Nutritional Stress in Dystrophic Savanna Soils of the Orinoco Basin
Biological Responses to Low Nitrogen and Phosphorus Availabilities
Danilo López-Hernández, Rosa Mary Hernández-Hernández, Ismael Hernández-Valencia and Marcia Toro
The llanos of Venezuela and Colombia have an extension of about 500,000 km2 and is the largest region of savannas located to the north of South America. These savannas have a high mean annual temperature (about 27°C) and relative high mean annual precipitation (over 900 mm), which are responsible for an active rate of chemical weathering and for the development of extensive well-weathered soils with strong P-fixing capacities. In the case of natural savannas (e.g., without a significant cattle raising activity), the main management tool is the regular burning of vegetation. Although fire frequency can be very variable, its influence on carbon and also on nutrient cycling (particularly N cycling) can be remarkable. The information presented suggests that the plants of the native savannas under frequent fires and strong seasonality are limited by N and/or P, yet the rates of many biological processes in this biome continue to be significant. The answer to this apparent paradox lies in the existence of the adaptations that the organisms of this biome have evolved to effectively overcome such low N and P availabilities. These strategies may be of two categories: (1) enhancing N and P conservation and efficiency of nutrient use and (2) enhancing N fixation and P incorporation and uptake. The former include nutrient translocation from old leaves to new leaves and roots, as an effective mechanism of element conservation together with the role of microbial biomass and pedo-fauna activities to generate nutrient “hotspots.” Within the latter, particular emphasis is given to the rhizosphere mycorrhizal environment and to microorganisms’ production of organic acids and biological N fixation. The aim of this chapter is to present information on the environment in which the well-weathered soils of the Orinoco savannas were developed, the main inputs–outputs of N and P in savannas, and how biological processes in those ecosystems can ameliorate N and P availabilities. Microorganisms have great potential to contribute to improving local fertility and so they might have potential use to generate biofertilizers.
Keywords
dystrophic soils; nutrient stress; fire; soil biota; N-fixing organisms; organic acids
15.1 Introduction
Savannas represent one of the largest extensions of land in the world. In South America, savannas occupy an area of more than 269×106 ha extended in Brazil (204×106 ha), Colombia (23×106 ha), Venezuela (25×106 ha), Guyana (4×106 ha), and Bolivia (13×106 ha) (Rippstein et al., 2001), thus giving to those biomes a high potential for agricultural expansion. In its natural form (with little human intervention), savannas are characterized by associations of herbaceous vegetation with the presence of scattered trees and with patterns in the seasonality of water availability defined by a marked dry season.
Within the areas occupied by savannas in South America, the most important ones are the Brazilian Cerrado and the savannas between Colombia and Venezuela, locally known as the Orinocos llanos. The llanos, with an extension of about 500,000 km2, is the largest region of savannas located to the north of South America (López-Hernández and Hernández-Valencia, 2008). In Venezuelan and Colombian llanos (Figure 15.1), we can find well-drained and flooded, eutrophic and oligotrophic savannas (Huber, 2007); however, the most extended are well-drained and dystrophic savannas also known as Trachypogon savannas due to the dominance of species of this genus (Ramia, 1967).
It is a well-known fact that not all the surface occupied by llanos is suitable for cropping, as soils suffer from physical and chemical limitations, which together with the marked climatic seasonality reduces the spectrum of possibilities for agricultural production (López-Hernández et al., 2005). Moreover, recently implanted crops in neotropical savannas, as in other regions of the tropics, are also subjected immediately to biological stress (pests) induced by fungi, bacteria, and endemic insects, which cohabit in the environment and which also compromise agricultural production (López-Hernández et al., 2005). Notwithstanding the above, the large tracts of savannas in South America are the main alternative to expansion into tropical areas of greater ecological fragility, e.g., slopes of mountains and the Amazon rainforest (López-Hernández, 1995).
Human presence in the savannas of South America is relatively recent, between 7000 and 9000 years before present (Berrio et al., 2002). After the encounter of cultures and for centuries, the activities of the sparse human populations settled in the savannas of the neotropics did not have a very marked effect on the landscape (López-Hernández, 1995). It has been pointed out that indigenous communities practice controlled burning; so, their knowledge is important to pursue strategies of prescriptive burning to control fuel loads (Rodriguez et al., 2009; Sleto and Rodríguez, 2013) and reduce carbon (C) emissions to the atmosphere. However, natural fires existed long before the emergence of humans and they are inextricably linked to the presence of savannas, as it has been recently reviewed by Beerling and Osborne (2006).
In the case of natural savannas (e.g., without significant cattle raising activity), particularly referred in this chapter, the regular burning of vegetation is a management tool. In this case, although fire frequency can be very variable (every 1 to 5 years) according to the type of savannas and to human uses, its influence on C and also on nutrient cycling (particularly nitrogen (N) cycling) can be remarkable indeed (López-Hernández and Hernández-Valencia, 2008). When savanna burns, a significant decline in the effective input of organic matter and nutrients to the soil is induced, as a result, N can be limited to plant growing (Hernández-Valencia and López-Hernández, 2002). In tropical savannas, phosphorus (P) also has been recognized as one of the most limited nutrients for plant production because of soil acidity and the high P adsorption induced by the reactivity of phosphate with iron (Fe) and aluminum (Al) sesquioxides, which are the final products of deep weathering (López-Hernández, 1977). Although important amounts of fertilizer have been added to some savanna soils as a management practice to overcome P adsorption, most of the added fertilizer is irreversibly fixed, so from an economical point of view the P fertilizer practice in the savannas is in general highly inefficient (Oberson et al., 1999). Thus, N and P cycling in savanna ecosystems is especially sensitive to frequent fires resulting in the loss of N and P.
For this reason, much research with emphasis in nutritional aspects has been carried out in savannas to characterize their ecological features and to assess the impact of different land uses. In this review, we would like to present information related to: (1) the environment in which the well-weathered soils of Orinoco savannas were developed, (2) the main inputs/outputs of N and P in well-drained savannas, and (3) how some biological processes in savanna ecosystems can ameliorate N and P availabilities for native plants.
15.2 Main environmental features of the savannas of the Orinoco basin
15.2.1 Climate in the Orinoco basin
Venezuela is under the influence of the Intertropical Convergence Zone (ITCZ) and consequently most of the climatological records for the meteorological stations located in the llanos region show a seasonal pattern for precipitation and temperature. Monthly mean temperature is isohyperthermic and ranges between 24°C and 29°C, with minimum values from December to February. According to the amount of precipitation, two seasons are recognized: (1) a dry season of 5–7 months (November to May) and (2) a rainy season (May to November) that concentrates at least 65% of annual total precipitation (Walter and Medina, 1971). The total annual precipitation ranges from 900 to 2041 mm with a tendency to increase toward the southern part of the Venezuelan territory, the Andean piedemont and the western plains (Duno de Stefano and Huber, 2007). The potential evapotranspiration is usually higher than the precipitation, with the predominance of semi-arid conditions, which favors the presence of xeromorphic species.
