 23.85 d | 0.1628 d |
| Na1 | 17.49 cd | 0.509 bc | 42.29 b | 126.68 c | 29.86 b | 37.28 bc | 0.1915 bc |
| Na2 | 15.78 d | 0.535 b | 33.65 c | 99.65 d | 21.53 c | 33.38 c | 0.1502 d |
| Se | 25.10 b | 0.453 c | 46.59 b | 139.38 bc | 30.04 b | 25.35 d | 0.1667 cd |
| Se + Na1 | 26.79 ab | 0.656 a | 57.00 a | 171.56 a | 39.77 a | 44.67 a | 0.2317 a |
| Se + Na2 | 20.75 c | 0.628 a | 47.69 b | 142.72 bc | 30.56 b | 39.09 b | 0.2026 b |
Na1, Na2, Se, Se + Na1, and Se + Na2 indicate 100 mM NaCl, 200 mM NaCl, 25 μM Na2SeO4, 100 mM NaCl + Na2SeO4, 200 mM NaCl + Na2SeO4 treatment, respectively. Values with different letters are significantly different at P < 0.05 applying LSD test.

16.6.2 Drought
For drought tolerance, one of the most important and basic points is conservation of water within the plant body. Silicon could affect this strategy precisely and differently in various cases. Silicon deposition in cuticles or endodermal cells can reduce water loss. In stems and leaves, Si is deposited as hydrated silica (SiO2•nH2O) by following the evapotranspiration path (Sangster et al., 2001); thus, irrigation with Si reduces evapotranspiration (Gao et al., 2006). In O. sativa, Si deposits are 0.1 μM thick (Ma and Takahashi, 2002) and Si could reduce the transpiration rate by 30% (Ma, 2004). Polar monosilicic acid and polymerized Si(OH)4 accumulated mainly in the epidermal cell walls and formed H-bonds between H2O and SiO2·nH2O, which might prevent water loss (Liang et al., 2008). Kaya et al. (2006) found that Si (2 mM Na2SiO3) increased leaf relative water content (RWC) by 26.5% in water-stressed corn (50% of FC). In Z. mays, Si decreased leaf transpiration under drought, thereby improving leaf water status (Gao et al., 2006). Chen et al. (2011) found that applying 1.5 mM Si to rice under drought-stress conditions increased transpiration rate by 19% in a drought-susceptible line and 53% in a drought-resistant line.
Generally, compared with normal plants, drought-adapted plants have well-developed root systems through which they uptake even the slightest amount of water from deeper soils. In S. bicolor, Si fertilization significantly improved the drought tolerance by promoting the formation of a good root system and water uptake (Sonobe et al., 2011). Proline, a key solute for maintaining osmotic adjustment under drought stress, has been increased by Si application (Gunes et al., 2008; Crusciol et al., 2009). Crusciol et al. (2009) report that the Si increased potato plant Pro levels. Kaya et al. (2006) found that 2 mM Na2SiO3 decreased 43% of Pro content relative to non-treated drought plants. Pulz et al. (2008) found that the use of calcium and magnesium silicates markedly decreased stem lodging and increased potato plant height and tuber yield in drought conditions (−0.05 MPa water potential). Shen et al. (2010) observed a reduced Pro level in soybean plants subjected to PEG stress (−0.5 MPa). Sorghum plants treated with Si (200 ml L−1) showed reduced drought damage and increases in leaf area index of 65%, specific leaf weight of 12%, leaf dry weight of 164%, shoot dry weight of 80%, root dry weight of 70%, and total dry weight of 75% (Ahmed et al., 2011). Silicon treatment under water deficit conditions resulted in 18, 17, 20, and 30% increases in dry mass in Z. mays, T. aestivum, G. max, and O. sativa (Janislampi, 2012).
Besides the positive effects on plant growth and development under drought, Si was effective in improving a number of physiological processes including photosynthesis, gs, water relations, and nutrient translocation. Si (1.70 mM) addition to drought (−0.5 MPa) affected soybean increased net photosynthetic rate by 21% and gs by 38%, while reducing transpiration by 29% after a week of drought, when compared to drought stressed plants. Increases in photosynthesis are supposedly associated with increases in activities of photosynthetic enzymes, chl content, and anthocyanin content observed in response to added Si (Shen et al., 2010). Silicon addition to S. bicolor increased gs and transpiration rate and alleviated the photosynthetic reduction caused by drought (Hattori et al., 2005). In S. bicolor, drought caused significant damage to different growth parameters of roots, shoots, leaves, and overall growth, but addition of Si (200 ml L−1) alleviated these negative effects by increasing chl contents by 52%, transpiration rate by 80%, and net photosynthetic rate by 62%; together, these changes resulted in a 75% higher total dry weight (Ahmed et al., 2011). Under drought stress, Si upholds the nutrient balance when compared to drought-stressed plants without Si (Gunes et al., 2008). Calcium and magnesium silicate fertilizer application increased P and Mn content and reduced N content in drought-stressed potato (Pulz et al., 2008). Sonobe et al. (2010) found that 1.78 mM Si (SiO2) increased sorghum root amino acid content and decreased tissue Ca content under drought imposed for 23 days by 15% PEG-6000 (−0.6 MPa) (Janislampi, 2012).