15.2.1.1 General characteristic of the soils of the Orinoco basin
Neotropical savannas are usually related to acid soils with high P-fixation capacity and consequently low P availability, low organic matter, nitrogen and exchangeable base contents (exchangeable bases <5 cmol kg−1 soil), but high Al saturation (López-Hernández and Hernández-Valencia, 2008). An analysis of the main chemical and physical characteristics of different savanna soils in Venezuela confirms this assumption (Table 15.1). Soil pH ranged between 4.8 and 6.3 units, whereas the highest values recorded for nutrients were 5.3 mg P kg−1 for available P, 1800 mg N kg−1 for total N, 1.7% for organic C, and 1.2 cmol exchangeable bases kg−1. The dystrophic nature of soils is a consequence of parent materials with low nutrient contents and/or strong weathering, which results in accumulations of resistant primary minerals (e.g., quartz), clays with low exchange activities, and the presence of amorphous and crystalline Fe and Al sesquioxides with strong P-fixation capacities.
Table 15.1
Soil Chemical and Physical Characteristics (0–30 cm) of Selected Trachypogon Savannas from Venezuela
| Location | pH | Available P (mg kg−1) | Total N (mg kg−1) | Total C (%) | Σ Exchangeable Bases (cmol kg−1) | Exchangeable Aluminum (cmol kg−1) | Sand (%) | Silt (%) | Clay (%) |
| Calabozo | 5.8 | 2.9 | 503 | 0.8 | 0.3 | 0.8 | 62.1 | 12.6 | 25.3 |
| Puerto Ayacucho | 5.5 | 4.8 | 393 | 0.6 | 1.2 | – | 93.0 | 2.8 | 4.2 |
| Uverito | 4.5 | 0.9 | 227 | 0.3 | 0.4 | 0.4 | 94.8 | 2.0 | 3.2 |
| La Iguana | 5.2 | 4.0 | 320 | 0.7 | 1.1 | 0.9 | 93.3 | 0.1 | 6.6 |
| Cabruta | 5.6 | 2.0 | 40 | 0.1 | 0.3 | 0.3 | 96.0 | 6.4 | 3.5 |
| Anzoátegui-A | 5.2 | 2.4 | 1100 | 0.8 | 0.5 | 0.5 | 61.5 | 15.0 | 23.5 |
| Anzoátegui-B | 5.3 | 5.3 | 1800 | 0.8 | 0.4 | 0.2 | 73.0 | 12.5 | 14.5 |
| Anzoátegui-C | 5.6 | 4.2 | 1100 | 0.8 | 0.3 | 0.1 | 71.5 | 15.0 | 13.5 |
| Anzoátegui-D | 5.4 | 3.2 | 1200 | 0.6 | 0.4 | 0.1 | 77.5 | 12.5 | 10.0 |
| Anzoátegui-E | 5.9 | 3.5 | 600 | 0.2 | 0.5 | 0.1 | 75.0 | 2.5 | 22.5 |
| Bolívar | 6.3 | 2.9 | 700 | 0.6 | 0.5 | 0.1 | 72.5 | 5.0 | 22.5 |
| Cojedes-A | 5.2 | 4.8 | 1100 | 1.2 | 0.6 | 0.5 | 56.8 | 10.5 | 32.7 |
| Cojedes-B | 5.1 | 2.9 | 1300 | 0.9 | 0.3 | 0.9 | 43.0 | 13.2 | 43.7 |
| Cojedes-C | 5.5 | 5.0 | 1400 | 1.7 | 0.6 | 0.7 | 55.8 | 11.5 | 30.5 |

Source: Calabozo (López-Hernández and Hernández-Valencia, 2008), Puerto Ayacucho (Sánchez et al., 1985), La Iguana (1988), Uverito (Hernández-Valencia and Bautis, 2005), Cabruta (Susach, 1984), Anzoátegui, Bolívar, Cojedes (Medina and Bilbao, 1991).
Soil texture in Trachypogon savanna is usually dominated by sands (43–96%) (Table 15.1) and coarse gravels but an increase in clay content could be found in the subsurface due to the accumulation of clays and the formation of argilic, kandic, oxic, or cambic horizons. On the other hand, a hardpan of laterite or plinthite could be present in soils with alternating periods of flooding and drought. Precipitation is seasonal and has an influence on soil water content with periods of excessive wet and eventually anaerobic conditions, alternated with periods where the soil becomes dry and below the permanent wilting point. Anaerobic conditions are usually found in subsuperficial layers where water drainage is poor due to high clay and silt content, or the presence of a lateritic layer or concretions (López-Hernández and Hernández-Valencia, 2011).
According to the Soil Taxonomy classification system (USDA, 2010), the soils of Trachypogon savannas are mainly identified as oxisols, ultisols, in those with argilic, oxic, kandic, and cambic subsurface horizons, with or without a lateritic hardpan, while entisols are related to sandy soils from alluvial sediments or eolian deposits (e.g., dunes). For the World Reference Base for Soil Resource (IUSS, 2007), soil types are mainly identified as ferralsols, acrisols, and lixisols.
Physical and chemical features of savanna soils have an influence on plant physiognomy and, consequently, on the use of water, nutrients, and, finally, on primary production. Savannas are dominated by grasses. Most herbaceous species have an intensive superficial root system, whereas trees have extensive roots able to exploit water and nutrients from deeper soil layers (Medina, 1993). February et al. (2013) found for a broadleaved woody savanna in Australia that regardless of the type of grass, both tree and grass roots are concentrated in the top 20 cm of the soil. While trees have greater root production and contribute with a finer root biomass, grass roots contribute with a disproportional amount of N and C to the soil relative to total root biomass. They postulate that grasses maintain soil nutrient pools and provide biomass for regular fires that prevent forest trees from establishing, while savanna trees are important for increasing soil N content, cycling, and mineralization rates.
Open savannas are related to superficial hardpans, which interfere with root tree development, or with flooded soils. On the other hand, tree density increases when the hardpan is absent or deeper and/or water availability increases in the tree root zone, but flooding is not present (Medina, 1993). A similar relationship between physiognomy and soil fertility has also been observed in Brazilian cerrado where open savannas are associated with less fertile soils, especially those with low P and calcium (Ca) availabilities and high Al percent saturation (Lopes and Cox, 1977). Both physical (fast drainage, presence of hardpan) and chemical (low nutrient availability and acidity) features are important constraints for agricultural production, and must be overcome by using machinery and great amounts of fertilizers and limestone (Lozano et al., 2012).
15.2.1.2 Vegetation of savannas in the Orinoco basin
The species of plants of the savannas, whether herbaceous or tree components, are adapted to oligotrophic conditions. The floristic variation in the ecosystem depends in good part on the higher or lower contents of clay, which can affect the water conditions of the soils and the biological transformation of organic matter. Productivity may vary depending on small changes in the physical, chemical, and biological characteristics affecting soil fertility. The general low productivity in the Orinoco basin, at the same time, contributes to the low levels of organic matter found in its soils, with values around 1 to 2% or less (Hernández-Hernández and López-Hernández, 2002).
Grass species are the most important floristic component of tropical well-drained savannas of the Orinoco basin. Legumes and sedges also coexist in the herbaceous layer; however, their coverage is lower than 10% in comparison with grasses (Velasquez, 1965; Susach, 1984). San José et al. (1985) studied seven sites of Trachypogon savannas from low to moderate fertility and found that the above-ground biomass of grasses ranged from 80 to 100%, while legumes reached a maximum of 3.8% in the location with the highest pH (5.5) and base saturation (81%). The lower biomass of legumes in neotropical savannas has been related to the high content of Al and, in turn, low P availability which hinders the formation of effective N-fixing symbiosis (Medina, 1987).
In Trachypogon savannas, species richness is about 285 species of angiosperms belonging to 55 families, but dominance is restricted to only a few like Poaceae, Papilionaceae, Cyperaceae, and Asteraceae, with Poaceae being represented by Trachypogon sp., the genus with the higher coverage (Riina et al., 2007). Other species of grasses are Axonopus canescens, A. anceps, Andropogon selloanus, Leptocoryphium lanatum, Paspalum carinatum, Sporobolus indicus, S. cubensis, and several species of the genus Aristida; legumes of the genera Mimosa, Cassia, Desmodium, Eriosema, Galactia, Indigofera, Phaseolus, Stylosanthes, Tephrosia, and Zornia; and sedges of the genera Rhynchospora and Bulbostylis. As previously described, tree presence is variable from absent in open savannas to dense in savanna parkland where crowns are closer, but with minimal overlapping. Trees can also be found scattered or in groups, and most of them are pyrophyte species like Byrsonima crassifolia, Curatella americana, and Bodwichia virgilioides (López-Hernández and Hernández-Valencia, 2008).
15.2.1.3 Effects of fire on nutrient cycling in savannas
Fire is considered one of the most important factors when explaining the origin and maintenance of savannas, and acts as a selective force which favors the dominance of fire-tolerant species (Sarmiento, 1984). Vegetation is burned during the dry season in order to eliminate senescent leaves, as a plague control, and to stimulate the production of new and more palatable pastures for stock breeding. Loss of nutrients by fires is related to burning efficiency, which in turn depends on the amount and the heterogeneity of the accumulated above-ground biomass, its moisture content, the wind speed, and the oxygen diffusion to the combustion zone (Bilbao and Medina, 1996). Beside burning efficiency, the long-term loss of nutrients by burning depends on fire frequency, which is usually annual, biennial, or triennial (Coutinho, 1988). The accumulation of high amounts of fuel load increases burning efficiency, but when fires have been burning in the same year, recent burn scars are very effective at stopping fires (Bilbao et al., 2010).
Fires produce strong mineralization and loss of nutrients. In the case of gaseous elements, like N and S, losses occur by volatilization and the spread of ashes, whereas in the case of sedimentary elements, like P and bases (potassium (K), sodium (Na), calcium (Ca), and magnesium (Mg)), losses by fire are due just to the spread of ashes. Hernández-Valencia and López-Hernández (2002) assessed the nutrient losses in the herbaceous layer of Trachypogon savanna, comparing the amount of N, P, Ca, Mg, and K, before the burning of the above-ground vegetation and after the combustion in the remnant tissues and ashes. Results indicated that fires expelled to the atmosphere as gases and ashes as follows: 1.7 kg P ha−1, 5.9 kg N ha−1, 3.7 kg Ca ha−1, 2.8 kg Mg ha−1 and 5.0 kg K ha−1, which represent more of 90% of the nutrient contents in the above-ground biomass, including litter. The high burning efficiency was related to the accumulation of dry foliages, its homogeneity, and the rapid fire spread enhanced by winds. In relation to their biogeochemical cycles, sedimentary nutrients like P, Ca, Mg, and K showed higher concentration in the ashes compared to the above-ground biomass; however, N was more diluted in the ashes due to its volatilization at high temperatures. Ash depositions returned to the soil between 21 and 34% of the sedimentary nutrients (P, Ca, Mg, and K), but only 1% N, and lowered the net losses to 1.1 kg P ha−1, 5.8 kg N ha−1, 2.4 kg Ca ha−1, 1.9 kg Mg ha−1, and 3.9 kg K ha−1; however, those measures have uncertainties because of the suspension of ashes by winds and the high variability in ash depositions.
15.3 Nutritional stresses in well-drained savannas—nitrogen as a limiting element
The soil characteristics, the climate, the geomorphology and net primary productivity in the savannas determine the low levels of N reported for soils of the Colombian and Venezuelan llanos. Neotropical savanna ecosystems include values of N, ranging from the extremely low of 0.2 g kg−1 in the first 10 cm of soils of Trachypogon Eastern Llanos, Venezuela, presenting more than 80% of sand composition (Gómez, 2004), to values of 1.2 g N kg−1 on savannas of higher clay contents in the Central Llanos, Venezuela (Hernández-Hernández et al., 2000). Hétier et al. (1989) showed N ranges between 0.6 g N kg−1 and 0.2 g N kg−1 for savannas of the Western Llanos, Venezuela, with up to 18% of clay at the surface and 40% of clay at 1 m deep.
The main form of total soil N is the organic, representing in many cases 96 to 98% of the soil (Sprent, 1999). In general, water-soluble (readily available) in well-drained Trachypogon savannas may reach around 30 to 40 mg N kg−1 soil, and within the mineral forms (López-Hernández, 2013), ammonium (NH4) is more abundant than nitrate (NO3). Although, NH4 and NO3 content changes during the rainy and dry seasons in savannas, in an ultisol, NH4 (20 mg NH4 kg−1) duplicates NO3 (10 mg NO3 kg−1) and this proportion is kept until 30 cm of depth (Lozano et al., 2011).
After the burning of savanna ecosystems, the fast combustion of the plant material and other organic debris together with the ash dispersion and volatilization of the element with gaseous cycles (e.g., C, N, and S) produce a drastic decline in the effective input of organic matter and nutrient to the soil (Frost and Robertson, 1985; Sanhueza and Crutzen, 1998; Hernández-Valencia and López-Hernández, 2002). Because many gaseous forms of N are formed under burning effects, N cycling is especially sensitive to frequent fires resulting in the net loss of N in savannas, unless losses are compensated by other mechanisms including biological fixation. Below we will examine the inputs and outputs of N in savannas and information related to some biological processes involved in savanna ecosystems which can ameliorate N deficiencies.
15.3.1 Main nitrogen inputs
15.3.1.1 Atmospheric depositions (wet and dry deposition)
In savannas, ash produced by fires in situ or in nearby areas can be deposited as dry deposition or washed out from the atmosphere and dissolved in the rain. In this way, precipitation can return nutrients lost by fires and other volatilization processes to the soil. Inorganic inputs of N from bulk precipitation are relatively low in savannas, unless the savanna is located near an industrial or polluted area (López-Hernández et al., 2013). Mineral N inputs (NO3+NH4) by precipitation in Venezuelan savannas ranged from 2.2 kg N ha−1 yr−1 in savannas of Calabozo (Montes and San José, 1989) to 6.2 kg N ha−1 yr−1 in a place more distant from urban activities at Estación Experimental, La Iguana (Table 15.2) according to Chacón (1988). Sanhueza and Crutzen (1998) also reported wet deposition of mineral N from 1.12 to 4.6 ha−1 yr−1 for the Orinoco region. Those precipitation inputs in inorganic N are very similar to the value presented for savannas of Loudetia located at comparable geographical latitude at Lamto, Ivory Coast, Africa (1.3–2.3 kg NO3 ha−1 yr−1 and 3.0 kg NH4 ha−1 yr−1) according to Villecourt and Roose (1978), and to the value presented by Bustamante et al. (2006) in a cerrado area protected from fire in Central Brazil. Detailed information on organic N sources in Trachypogon savannas located in Central Venezuela is almost non-existent, although Pacheco et al. (2004) reported that soluble organic N inputs may be considered an important contribution, making up 75% of total N in rainfall; however, more work needs to be done to confirm this information. Nonetheless, in Lamto savannas (Ivory Coast), soluble organic N inputs have been reported to be as much as 14.5 kg ha−1 yr−1 (Villecourt and Roose, 1978; Abbadie, 2006). In general, typical mineral N inputs in Africa are reported to be very low (Giller et al., 1997).