Silicon can enhance the antioxidative machinery through effects on both its enzymatic and non-enzymatic components, thereby reducing drought-induced oxidative stress. Silicon enhanced the stability of lipids and protected the structural and functional integrity of cell membranes in rice plants against drought (Agarie et al., 1998). Supplementation of drought-stressed Cicer arietinum with Si significantly increased activity of some antioxidant enzymes (SOD, CAT, APX) in shoots as well as some non-enzymatic antioxidant activity, which reduced lipid peroxidation (MDA), lipoxygenase (LOX) activity, and Pro and H2O2 accumulation (Gunes et al., 2007a). Silicon (1.7 mM Si) application significantly reduced the H2O2 levels and thus reduced membrane damage as indicated by reduced lipid peroxidation and osmolyte leakage in drought (−0.5 MPa)-affected G. max plants. The probable reason for this alleviation of oxidative damage was modulation of SOD, CAT, and peroxidase (POD) activity induced by Si (Shen et al., 2010). Gong et al. (2005) also observed Si (2.11 mM Na2SiO3) increased SOD and CAT activity in wheat under drought, which alleviated oxidative damage and improved other indicators of physiological status. According to Gunes et al. (2008), Si applied to the soil reduced sunflower tissue H2O2 content.
Several plant studies that focused on the protective effects of Se in under drought stress indicated that the effects of Se are due to its ability to regulate the water status of plants under water deficit. Kuznetsov et al. (2003) reported that the addition of 0.1 or 0.25 mM Se caused a 2–6% increase in leaf water content, thereby increasing the drought resistance. The Se-induced improvement in leaf tissue water status was accompanied by a sharp (two- to four-fold) inhibition of stress-induced accumulation of Pro and a significant inhibition of POX activity (Kuznetsov et al., 2003). These results support the suggestion that Se exerts an antioxidant effect via a decreased concentration of intracellular ROS, which induces the de novo biosynthesis of Pro and POX production. The Se-induced effect on the content of intracellular water was also less pronounced at the optimal rate of water supply. In buckwheat (Fagopyrum esculentum), Tadina et al. (2007) observed that plants under water deficit exhibited significantly lower gs, while Se supplementation significantly improved gs. Selenium also improved the efficiency of PSII, which was attributed to the improvement in plant water status. Yao et al. (2009) suggested that low concentrations of Se (1.0, 2.0, and 3.0 mg Se kg−1) favored the growth of T. aestivum seedlings under drought. In addition, Se supplementation increased the Pro, carotenoid, and chl content and the activities of antioxidant enzymes (namely, POD and CAT), which in turn decreased the MDA content. In B. napus affected by late season drought stress, foliar application of Se had significant and additive effects on vegetative and reproductive parameters. It increased plant height and improved pollen survival and fertilization, which resulted in higher numbers of pods and seeds; ultimately, the seed yield, biological yield, harvest index, and oil yield improved (Zahedi et al., 2009). While studying T. aestivum seedlings under drought conditions, Xiaoqin et al. (2009a) observed 12, 15, and 12% increases in the fresh weight of the shoot, root, and total biomass, respectively, following supplementation with exogenous Se (0.5 mg kg−1). Selenium-induced drought tolerance was also indicated by the decrease in the shoot/root ratio and enhanced activities of POD and CAT (Xiaoqin et al., 2009a). In another study, Xiaoqin et al. (2009b) showed that exogenous Se (1.0 and 2.0 mg kg−1) increased root activity, Pro content, and POD and CAT activities and reduced the MDA content of T. aestivum seedlings. Selenium application at 1.0 and 2.0 mg kg−1 increased the chl (a+b) content by 26 and 32%, carotenoid content by 6 and 9%, and total biomass by 12 and 36%, respectively. Valadabadi et al. (2010) observed that drought stress markedly reduced the physiological and growth indices (namely, dry weight, leaf area index, relative growth rate, and crop growth rate) of B. napus, while application of Se (30 g L−1) improved those indices and mitigated the stress-induced damage.
Wang et al. (2011) examined the effect of Se (5 μM Na2SeO4) on the AsA–GSH cycle in Trifolium repens seedlings subjected to PEG-induced water deficit. They observed that Se application decreased the contents of H2O2, TBARS, DHA, and GSSG; increased the levels of GSH and AsA; and inhibited the decreases of AsA/DHA and GSH/GSSG ratios when compared to control plants. Selenium supplementation significantly increased the activities of MDHAR, DHAR, and GR. Among the enzymes, GR showed the highest increase in activity compared to DHAR and MDHAR. In our laboratory, we studied the beneficial role of Se pretreatment (25 μM Na2SeO4, 48 h) in B. napus seedlings under drought stress (10 and 20% PEG-6000) (Hasanuzzaman and Fujita, 2011b). The seedlings exposed to drought stress showed significant increases in GSH and GSSG content; however, the AsA content increased only under moderate stress (Table 16.8). The MDHAR and GR activity increased only under moderate stress (10% PEG). The activities of DHAR, GST, and GPX significantly increased at all levels of drought, while CAT activity decreased (Table 16.9). Drought stress resulted in a marked increase in the levels of H2O2 and MDA (Table 16.8). In contrast, Se-pretreated seedlings exposed to drought stress showed a rise in AsA and GSH content, and up-regulated activities of CAT, APX, DHAR, MDHAR, GR, GST, and GPX when compared with the drought-stressed seedlings without Se. In turn, the Se-treated seedlings showed a considerable decrease in the levels of H2O2 and MDA and considerable alleviation of oxidative stress (Tables 16.8 and 16.9; Hasanuzzaman and Fujita, 2011b). Nawaz et al. (2013) found beneficial role of Se priming in conferring drought stress tolerance. In their experiment, seeds of T. aestivum were soaked in distilled water or Na2SeO4 solutions (25, 50, 75, and 100 μM) for 30 or 60 min, followed by re-drying and subsequent sowing. Priming with Se significantly increased root length, stress tolerance index, and total biomass of germinated seedlings.