Table 15.2
N Inputs and Outputs (kg N ha−1 yr−1) in Savannas of La Iguana (Venezuela)
| Location | Unburned Savanna | Burned Savanna |
| Wet deposition | 6.2 (mineral) | 6.2 (mineral) |
| Dry deposition | 10.5* | 10.5* |
| N2 fixation | ||
| Soil–plant system | 7.8 | 13.7 |
| Microbial crusts | 4.0 | 2.4 |
| Legumes | ? | ? |
| Σ inputs | 28.5 | 32.8 |
| Fire | 0 | 8.2 |
| Leaching | 2.1 | 2.1 |
| Denitrification, NO and N2O* | 7.5* | 7.5* |
| Σ outputs | 9.6 | 17.8 |
Source: *Sanhueza and Crutzen, 1998 and Cárdenas et al., 1993.
As to dry deposition, Sanhueza and Crutzen (1998) presented information concerning the Venezuelan savannas during the wet and dry seasons with values ranging from 4.5 to 10.5 kg N ha−1 yr−1) (Table 15.2), most of them NH4++NH3 forms. In the savannas of Lamto, Abbadie (2006) reported a dry deposition of 3.5 and 2.1 kg N ha−1 yr−1 for the ammonium and nitrate form, respectively.
15.3.1.2 Biological nitrogen fixation
In the savannas of the Orinoco basin, fires volatize every year up to 19–30% of the N required for the net primary production of savanna herbaceous vegetation (Chacón et al., 1992; López-Hernández et al., 2006). N volatilized by fires and N losses due to leaching and erosion are not compensated for by the scarce inorganic N inputs from the precipitation mentioned above. This situation could cause a progressive reduction in the potential productive capacity of savannas in the absence of additional mechanisms of N input, apart from precipitation (Abbadie, 1983; Chacón, 1988; Chacón et al., 1991; Cook, 1994). Consequently, N budgets in dystrophic savannas must be balanced by biological N fixation.
In general, N fixation can occur in savannas from three different sources: (1) rhizobium-legume symbiosis; (2) organisms located in the rhizosphere of grasses; and (3) microbial crusts on the soil surface formed by cyanobacteria.
15.3.1.2.1 Nitrogen fixation by rhizobium Symbiosis
Neotropical savannas are characterized by their great diversity of herbaceous and woody leguminous species, although their relative importance in the plant canopy of well-drained savannas is small compared with the herbaceous component (Chacón et al., 1992). Previous studies in Trachypogon savannas indicated that, although most of the native leguminous species formed nodules, they seemed inactive or with reduced N fixation compared to cultivated legumes due to the soil acidity and poor base contents (Chacón et al., 1991; Medina and Bilbao, 1991). Few studies have been done to document N fixation by native legumes under natural conditions in Orinoco’s savannas, although evidence through natural abundance of 15N and relative abundance of ureids suggest N fixation for a few species (Medina and Bilbao, 1991; Izaguirre-Mayoral et al., 1992). Therefore, this mechanism of N fixation seems to be less crucial for the N economy of savannas than the other N-fixing mechanisms mentioned above; however, Bustamante et al. (2006) have emphasized that to have a reliable assessment of legumes to the N budget of savannas it is necessary to have information related to legume density and seasonal N fixation.
15.3.1.2.2 Nitrogen fixation by organisms located in the rhizosphere of savanna grasses
Nitrogen fixation by non-symbiotic organisms associated with the rhizosphere, rhizoplane, and endorhizosphere could be an important source of available N in nutrient-poor soils of savannas. Estimates of N fixation by the acetylene reduction method (nitrogenase activity, NA) in the soil–plant system in savannas of Trachypogon plumosus and Paspalum carinatum located at Estación Experimental La Iguana, Central Venezuela (Table 15.2) reported values of 13.7 and 7.8 kg ha−1 yr−1 for burned and protected plots (López-Hernández et al., 2006), respectively. During the dry season, the low water availability in savannas could be a major limiting factor for microbial and NA activity. As in the case of protected plot (unburned), the increase in NA in the dry season indicates the effect of rhizosphere conditions (moisture content, exudates, pH) on associated microorganisms.
Similar values for NA have been reported by Balandreau and Villemin (1973) and Balandreau (1975) in Loudetia and Andropogon savannas (12 kg ha−1 yr−1 and 9 kg ha−1 yr−1, respectively) in the Ivory Coast (Africa). Non-symbiotic N fixation has also been reported to have a significant importance to N budget in West African savannas (Robertson and Rosswall, 1986) with a contribution of Ca (approximately 12 kg ha−1 yr−1). In general, rhizospheric N fixation becomes significant in areas affected by limited N availability, a situation very common in well-weathered tropical savannas. These reports make clear that N2 fixation mediated by leguminous symbionts seems to be relatively low as compared to the activity of free-living organisms associated with the grass roots.
15.3.1.2.3 Nitrogen fixation by microbial crust system
The well-drained savannas present pedoclimatic features, which can lead to extreme conditions of water stress during certain months of the year (dry season), contributing to the formations of biocrusts, typical of arid and semi-arid ecosystems on the surface of the soil (Chacín et al., 2011). According to Schlesinger et al. (1996) and Housman et al. (2007), their presence produces islands of fertility in the soil that can be modulated by climatic and edaphic factors as well as management. Normally, in Orinoco savannas, the soil crusts are located between patches of Trachypogon tillers and other grasses, contributing to the fertility of the soil and affecting decomposition and mineralization of biological processes and biogeochemical cycles. Soil crusts function as bridges of transfers of resources between plants, through their roots in connection with mycorrhizal fungi that form networks, and carry on in a bidirectional way C and N between plants and crusts. Symbiotic associations are established according to the needs of the fungi and the primary producers, which use the C and transport and transform nutrients such as N (Allen, 2007). Their presence implies clear effects on the N cycling, either through biological fixation by algae and cyanobacteria that form the crust (López-Hernández et al., 2006), or via decomposition of organic compounds from metabolites of biocrust organisms and the accumulation of organic material that are concentrated in crusts (Hernández-Hernández et al., 2013). The soils of the Venezuelan savannas, with varying degrees of index of aridity, have crusts dominated by cyanobacteria, with N values between 0.6 and 0.9 g kg−1 compared with 0.2 and 0.3 g N kg−1 for the bare soil (Hernández-Hernández et al., 2013), whereas the values for available P were not much different in the soil with crusts (0.54–0.94 mg kg−1) in relation to the bare soils (0.59–0.62 mg kg−1). Concerning the inorganic N forms, ammonium forms were always higher compared to nitrate forms (30 mg NH4 kg−1 vs. 5 mg NO3 kg−1) in both crusted soil and bare soil (16 mg NH4 kg−1 vs. 9 mg NO3 kg−1), respectively (Chacín et al., 2011).