Table 16.8
Malondialdehyde (MDA), H2O2 Content, Reduced Ascorbate (AsA), Reduced Glutathione (GSH), and Oxidized Glutathione (GSSG) in Rapeseed Seedlings Induced by Se under Drought Stress Conditions
| Treatment | MDA Content (nmol g−1 Fresh Weight) | H2O2 Content (nmol g−1 Fresh Weight) | AsA Content (nmol g−1 Fresh Weight) | GSH Content (nmol g−1 Fresh Weight) | GSSG Content (nmol g−1 Fresh Weight) |
| Control | 21.16 c | 4.44 c | 4970.52 d | 283.31 d | 8.48 d |
| D1 | 30.77 b | 5.92 b | 5923.34 bc | 439.93 bc | 18.26 c |
| D2 | 42.06 a | 7.36 a | 5203.01 d | 414.97 c | 32.61 a |
| Se | 19.87 c | 4.69 c | 5476.88 cd | 269.73 d | 9.84 d |
| Se + D1 | 23.42 c | 5.00 c | 7006.01 a | 514.72 a | 17.61 c |
| Se + D2 | 29.46 b | 5.82 b | 6295.85 b | 467.83 b | 21.52 b |
D1, D2, Se, Se + D1, and Se + D2 indicate 10% PEG, 20% PEG, 25 μM Na2SeO4, 10% PEG + Na2SeO4, and 20% PEG + Na2SeO4 treatment, respectively. Values with different letters are significantly different at P < 0.05 applying LSD test.
Table 16.9
Activities of Antioxidant Enzymes in Rapeseed Seedlings Induced by Se under Drought Stress Conditions
| Treatment | CAT Activity (μmol min−1 mg−1 Protein) | APX Activity (μmol min−1 mg−1 Protein) | MDHAR Activity (nmol min−1 mg−1 Protein) | DHAR Activity (nmol min−1 mg−1 Protein) | GR Activity (nmol min−1 mg−1 Protein) | GST Activity (nmol min−1 mg−1 Protein) | GPX Activity (nmol min−1 mg−1 Protein) |
| Control | 24.98 a | 0.344 bc | 37.40 c | 201.73 e | 33.67 c | 45.17 c | 0.1177 d |
| D1 | 18.79 b | 0.366 b | 41.09 b | 233.38 cd | 41.54 b | 56.52 b | 0.1390 c |
| D2 | 16.02 b | 0.307 c | 34.42 c | 261.90 bc | 39.25 bc | 59.24 b | 0.1402 c |
| Se | 25.52 a | 0.343 bc | 37.48 c | 212.83 de | 36.52 bc | 49.98 c | 0.1306 c |
| Se + D1 | 25.32 a | 0.432 a | 45.30 a | 270.46 b | 54.48 a | 67.39 a | 0.1748 a |
| Se + D2 | 23.68 a | 0.453 a | 44.66 a | 314.15 a | 51.89 a | 69.73 a | 0.1595 b |

D1, D2, Se, Se + D1, and Se + D2 indicate 10% PEG, 20% PEG, Na2SeO4, 10% PEG + Na2SeO4, and 20% PEG + Na2SeO4 treatment, respectively. Values with different letters are significantly different at P < 0.05 applying LSD test.
16.6.3 High temperature
Reports on the effects of Si on HT stress are scare. However, some findings have indicated that Si exerts a beneficial effect under heat stress. Agarie et al. (1998) observed that relative to O. sativa plants subjected to heat stress alone, plants grown in 100 ppm SiO2 showed significantly reduced heat stress symptoms, determined by a 2.5-fold reduction in stress-induced electrolyte leakage. This result suggested that Si might have beneficial effects in maintaining thermal stability of lipids in cell membranes, thereby helping to maintain structural and functional integrity of cell membranes. Besides decreasing the electrolyte leakage, Si addition also increased the levels of polysaccharide in the cell walls, indicated by an increased ratio of cell wall polysaccharide to total carbohydrate. Thus, Si also helped to improve the cell wall structure (Agarie et al., 1998). In O. sativa, Si supplementation increased the number and diameter of pollen grains relative to heat treatment (39°C) alone (Li et al., 2005). Silicon application resulted in a dramatic increase in partial dehiscence (135% higher) of the anthers of rice flowers relative to heat stress alone. This consequently developed into a marked increase in fully dehiscence anthers, which was 111% higher in plants in the Si-fertilized treatment when compared to plants exposed to heat stress alone. Anther cracking rate was increased by 130% and the stigma pollination probability was increased by 66%, which enhanced the fertilization of rice flowers (Li et al., 2005). In Euphorbia pulcherrima Willd. “Ichiban,” Si-treated (50 mg L−1 Si, either as K2SiO3, Na2SiO3, or CaSiO3) plants were more tolerant to HT (35±1°C) compared to control plants (Son et al., 2011).
Very few reports have also appeared regarding the protective role of Se under HT. Djanaguiraman et al. (2010) investigated the effects of Se foliar spray (75 mg L−1) on leaf photosynthesis, membrane stability, antioxidant enzyme activity, grain yield, and yield components in S. bicolor plants grown under HT stress (40°C /30°C). They observed that HT stress decreased chl content, chl a fluorescence, photosynthetic rate, and activities of antioxidant enzymes. Heat stress also promoted oxidative stress and membrane damage, which ultimately affected the grain yield negatively. However, Se supplementation prevented membrane damage and improved the antioxidant defense system, which helped in attaining a higher grain yield. Overall, Se application significantly increased photosynthetic rate, gs, and transpiration rate by 13.2, 12.4, and 8.11%, respectively, compared with the unsprayed control. In addition, foliar spray of Se significantly reduced O2•− content, H2O2 content, MDA level, and membrane injury by 11.5, 35.4, 28.4, and 17.6%, respectively, compared with control plants. Moreover, Se application increased CAT activity in both control and HT stress; however, the maximum increase was observed in HT stress. Across the days of observation, Se application increased CAT and POX enzyme activity by 25.9 and 23.6%, respectively, under HT stress, and 9.2 and 3.3%, respectively, at the control temperature. As a result, an Se spray significantly increased filled seed weight and seed size by 26.3 and 10.7%, respectively, over the untreated controls (Djanaguiraman et al., 2010).
16.6.4 Low temperature
Many reports have indicated a protective role for Si under low temperature (LT) or chilling stress. Silicon at both 0.1 and 1.0 mM improved the shoot dry weight and water status of wheat leaves under a freezing stress of −5°C (Liang et al., 2008). Silicon-treated plants O. sativa, Z. mays, C. sativus, H. annuus, and Benincasa hispida grown hydroponically showed enhanced tolerance to LT (0–4°C) in terms of root nutrient absorbing capability and prevention of wilting (Liang et al., 2006b). Lemon fruits dipped in 0, 50, 150, and 250 mg L−1 solutions of K2SiO3 for 30 min showed no chilling injury symptoms when stored at −0.5°C for 28 d. Only 27% of the fruits showed chilling injury when dipped at 50 mg L−1 K2SiO3, whereas 97% of untreated fruits were injured and showed significant weight loss. Furthermore, treatment with 50 mg L−1 reduced the occurrence of chilling injury symptoms (Mditshwa et al., 2013).
The homeostasis of soluble sugar concentration is considered to be one of the osmosis-regulated conditions in plant tissues that confer tolerance in freezing-stressed plants. Compared with the non-Si-amended freezing treatment, the Si-amended freezing treatment decreased the content of soluble sugar significantly in wheat but enhanced freezing tolerance (Zhu et al., 2006; Liang et al., 2008). Silicon also maintained balanced Pro levels for conferring higher chilling tolerance in T. aestivum (Zhu et al., 2006; Liang et al., 2008). Under LT, Si improved the photosynthesis and its related parameters. In the wheat leaf, photosynthesis and water-use efficiency were significantly inhibited under freezing stress but these processes were restored by Si (Zhu et al., 2006). Treatment of T. aestivum with exogenous Si increased the net photosynthetic rate, gs, transpiration rate, chl content, and chl a/b ratio and decreased the soluble sugar content (Zhu et al., 2006).
Silicon might play a role in maintaining moisture (Gong et al., 2003; Tesfay et al., 2011). Silicon-treated avocado fruit maintained a higher water status compared with non-Si-treated plants during post-harvest storage at 5.5°C. In fruit peel, deposition of Si has been reported to cause impregnation of the intercellular parts, which might cover the fruit stomata with an Si layer and reduce fruit respiration (Hammash and El Assi, 2007); this would concomitantly result in decreased weight loss of avocado under chilling storage of 5.5°C. This Si deposition could also affect the membrane integrity and fruit firmness (Tesfay et al., 2011). Phenolic and flavonoids are correlated with chilling tolerance. Silicon in avocado could also adjust polyphenol oxidase (PPO) activity to regulate total phenolics and increase free polyphenol concentrations in the mesocarp, thereby improving chilling tolerance and thus fruit firmness. A reduced weight loss was observed when compared to untreated fruit following exposure to 5.5°C temperature (Tesfay et al., 2011). Uninjured fruits had higher phenolic and flavonoids contents compared to injured fruit. The chilling susceptible lemon fruit contained lower phenolic and flavonoid concentrations, with higher weight loss and electrolyte leakage, when compared with chilling-resistant lemons (Mditshwa et al., 2013). This electrolyte leakage reduction might be due to Si-induced improvement of antioxidant enzyme activities involved in plant stress defense systems (Crusciol et al., 2009; Keeping and Reynolds, 2009). Low temperatures—both chilling and freezing—induce the production of ROS (Xu et al., 2008), which damages membrane lipids and other cellular and subcellular organelles and leads to cell death (Molassiotis et al., 2006) and exogenous Si application was found beneficial for improving these effects.
Exogenous Si supplementation reduced the percentage of withered cucumber leaves under chilling (15/8°C) stress, which was supposedly correlated with Si deposition in leaves. The other reasons might be improved antioxidant activities including SOD, GPX, APX, MDHAR, GR, GSH, AsA, which would lower the levels of H2O2, O2•−, and MDA and reduce the leaf withering percentage (Liu et al., 2009). The addition of Si (1.0 mM) significantly suppressed H2O2, free Pro, and MDA under freezing stress (−5°C) in T. aestivum, which might be due to the observed increases in SOD and CAT activities of 39 and 59%, respectively, and also to the significant augmentation of non-enzymatic antioxidants (namely, AsA and GSH) (Liang et al., 2008). In avocado fruit, K2SiO3 application increased the CAT activity, reduced lipid peroxidation, and improved mesocarp electrical conductivity when fruit was stored at 5.5°C for 17 d (Tesfay et al., 2011).