These values are closely related to a more active cycling of labile compounds of C in the soil of savannas in the presence of crusts. There is a greater biological activity in soils of savannas with crusts up to six times greater than in a soil without crusts (Chacín et al., 2011). Also, the crusts give physical protection to the impact of rain drops in the wet season and improve the structure of soils (Chamizo et al., 2012).
Taking into account the microbial crust cover and NA measurements, López-Hernández et al. (2006) have estimated N2 fixation rates by microbial crusts of 2.4 and 4.0 kg ha−1 yr−1 in burned and protected Trachypogon savannas, respectively (Table 15.2). In any case, fixation should be less than 13.5 kg ha−1 yr−1, a value which would be met if the maximum NA recorded was maintained during the 214 days that cyanobacteria remained active. Moisture content in the microbial crusts strongly influences NA activity, which is limited to the rainy season. During the dry season no NA was recorded in Trachypogon savannas. This could indicate that cyanobacteria are very active and responsible for N fixation during the wet season, whereas between rainy seasons they are dormant due to the severe drying of the soil. The larger plant cover in the protected plot maintains, at a microclimatic level, a relative higher crust moisture content creating more favorable conditions for NA during the rainy season (Stewart et al., 1977).
15.3.2 Main nitrogen outputs
15.3.2.1 Losses by fires
Burning of vegetation during the dry season is the rule in savannas (Vareschi, 1962; Vuattoux, 1976). This factor is supposed to be pivotal for the origin and maintenance of savannas (Beerling and Osborne, 2006) and behaves like a selective force which maintains the dominance of fire-tolerant species.
After the fire’s passage, more than 90–95% of the herbaceous aerial cover is lost, and most of the organic elements are transformed into CO, CO2, NO, and NO2 (Sanhueza and Crutzen, 1998). In unfertile neotropical Trachypogon savannas located at La Iguana Experimental Station, Venezuela, losses of 8.2 kg N ha−1 yr−1 have been measured (Table 15.2), whereas in the more fertile soils of Lamto Experimental Station in Ivory Coast, Africa, losses of 17 and 24 kg N ha−1 yr−1 have been reported for Loudetia and Andropogon savannas, respectively (Abbadie, 1983, 2006). The higher N losses from Lamto savannas are in good agreement with their higher above-ground primary production. Losses of N by biomass burning at La Iguana savannas are in accordance with more detailed information of gas emissions (NH3, N2, NOx, RCN) in other savannas of the Orinoco basin presented by Sanhueza and Crutzen (1998) and Bustamante et al. (2006), ranging from 8.8 to 26.5 kg N ha−1 yr−1.
15.3.2.2 Nitrogen losses by leaching
The loss of N by internal drainage is relatively small in well-drained savannas, which is understandable since the water-soluble levels of N forms are always scarce, except perhaps at the beginning of the rainy season when substantial organic matter decomposition occurs. The losses of N in the savannas of Estación Experimental La Iguana in Guarico Central were estimated at 2.1 kg N ha−1 yr−1 (Chacón et al., 1991; Table 15.2), whereas for a Loudetia–Andropogon savanna at Lamto, Ivory Coast, 5.6 kg N ha−1 yr−1 was reported to leave the system through internal drainage, mostly in organic form (Villecourt and Roose, 1978; Abbadie, 2006) coming from microorganisms or recent dead plant material. In savanna ecosystems, information on nutrient losses by leaching is scarce and perhaps related to methodological inconveniences for designing and installing an effective system to catch leachates (López-Hernández and Hernández-Valencia, 2011). As a consequence, more accurate measures are still needed.
15.3.2.3 NH3 losses
The mineralization of the soil organic matter in native soils might produce ammonium and at high pH some of the NH4+ exists as NH3. However, in acidic, well-weathered savanna ecosystems, soil emissions of NH3 are considered negligible (Abbadie, 1983; Sanhueza and Crutzen, 1998; López-Hernández, 2013).
NH3 emissions in the budget presented in Table 15.2 are not included since animal waste and fertilizer applications are negligible, particularly in the case of the experimental site located at La Iguana Experimental Station.
15.3.2.4 Denitrification and emissions of NO and N2O
Losses by denitrification in savannas of the Orinoco basin should be insignificant due to the low levels of nitrate and organic matter and the acidic pH of the soils. Similar information was presented by Abbadie (2006) in Lamto savannas, who reported that denitrification is highly variable in the space between soils. NO3 pools in the soils of the tropical savannas of South America are low, which means that the rates of nitrification are also negligible; nonetheless, the levels of the intermediate NH4 are, in general, significant.
In the savannas of the Orinoco region, Sanhueza and Crutzen (1998) have estimated an annual production of 0.18–0.63 kg ha−1 and 0.36–6.3 kg ha−1 for N2O and N2, respectively. However, in Orinoco savannas, soil emissions of NO are largely uncertain, ranging from 0.3 to 3.0 kg ha−1 yr−1 depending on the amount and distribution of rainfall (Cárdenas et al., 1993); all together N2O, N2, and NO losses in Trachypogon savannas account for about 7.5 kg ha−1 yr−1 (Table 15.2).
15.4 Nutritional stresses in well-drained savannas—phosphorus as a limiting element
The total content of P in soils of the savannas of the Orinoco basin is in general low and corresponds to well-weathered soil according with the model of Walker and Syers (1976) for ecosystem development. Values can be as low as 50 mg kg−1 in sandy Quartzipsamments and above 600 mg kg−1 in some clay soils.
Soil P can be found in different forms of organic (Po) and inorganic phosphorus (Pi), which can be discriminated as: (1) active or labile forms represented by Pi soluble or sorbed on soil surfaces and labile Po (easily mineralizable Po) associated to the soil microorganisms and organic matter, (2) Pi associated with amorphous and some crystalline Al and Fe phosphates and Po in humic and fulvic acids with lower availability than labile forms and involved in long-term transformations, (3) calcium-bound Pi which predominates in primary minerals like apatite-type minerals or alkaline soils, and (4) recalcitrant P, composed by the more chemically stable organic P and insoluble inorganic P and represented by P in particulate organic matter, occluded or strongly fixed in the mineral matrix (López-Contreras et al., 2007). Plants absorb soluble inorganic P ion forms (HPO4−, HPO42−) from the labile fraction, which is usually termed as available P. The amount of available P is highly dependent on pH and a maximum is reached at neutral values (6–7 pH units), but despite this favorable condition, available P rarely surpasses 15% of the total P content (Brady and Weil, 2008).