Under LT stress, Se also exerted beneficial effects at low concentration. In C. sativus, Se-treated seedlings avoided the membrane damage caused by LT stress (10°C/5°C, 24 h), which was attributed to Se-induced higher Pro levels and higher shoot biomass (Hawrylak-Nowak et al., 2010). In contrast, the seedlings without Se supplementation showed higher membrane damage, as indicated by higher MDA content (Hawrylak-Nowak et al., 2010). Different levels of Se (0.5, 1.0, 2.0, 3.0 mg Se kg−1) were imposed on T. aestivum subjected to LT stress (4°C); the lower concentration of Se (1 mg Se kg−1) was found to be more beneficial as it enhanced the content of anthocyanins, flavonoids, and phenolic compounds and activities of antioxidant enzymes (namely, POD and CAT), which reduced oxidative damage and concomitant reduction in the levels of MDA and O2•− (Chu et al., 2010). Soaking of T. aestivum seeds with Se (5 mg L−1 for 5, 10, and 15 h) made the seedlings tolerant to LT stress (3 or 5°C). Se improved the shoot and root length, total biomass, and the contents of chl, Pro, and soluble sugars. Low temperature caused membrane damage, electrolyte leakage, and increased lipid peroxidation. Se supplementation, through enhancement of different antioxidant components like anthocyanin and AsA and promotion of enzymatic activities of CAT and PPO, significantly reduced oxidative damage. Furthermore, the Se-induced cold tolerance was confirmed by the appearance of novel protein bands (Akladious 2012). According to Djanaguiraman et al. (2005), Se was able to increase tolerance of G. max plants to LT stress by promoting antioxidant capacity and it improved growth and developmental processes of that plant under LT. Abbas (2012) found that SeO42− at low concentrations (3 and 6 mg L−1) enhanced growth, levels of chl, anthocyanin, sugar, Pro, and AsA, and enzymatic activities in S. bicolor seedlings subjected to LT stress. However, high levels of SeO42− (12 mg L−1) resulted in toxic effects. Low SeO42− (3 and 6 mg L−1) also diminished lipid peroxidation by enhancing the activities of APX and GPX.
16.6.5 Heavy metals
Among all the abiotic stresses, the effects of Si on heavy metal (HM) stress is probably the most widely studied. Silicon has been shown to reduce HM content within different plants subjected to HM stresses. Silicon-induced modification of HM binding properties of the cell walls may be an important mechanism for alleviation of HM toxicity (Ye et al., 2012). Proposed mechanisms include coprecipitation of Si and HMs in the cell wall, which would reduce the concentration of biologically active HMs (Neumann and Zur Nieden, 2001); reducing the cell wall porosity by depositing Si in endodermal cell walls; and limiting apoplasmic transport (Shi et al., 2005; da Cunha and do Nascimento, 2009). Silicon addition is documented as reducing the uptake and translocation of HMs (Shi et al., 2005; Nwugo and Huerta, 2008; Kaya et al., 2009).
When Si was applied under Cd stress, it improved the growth parameters in maize including fresh weight, dry weight, primary seminal root, and leaf area, when compared to Cd treatment alone. Silicon might have exerted these positive effects because it increased the cell wall extensibility. Again, by increasing apoplasmic concentration of Cd, Si would considerably decrease the symplasmic content in maize shoots, which might affect other physiological processes related to plant growth (Vaculík et al., 2012). Cucumis sativus L. plants treated with Si accumulated higher biomass against Cd stress. The reasons mentioned included the possibility that Si could improve photosynthetic rate by protecting the chloroplast from disorganization and by increasing pigment contents. Application of Si also alleviated the inhibition of photosynthesis and Fv/Fm (ratio of the variable fluorescence to the maximum fluorescence) and actual quantum efficiency of PSII under Cd stress. Moreover, Si alleviated the inhibition of the enzymes of N metabolism including nitrogen reductase (NR), glutamine synthetase (GS), glutamate synthase (GOGAT), and glutamate dehydrogenase (GDH) (Feng et al., 2010). In rice (O. sativa L.) under Cd (0, 2, and 4 μM) stress, Si (2 and 4 mM) supplementation increased shoot and root biomass by 125–171% and 100–106%, respectively (Zhang et al., 2008). Silicon can modify the development of root tissues, thereby reducing the uptake and concentration of Cd in other plant parts. Si can also bind toxic Cd ions in the apoplasmic space including the cell wall, or detoxify Cd by sequestering it in vacuoles (Vaculík et al., 2012). Si influenced the development of Casparian bands and suberin lamellae as well as vascular tissues in root, thereby helping to decrease symplasmic and increase apoplasmic concentrations of Cd in maize shoots. These apoplasmic barriers enhanced binding of Cd to the apoplasmic fraction in Z. mays shoots (Vaculík et al., 2012).
Silicon also increased growth parameters in T. aestivum and H. vulgare against boron (B) toxicity (Inal et al., 2009). For B detoxification, some assumptions were confirmed for decreases in B concentrations including the formation B–SiO44− complexes in the soil and reduction of B translocation from the roots to shoots (Gunes et al., 2007b). Silicon has ameliorative effects as it irreversibly precipitates as amorphous silica (SiO2•nH2O) in the cell walls and lumen, which reduces the HM-induced membrane damage (Epstein, 1999). In O. sativa, Si also decreased shoot Cd concentrations by 30–50% and Cd distribution ratio in shoots by 25.3–46%, compared to the treatment without Si supply (Zhang et al., 2008). The mitigation of Cd concentration and its toxicity by Si was also predicted in other plant species including T. aestivum (Rizwan et al., 2012), B. rapa (Song et al., 2009), A. hypogaea (Shi et al., 2010), and C. sativus (Feng et al., 2010). Silicon reduced the chromium (Cr) concentration in O. sativa shoots by 41% and in roots by 30% in plants grown in Cr (100 μM)-enriched medium; these effects were due to the dramatic decrease in the translocation factor by 16% (Zeng et al., 2011).
Kidd et al. (2001) suggested that the development of aluminum (Al) tolerance in Z. mays may occur by an enhanced exudation of phenolic compounds, which leads to complexation of Al. Zinc (Zn) could be detoxified by forming bonds with Si; this was found to be localized in the intercellular space, cytoplasm, nucleus, and vacuolar vesicles of leaf mesophyll cells (Neumann and Zur Nieden, 2001; da Cunha and do Nascimento, 2009). However, Al detoxification was reported to occur by reduction of the Al3+ content in symplasm and the formation of hard soluble aluminosilicates or hydroxyaluminosilicates in the apoplasmic space and outer epidermal cell walls (Hodson and Sangster, 1993; Hodson and Evans, 1995; Wang et al., 2004). Prabagar et al. (2011) found that the formation of aluminosilicate complexes in the cell wall and apoplasm of Norway spruce (Picea abies) plants create a significant barrier to Al penetration and Si-induced cell damage. Chromium-induced reductions of seedling height, dry biomass, and soluble protein content were alleviated by Si application, which was due mainly to reduced Cr uptake and enhanced antioxidant activity (Zeng et al., 2011). In Norway spruce, the presence of 1.0 mM Si reduced the cell death due to Al stress. Silicon reduced the maximum percentage of cell death by 50, 45, and 35% at Al concentrations of 0.2, 0.5, and 1.0 mM, respectively, after 48 h of exposure (Prabagar et al., 2011). In H. vulgare, 2 mM Si application enhanced plant growth relative to the control, and alleviated Cr (100 μM) toxicity, as reflected by a significant increase in growth and photosynthetic parameters, such as SPAD value, net photosynthetic rate, cellular CO2 concentration, gs, transpiration rate, and chl fluorescence efficiency (Ali et al., 2013). In Zea mays, Si treatment prior to exposure to excess manganese (Mn) caused increased thickness of the leaves and epidermal cells when compared to the control plants without Si pretreatment. Silicon-pretreated maize plants maintained higher concentrations of chl a and b and carotenoids when compared to plants exposed to Mn stress treatment alone and Si pretreatment also helped to maintain maximum quantum efficiency of PSII photochemistry, the actual efficiency of PSII electron transport, and the rate of photosynthetic O2 evolution (Doncheva et al., 2009).
Silicon can reduce HM-induced oxidative damage. Silicon improved growth characteristics of Vitis vinifera rootstock under B as indicated by decreases in stomatal resistance, lipid peroxidation, membrane damage, and MDA and H2O2 levels and increases in CAT and SOD activity (Soylemezoglu et al., 2009). Similarly, LOX (224%) and APX (61%) activity were increased in H. vulgare by Si (280 mg kg−1) under sodic-B toxicity conditions, which were correlated with decreased peroxidation of lipid (MDA) by 42%, H2O2 by 42%, and membrane permeability by 21% and increased Pro content (53%). Together, these changes could result in increases in fresh (35%) and dry weight (42%) in H. vulgare plants. Silicon also decreased the B (18%) and Na (8%) concentration (Gunes et al., 2007a). Silicon alleviates Cr toxicity in rice plants by inhibiting the uptake and translocation of Cr, together with enhanced activities of antioxidant enzymes, SOD, POD, CAT, and APX, which help to reduce TBARS content (Zeng et al., 2011). Alleviation mechanisms against Mn toxicity by Si include Mn adsorption by cell walls, active removal of excess Mn by soluble Si in the apoplasm, and enhanced enzymatic and non-enzymatic antioxidant levels (Horst et al., 1999; Iwasaki et al., 2002a,b; Rogalla and Römhled, 2002; Shi et al., 2005). Song et al. (2011) proved that Si improved antioxidant defense capacity and membrane integrity to prevent Zn toxicity in O. sativa. Silicon promoted the expression of free radical metabolizing enzymes, which amplified plant biomass parameters and decreased copper (Cu) uptake and leaf chlorosis (Nowakowski and Nowakowska, 1997; Li et al., 2008; Khandekar and Leisner, 2011).
Selenium has been documented to reduce metal toxicity in several research studies. The modes of action were varied and are still unclear; however, some suggested reasons included improvement of the antioxidant defense system, reduction of metal uptake, formation of non-toxic Se–metal complexes, and phytochelatin activity (Vorobets and Mykiyevich, 2000; Sun et al., 2010). Moreover, Se is effective at sustaining physiological activities, growth, and developmental processes even in HM toxic environments (Pedrero et al., 2008; Cartes et al., 2010). In B. oleracea, Cd phytotoxicity resulted in elevated MDA level and decreased photosynthetic pigment and tocopherol concentrations, but Se treatment effectively alleviated these adverse effects (Pedrero et al., 2008). In B. napus, Se (2 μM) conferred tolerance to Cd (400 and 600 μM) stress by reducing lipid unsaturation and peroxidation, modulating the activity of antioxidative enzymes (SOD, CAT, APX, GPX), and preventing Cd-induced changes in the DNA methylation pattern (Filek et al., 2008). Sun et al. (2010) summarized the mechanisms for the positive role of Se on Cd toxicity as: (1) removal of Cd from metabolically active cellular sites, (2) induction of Se to scavenge the Cd-induced ROS generated, and (3) the regulation phytochelatin synthesis-associated enzymes induced by Se. These three actions mitigated the effects of Cd on A. sativum. In our recent study, we observed that rapeseed seedlings grown under Cd stress (0.5 and 1.0 mM CdCl2) showed substantial increases in MDA and H2O2 levels. The AsA content of the seedlings decreased significantly upon exposure to Cd stress. The amount of GSH increased only at 0.5 mM CdCl2, while GSSG increased at any level of Cd with concomitant decreases in the GSH/GSSG ratio. The activities of antioxidant enzymes also reduced under Cd stress. Importantly, Se-pretreated seedlings exposed to Cd showed increases in the AsA and GSH contents; GSH/GSSG ratio; and the activities of APX, MDHAR, DHAR, GR, GPX, and CAT. However, in most of the cases, pretreatment with 50 μM Se showed better results compared to 100 μM Se. These results indicated that the exogenous application of Se at low concentration increased the tolerance of the plants to Cd-induced oxidative damage by enhancing their antioxidant defenses. The phenotypes of the Se-supplemented seedlings were also superior to the seedlings subjected to Cd stress without Se (Figure 16.7).