Figure 15.2 shows the distribution of the soil P fractions mentioned above in four different Trachypogon savannas in Venezuela, expressed as a percentage of total P. Despite the differences in textural conditions and climatic regimes, the picture resembles a similar pattern, with a dominance of recalcitrant and very low contents of associated P to primary minerals or calcium bound P, as usually found in highly weathered soils. Available plant P, which is included in the labile inorganic P pool, accounted for less than 13%. Their absolute values are below 5 mg kg−1 soil and could be considered low for agricultural purposes. However, labile organic P accounted for 2 to 9% of total P, whereas moderately labile organic P accounted for 5 to 30% of total P. Both forms can perform as P suppliers in the short (labile) to long (moderately labile) term.
As presented above, the periodical burning of savanna ecosystems produces a drastic decline in the effective input of organic matter and nutrient to the soil. In the case of elements with sedimentary biogeochemical cycles (e.g., P and nutrient bases K, Ca, and Mg), the gaseous losses are irrelevant or non-existent; however, part of the in situ produced ashes can be lost by wind dispersion and lateral movement with precipitation waters (Hernández-Valencia and López-Hernández, 2002), therefore inducing a potential P limitation to plants. Moreover, the scarcely available P forms deposited in ashes can be easily adsorbed to insoluble P forms for the soil matrix containing Al and Fe sesquioxides, which easily adsorb or precipitate orthophosphate (López-Hernández, 1977). Now we will examine the inputs and outputs of P in savannas, and information related to some biological processes involved in savanna ecosystems, which can ameliorate P deficiencies.
15.4.1 Main phosphorus inputs
In burned savannas, ash produced by fires and dust are settled down or are washed out from the atmosphere by rain and dissolved or suspended in the droplets. Inorganic inputs of P from bulk precipitation are relatively low in the well-drained savannas of Venezuela, because most of them are located far from industrial or polluted areas. This was demonstrated for Estación Biológica de los Llanos, where input values ranged from 0.31 to 0.52 kg ha−1 yr−1 (Table 15.3) and water P concentration from 0.03 to 0.10 mg L−1 (Montes and San José, 1989; Hernández-Valencia, 1996). The pattern observed in P inputs via precipitation has two peaks, one at the beginning of the rainy season and the other during the period of heavy rainfall.
Table 15.3
Annual P Budget for a Well-drained Savanna in Calabozo (Venezuela)
| Transferences | kg P ha−1 |
| Inputs | |
| – Precipitation | 0.5 |
| Outputs | |
| – Fire | 1.1 |
| – Cattle extraction | 0.1 |
| – Leaching | 0.3 |
| Balance | −1.0 |

Source: Hernández-Valencia, 1996.
At a global scale, it has been suggested that substantial amounts of P can be transported over extremely long distances in the form of dust (Swap et al., 1992; Okin et al., 2004). In the case of tropical forest ecosystems, it has been registered that dust inputs originating in the Sahara/Sahel region in Africa can incorporate 1 to 4 kg P ha−1 yr−1 in the northeastern Amazon basin, concluding that the high productivity of the Amazon forest is fueled, at least partially, from this transported dust. Is a similar situation occurring in the relatively nearby savannas of the Orinoco basin? We do not have enough information to corroborate this assertion; however, any P input in savannas of the Orinoco is important since the extremely weathered environments of some regions have a concomitant serious P depletion.
P released from parent material by weathering has not been assessed in well-drained savannas; however, we consider it negligible, because the sediments are mainly composed of P forms very resistant to weathering (Hernández-Valencia and López-Hernández, 1999).
15.4.2 Main phosphorus outputs
15.4.2.1 Losses by fires
In tropical savannas, burning of vegetation produces a strong mineralization pulse and loss of nutrients once ashes are spread outside the ecosystem. Studies in Calabozo savannas in Estación Biológica de los Llanos, comparing the P content in the above-ground biomass before and after the burning of the vegetation, indicated that about 1.7 kg P ha−1 was expelled to the atmosphere (Table 15.3). This amount represents an important part of the above-ground biomass, including litter (Hernández-Valencia and López-Hernández, 2002). Although temperature was not recorded during the experimental period, the high burning efficiency (more than 90%) seems to be related to the accumulation of dry foliage and the rapid fire spread that was enhanced by winds. Ash deposition returned about 21% of the P transferred to the atmosphere by fires to the soil and lowered the net losses to 1.1 kg P ha−1. However, those measurements could have significant uncertainties, because of the suspension of ash by winds and the high variability in the measured ash depositions. These results demonstrate the role of fire as net exporter of P and its contribution to the global balance of other elements in the atmosphere (i.e., C, S, and N). Also the need to manage fire to reduce carbon and other nutrient emissions from savanna ecosystems is emphasized. Reducing fire frequency could be an easy way to address this situation; if fires were produced on a biennial or triennial basis, the associated N and P losses could be compensated by inputs through precipitation, as was shown for the Brazilian Cerrado (Coutinho, 1988).
15.4.2.2 Losses by leaching
Nutrient losses from soil by leaching are likely in well-drained savannas due to high permeability of the soil. Leaching is usually restricted to the wet season and when an excess of water drains out of the rooting zone (Hernández-Valencia, 1996). For well-drained savannas the information on P losses by leaching is scant. At Estación Biológica de los Llanos, P concentrations were below 0.1 μg L−1 in leachates sampled at 1 m depth; these values are typical for tropical weathered soils with concomitant high P-sorbing capacities (López-Hernández et al., 1981). Drainage out of the rooting zone (>1m) was recorded in three of the six rainy-season months and P concentration did not present any seasonal pattern. The amount of P that is lost by leaching ranged between 0.23 and 0.29 kg P ha−1 yr−1 (Table 15.3).
15.5 Strategies that are used by native savanna plants to enhance nitrogen and phosphorus conservation and uptake
The information presented suggests that the plants of the native savannas under frequent fires and strong seasonality are limited by N and P or both, yet the rates of many biological processes in this biome continues to be significant; a similar situation occurs in the case of the tropical rain forests with strong P limitation (Reed et al., 2011). The answer to the apparent paradox lies in the existence of the adaptations that the organisms of this biome have evolved to effectively overcome such low N and P availabilities. These strategies may be of two categories: (1) enhancing N and P conservation and efficiency and (2) enhancing N fixation and P incorporation and uptake. Biological N fixation was thoroughly discussed above.
15.5.1 Enhancing nitrogen and phosphorus conservation and efficiency
15.5.1.1 Nutrient uptake, translocation, and biomass production
The mechanisms involved in the adaptation of savanna plants to soils of low nutrient availability have scientific and practical importance (Medina and Bilbao, 1991). Natural selection favors species with competitive ability to overcome oligotrophic conditions. Most of them usually have low nutrient demands, low nutrient contents in their tissues, and, in turn, a low organic matter production. Since savannas are areas of stock breeding, intensive animal production is restricted by the poor production and palatability of forages (López-Hernández and Hernández-Valencia, 2008).
A review of 15 studied sites corroborates the mean low productivity values for Trachypogon savannas (López-Hernández and Hernández-Valencia, 2008), where above-ground production (3.78 mg ha−1 yr−1) surpassed mean values for below-ground production (2.64 mg ha−1 yr−1) and finally the overall primary production reached 6.74 mg ha−1 yr−1, values that are within the lowest in the world production of savanna ecosystems (Sarmiento 1984; López-Hernández et al., 2012).