The effect of Se (5 or 10 μM of Na2SeO4) on Pteris vittata plant was studied under arsenic (As, 150 or 300 μM of Na2HAsO4) stress. Selenium was effective at increasing the levels of thiols and GSH (increased by 24%) and decreasing lipid peroxidation (by 26–42%) in a dose-dependent manner (Srivastava et al., 2009). In ryegrass, Cartes et al. (2010) proposed two probable mechanisms for Se-induced alleviation of Al toxicity: (1) the improved dismutation of O2•− to H2O2, and (2) the activation of antioxidant enzymes (POD, a H2O2 scavenger). In addition, a low concentration of Se (2 μM) also improved root growth and decreased lipid peroxidation in plants grown under Al stress (0.2 mM Al). In contrast, a higher concentration of Se (>2 μM) caused phytotoxicity. Ribeiro et al. (2011) observed that the growth of seedlings of Stylosanthes humilis was inhibited by Al3+, while elongation was recovered with Na2SeO4 at 1.0 μM. Methyl viologen and H2O2, which are ROS-generating compounds, also inhibited seedling elongation and again growth was restored by SeO42−. Selenium-induced enhanced oxidizing ability, resistance to oxidation, and reduced membrane lipid peroxidation of rice roots under iron (Fe) stress was reported by Qi et al. (2004) and Peng et al. (2002).
16.6.6 UV radiation
Although studies are scarce, Si has been proved to be beneficial for improving different growth, developmental, physiological, and biochemical parameters in different plants growing under ultraviolet radiation stress. In G. max seedling, Si (1.70 mM) application increased photosynthesis by 21.0 and 21.5% under UV-B radiation (UV 290–320 nm) and the combination of UV-B and drought treatment, respectively, with increases seen in shoot dry matter of 23%, root dry matter of 42%, and root-to-shoot ratio of 16%. Si could also improve the relative water content (RWC) by 19% after a week of Si application in a combined treatment of UV and drought (Shen et al., 2010). In O. sativa, Si increased plant height and leaf dry matter relative to treatment without Si. More significant results were obtained for total leaf area and specific leaf area both at the boot and milk ripe stage. The increased values for these two parameters were 208 and 113% for the boot stage and those for the milk stage were 51 and 25%, respectively (Goto et al., 2003). Exogenous Si application could modulate the plant’s internal Si and uptake of other nutrients and their contents in soybean plant. Increases in leaf N and P of 9 and 16%, respectively, and decreases in leaf Mg content of 9% and Ca of 24% were seen in rice plants under UV stress. UV-B radiation also increased the allocation of P, potassium (K), and Ca to roots compared with stem and leaves, presumably to ensure sustained nutrient uptake under stress. Silicon application improved the uptake of P and Mg by 11%, which favored the partitioning of dry mass to shoots under UV-B radiation and the allocation of tissue P and Ca to roots (Shen et al., 2009). Gao et al. (2003) also reported that P, Ca, Na, and Si uptake was increased in rice leaves by exogenous Si application under UV stress. The Si-treated rice plants also showed lower cinnamyl alcohol dehydrogenase (CAD) levels in the cell walls of sclerenchyma, vascular bundle sheath, and metaxylem vessel cells, which might the reason why Si-treated rice plants have a lower UV absorbance of around 280 and 320 nm in the leaf blades compared with plants without Si treatment (Goto et al., 2003). After a week of Si application in UV-B stress conditions, net photosynthetic rate in G. max seedlings was significantly increased by 18%, which might be associated with increased gs (by 15%), and transpiration (by 4%) and significant increases in photosynthetic pigments like chl and anthocyanin in G. max seedlings grown under UV-B stress (Shen et al., 2010).
Within the plant, a protective accumulation of anthocyanins and other UV-absorbing compounds like flavonoids and total phenols occurs in response to UV radiation; these compounds may act as solar screens in the leaf (Alexieva et al., 2001; Li et al., 2004; Shen et al., 2010). Phenolics and flavonoids are effective in reducing UV radiation-induced damage as they prevent the accumulation of ROS, as was seen in Arabidopsis (Bieza and Lois, 2001). In the rice leaf surface, more specifically in the epidermal cells, phenolic compounds are effective protectors against UV-induced damage as they absorb high amounts of UV radiation. Some insoluble compounds of Si are deposited in the leaf epidermis and act as promoters of constitutive phenols (Goto et al., 2003; Li et al., 2004). UV radiation promoted Si deposition in rice leaves (IRRI, 1991). In the rice leaf epidermis, soluble and insoluble UV-absorbing compounds were increased by 21 and 67%, respectively, due to Si treatment (compared to Si-untreated leaves). Moreover, the cell walls and cell lumina of the epidermis contained more Si-rich insoluble UV-absorbing compounds. This led Li et al. (2004) to conclude that Si increased phenolic compounds in the epidermis, which rendered UV tolerance as evidenced by healthy leaves free from the brown spots and strips of UV damage symptoms present in leaves not treated with Si. At the mature stage, rice glumes consist of about 20% silica on a dry matter basis (Okuda and Takahashi, 1961), which was considered as vital for protecting the kernel from severe damage from UV radiation (Ebata et al., 1989). In G. max, UV-B caused substantial membrane damage, as assessed by lipid peroxidation and osmolyte leakage, but Si application significantly reduced the H2O2, lipid peroxidation, and membrane damage, and ultimately reduced the leakage of electrolyte. This might have been due to modulation of antioxidant enzymes, phenols, and anthocyanins in relation to other physiological parameters (Shen et al., 2010). Silicate application can increase silica deposits and decrease CAD activity and ferulic and p-coumaric acid contents in rice plants, and these responses are closely related to alterations in the UV defense system (Goto et al., 2003).
Selenium improves plant growth and survival under UV radiation, as reported in several studies. Valkama et al. (2003) investigated the possible ameliorative effects of Se addition (0.1 and 1 mg kg−1) to soil on the detrimental effects of enhanced UV-B radiation in strawberry (Fragaria × ananassa) and barley (H. vulgare) plants. In contrast, higher concentrations of Se had no protective effects and even increased the sensitivity to UV-B radiation. In buckwheat, Se improved the effective quantum yield of PSII under UV-B radiation; thus, plant height and biomass production were increased (Breznik et al., 2005). Selenium might play positive roles in recovery of the photosynthetic and respiratory machinery, as documented in Euglena gracilis. During exposure to different UV radiation (UV-A, 320–400 nm, of 1.02 W m−2 plus UV-B, 280–320 nm, of 0.73 W m−2), no effect was seen for Se (10−7, 10−8, 10−9, and 10−10 M, Na2SeO3.5H2O) in E. gracilis. However, after a 24 h recovery period, Se increased photosynthesis and light-enhanced dark respiration by its protective effects against photodamage and oxidative stress (Ekelund and Danilov, 2001). Se (0.01 and 0.05 mg kg−1 soil) improved the antioxidative capacity, protected chloroplast enzymes, and increased shoot yield in Lactuca sativa under combined UV-B and UV-C stress (Pennanen et al., 2002). Significant increases in the activities of POD and SOD, together with reduced MDA and O2•− levels, were documented in T. aestivum under UV-B radiation. Selenium also increased root activity and flavonoid and Pro contents in this plant (Yao et al., 2010a,b). Selenium also showed positive effects and increased yield in ryegrass (Hartikainen et al., 2000), lettuce (Xue et al., 2001), potato (Turakainen et al., 2004), and pumpkin (Germ et al., 2005) exposed to UV-B radiation.
16.7 Conclusion and future prospects
The mechanisms underlying the responses and tolerance of plants to different abiotic stresses are still unclear, and require further critical physiological and molecular studies. Exploring the most effective and easiest ways to overcome stressors is one of the emerging tasks for plant scientists. The published literature indicates that Si and Se have vital roles in a wide spectrum of physiological processes in plants. In particular, they have prominent roles in the protection of plants from abiotic stress-induced damage. Although much research has been published on the effects of Si on plants under abiotic stress, the underlying physiological mechanisms by which Si could protect plants from stressful conditions are elusive. The potential of exogenous application of Si has gained significant attention in recent times, and numerous plant studies have revealed its protective roles in oxidative stress tolerance. However, the exact dose of application and methods of application are still under study. Like Si, the facts about Se are also intriguing, enigmatic, and challenging (even capricious) for researchers. Although a number of reports hint at a protective role for Se under abiotic stress conditions, research conducted to date on the physiological role of Se under stressful condition is scarce. Controversy remains regarding the question of whether Se is an essential plant micronutrient. On a cautionary note, the appropriate concentration of exogenous Se is still a matter of research. Complete elucidation of the role of Se as well as detailed protective mechanisms would be helpful for developing stress tolerance in plants. Despite the widespread occurrence of Se deficiency globally, Se toxicity (selenosis) is a problem in some areas. Some soils and mineral deposits are naturally Se rich, and exploitation of these seleniferous soils or fossil fuels can lead to toxic accumulation of Se in the environment. Selenium contamination of sediments, soils, and drainage water is a particular issue in arid seleniferous areas with intensive crop irrigation. Effective enrichment of agricultural crops with Se via soil using Se-enriched fertilizers can be challenging due to varying soil Se concentrations, soil types, soil redox potentials, soil pH, microbiological activity, etc. Furthermore, the high cost of Se fertilizer, in combination with the modest incorporation rate, should be considered. However, the appropriate application of exogenous Se can be an effective protectant for plants for combating abiotic stress. Notably, findings from experimental field research studies are still scarce. Field studies that confirm the ability of exogenous applications of both Si and Se to mitigate abiotic stress are likely to prop up their extended practical application to crop plants.
Acknowledgments
We wish to thank Md. Mahabub Alam, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan, for providing several supporting articles and suggestions for improving the manuscript. We are also highly thankful to Mr. Anisur Rahman and Mr. Md. Hasanuzzaman, Department of Agronomy, Sher-e-Bangla Agricultural University, Dhaka-1207, Bangladesh, for their critical reading of the manuscript draft.