Organic matter production by the herbaceous layer shows a seasonal trend correlated with precipitation and soil water availability (Hernández-Valencia and López-Hernández, 1997). The burning of the vegetation during the dry season stimulates plant growth and the demand for nutrients increases. As a consequence, due to their higher metabolic activity the youngest tissues reach the highest nutrient concentrations at that growth period. As long as the rainy season proceeds, biomass and nutrient storage increases and nutrient concentration decreases due to dilution or translocations from old to young leaves. Thus, the translocation to young leaves and roots can operate as efficient mechanisms for nutrient conservation. In the dry season, organic matter productivity and nutrient uptake decrease, therefore the nutrient contents in foliage reach their lowest concentrations. This behavior has been found for N, P, and S, while Ca and Mg show the opposite behavior (Medina et al., 1978; Hernández-Valencia and López-Hernández, 2000). In general, low levels of N, P, Ca, and S are commonly found in the foliage of grasses in well-drained savannas in South America. The maximum amounts recorded after fires (dry season) ranged between 6.2–8.9 mg N g−1 and 1.8–3.1 mg P g−1 while the maximum at the standing crop (wet season) ranged between 0.9–8.1 mg N g−1 and 0.9–2.0 mg P g−1 (Medina et al., 1978; Sánchez et al., 1985; Hernández-Valencia and López-Hernández, 2000).
Information about N and P uptake for primary production is scarce when compared to productivity data. Comprehensive studies on N cycles estimated values from 27.0 to 48.0 kg N ha−1 yr−1 and from 29.6 to 30.3 kg N ha−1 yr−1 for the unburned and burned savannas, respectively (López-Hernández and Hernández-Valencia, 2008), while in the case of P, reported phosphorus uptake was about 5.9 to 8.8 kg P ha−1 yr−1 in burned savanna (Hernández-Valencia and López-Hernández, 2000). According to Medina (1987), the ability of savanna grasses to extract nutrients from poor soils is probably more related to a dense root system for exploring the soil volume and, therefore, a higher root/shoot ratio than more fertile soils. Data from seasonal savannas of Venezuela indicate that the root/shoot at maximum standing crop ranged from 0.22 to 1.75 (Table 15.4). Unburned savannas showed lower root/shoot ratios due to the accumulation of above-ground biomass.
Table 15.4
Root/shoot Ratios for Different Trachypogon Savannas from Venezuela at the Maximum Standing Crop
| Location | Root/Shoot Ratio | |
| Burned savannas | Calabozo | 0.22–0.94 |
| Cabruta | 1.75 | |
| La Iguana | 0.95–1.32 | |
| Unburned savannas | Calabozo | 0.23–0.43 |
| Cabruta | 0.57 | |
| La Iguana | 0.17–0.34 |

Source: Calabozo (López-Hernández and Hernández-Valencia, 2008), La Iguana (1988), Cabruta (Susach, 1984).
There is no information about nutrient uptake of savannas trees in the Orinoco basin; however, several patterns have been distinguished among grasses and trees (Medina, 1993). Grasses and woody plants from semi-arid savannas in general have higher nutrient contents than those from dystrophic humid tropical savannas; however, trees demand more nutrients than xeromorphic C4 species, and thereby are associated with soils with better nutritional status. Evergreen trees are more frequent in dystrophic savannas, especially if fire is frequent, and produce leaves with lower nutrient contents than deciduous trees. Leguminous trees have usually higher N and P content and consequently higher N and P demands than non-legumes, which explains the low coverage of legumes observed in most P-depleted savannas. It has also been demonstrated that trees improve nutrient contents in the soil beneath them in comparison with the soil associated with the herbaceous layer of a treeless savanna (Table 15.5; Hernández-Hernández, 2008). The input of litter with a richer nutrient content has been argued as a mechanism to explain this finding (Kellman, 1979), but also it must be considered that trees can concentrate nutrients beneath the soil by pumping them from the subsurface to the topsoil, by acting as traps of dust and ashes suspended in the air and by using perching bird droppings.
Table 15.5
Total N Contents and Available P in Soils of Well-drained Savannas of the Orinoco Basin under the Canopies of Dominant Tree Species and Trachypogon plumosus
| Specie | Total N (g kg−1) | Available P (mg kg−1) |
| Byrsonima crassifolia | 0.63±0.01 | 4.07±0.09 |
| Curatella americana | 0.69±0.01 | 2.74±0.08 |
| Bowdichia virgilioides | 1.14±0.01 | 7.01±0.08 |
| Trachypogon plumosus | 0.65±0.11 | 2.70±0.08 |
15.5.1.2 Role of microbial biomass
The microbial biomass (MB) mediates the transformation (mineralization–immobilization) of biogenic nutrients (C, N, P, and S) between the inorganic and organic forms; therefore, MB plays an important role in nutrient cycling. It also acts as an important sink for plant nutrient conservation and utilization in tropical savannas. In the soils of the Orinoco savannas, in general, the contents of soil microbial biomass carbon (C-MB) are low (100–200 mg kg−1), which is in agreement with the low soil organic matter content of the soils (López-Hernández and Hernández-Valencia, 2008).
15.5.1.2.1 Microbial forms of nitrogen
In savannas, it has been reported that very low values of microbial N respond to a typical temporal variability of the ecosystem, which tends to increase significantly during rainy season. In Venezuelan eastern savannas, Gómez (2004) has reported microbial biomass N (N-MB), in the dry season, ranging from 3 to 6 mg kg−1 in a sandy soil, those values being lower than in savannas with higher silt–clay components, where the contents of microbial N are approximately 35 mg kg−1 (Hernández-Hernández et al., 2012). In general, it can be emphasized that in the Venezuelan savannas the N-MB followed a similar pattern to the C-MB, e.g., low values (around 10–30 mg kg−1) associated with the low levels of organic matter. Nutrients in microbial biomass have been shown to provide pulses of nutrient release in seasonally dry ecosystems (Hernández-Hernández and López-Hernández, 1998); thus, this source of N for primary production could indicate the potential provision and conservation role in the N economy of the savanna.
15.5.1.2.2 Microbial forms of phosphorus
In the case of P, microorganisms release available P and readily mineralizable organic labile P after cell lysis. Microorganisms also produce enzymes like phosphatases that catalyze the conversion of organic P to inorganic available P for plant requirements. Values of microbial P for Trachypogon savannas range from 2.6 to 12.4 mg kg−1 (Hernández-Valencia and López-Hernández, 1999; López-Contreras et al., 2007), and account for about 6 to 10% of total soil P content. These values are higher than available P pools and show the importance of microorganisms in the transformation, storage, and supply of P for plants in these P-depleted soils. At Estación Biológica de los Llanos, microbial P ranged between 3.7 and 13.7 mg kg−1 and no seasonal trends were observed (Hernández-Valencia, 1996). However, Singh et al. (1991) found for Indian savannas that microbial C, N, and P decreased in the rainy season and then increased in the dry season. According to those authors, the higher soil moisture during the rainy season favors cell lyses and nutrient release through microbial activity.
15.5.1.3 Role of pedo-fauna in the generation of biogenic “hotspots” in Trachypogon savannas
In savanna ecosystems, termites and earthworms are among the most conspicuous components of soil macro-fauna and play an important role in the decomposition processes of organic matter and nutrient cycling (Lavelle et al., 1994; López-Hernández, 2001; Chapuis-Lardy et al., 2011).
Invertebrates belonging to macro-fauna are key species of soil functioning. They participate in litter decomposition, mix organic and mineral matter, create and maintain soil structure by digging burrows and modifying aggregation, and regulate microbial diversity and activity (Lavelle et al., 1994; Renard et al., 2013). The gut and biogenic structures (burrows, casts, nests) of earthworms and termites are specific habitats where soil microbial activities are either stimulated or attenuated (Martin, 1991). Earthworms are important actors in the regeneration of compacted soils by reduced tillage systems (Capowiez et al., 2012) and freshly egested earthworm casts, i.e., the by-products of gut passage, are generally characterized by an intense mineralization of organic matter and the release of nutrients for plants (Lavelle et al., 1994; López-Hernández et al., 1993; Ngo et al., 2012). Conversely, the mineralization in old casts is reduced, which allows for carbon and nutrient storage in soil in the long term (Martin, 1991; Lavelle et al., 1994). Thus, biogenic structures produced by earthworms (casts), ants, and termites have been considered as “hotspots” for nutrients and places to potentially start a successional development from savannas to a “micro-forest” (López-Hernández et al., 2012; Erpenbach et al., 2013).
15.5.1.3.1 Nitrogen contents in biogenic structures of earthworms (casts) and termite mounds and adjacent soils
In Trachypogon savannas using a conservative estimate of earthworm population density, it has been estimated that 3 and 34 kg ha−1 yr−1 of inorganic N in the savanna and introduced pasture, respectively, may be released in fresh casts (Decaëns et al., 1999). If underground and above-ground cast productions are taken into consideration (e.g., 14 and 114 Mg ha−1 for the natural and the introduced-pasture savanna, respectively) then a significant contribution to the N budget (internal recycling) in those ecosystems derives from deposition of casts, particularly in the man-affected savannas.
The role of termite activity in the N economy in savannas is more difficult to assess. Termites are recognized as the most important decomposers in tropical forest and savannas, strongly affecting soil organic matter and nutrient dynamics. In general, an increase in N content has been reported in mounds (which act as hotspots) compared with the associated soils, and it may affect ecosystem processes on certain spatial and temporal scales (Decaëns et al., 1999; López-Hernández, 2001).
15.5.1.3.2 Phosphorus contents in biogenic structures of earthworms (casts) and termite mounds and adjacent soils
Earthworm activity creates a series of geochemical and biological effects that are especially important for the cycling of P in soils (Chapuis-Lardy et al., 2011). The availability of P is strongly influenced by the physical adsorption or fixation of P in soil, which is much related to soil type (Figure 15.3). López-Hernández et al. (1993), using isotopic techniques, reported higher P availability (P in solution) and E1 (P isotopically exchanged in 1 min) in fresh casts of P. corethrurus for two soils, one from the savanna of Lamto, Ivory Coast, with a moderate to low P-sorption capacity, and the other from Laguna Verde, Veracruz, Mexico, with a high P-sorption capacity. These changes in P availability were ascribed to (1) the greater pH of the gut content, (2) changes in sorption complexes induced by competition for sorbing sites between orthophosphates and carboxyl groups of a mucus glycoprotein produced by the earthworm in its gut, and (3) an increase in microbial activity during digestion (López-Hernández et al., 2012).
Earthworm casts usually contain larger amounts of organic P (e.g., for P. corethrurus; Chapuis-Lardy et al., 1998), probably derived from a selective ingestion of soil particles (López-Hernández et al., 1993; Chapuis-Lardy et al., 1998). Several studies have reported increased enzymatic activity and the stimulation of microbial activity in earthworm casts (Sharpley and Syers, 1976; López-Hernández et al., 1993; Brossard et al., 1996; Le Bayon and Binet, 2006). Devliegher and Verstraete (1996) showed that the increase of inorganic P in soils in the presence of earthworms was due to the production of alkaline phosphatases by earthworms (Satchell and Martin, 1984; Park et al., 1990) and a stimulation of microbial acid phosphatase production by earthworm activity (Satchell and Martin, 1984).
A similar role in the decomposition processes of organic matter for tropical and subtropical environments has been ascribed to termites in Africa and Australia; however, in neotropical savannas the importance of termites is considered secondary (Chapuis-Lardy et al., 2011). In a comprehensive P fractionation study done in soil and associated termite mounds of Nasutitermes ephratae, a common plant debris-feeding termite from savannas of the Orinoco Llanos (López-Hernández, 2001), it was found that all P concentrations were significantly higher in the Nasutitermes mounds than in the adjacent soils. Evidence suggests that nutrients accumulate in the mound from the nearby areas; therefore, the abundant termite populations could play an important role in controlling nutrient cycling in tropical savannas, in which available nutrients can often be a limiting factor for plant growth and development.
15.5.2 Enhancing nitrogen fixation and phosphorus acquisition and uptake
Under increasing acidity, soil exchangeable aluminum (Al+3) tends to increase to toxic levels with a concomitant deficiency in available forms of P (López-Hernández, 1977). Savanna plants need to cope with the minuscule amounts of P existing in the soil solution through a series of adaptations that in many cases involve the abundant microorganism population living in the rhizosphere zone. The diversity and abundance of microorganisms influence the function of the ecosystems through soil nutrient cycling (N, P, and C) (Pineda et al., 2013). Many of those microorganisms cohabit in the rhizosphere soil zone where roots develop and the microorganisms grow actively (Richardson et al., 2009; Bhattacharyya and Jha, 2012). In natural conditions, most of the tropical plants are adapted to different ecological niches associated with soil microorganisms such as arbuscular mycorrhiza (AM) (Janos, 1985; Mora et al., 2013), phosphate solubilizing bacteria (PSB) (Toro et al., 1996, 1997; Mora et al., 2013), and symbiotic N-fixing bacteria as Rhizobium (Izaguirre-Mayoral et al., 1992; Mora et al., 2013), which can play a key role in nutrient cycling and in the protection of the plant to environmental stress (Olivares et al., 2013).
These microorganisms have great potential to contribute to improving local fertility problems and consequently they might have a potential use to generate biofertilizers (Richardson et al., 2009; Zhang et al., 2010; Olivares et al., 2013). Next, we analyze the presence and functionality of some of these microbial groups in the savanna soils, and their relevance to P and N nutrition.
15.5.2.1 Mycorrhizal association
Mycorrhizal association is a symbiosis established since ancient times between fungi of the phylum Glomeromycota (Schüßler and Walker, 2010) and the roots of plants; 80% of the land plants possess such association (Smith and Read, 2008; Brundrett, 2009). These fungi are symbionts that are forced and require a source of carbon from the host to complete their life cycle; the resulting structure in the root is known as arbuscular mycorrhiza (AM), frequently present in terrestrial ecosystems. Only some families have lost the ability to form this symbiosis (10%), e.g., Chenopodiaceae and Brassiceae, among others (Smith and Read, 2008; Brundrett, 2009), consequently, AM presence is widely extended in nature.
The AM exert a beneficial effect on plant growth and development, because the fungi develop an extensive network of mycelium out of theroots, able to take elements like P, usually depleted in the rhizosphere due to slow diffusion in soil solution and fast root uptake (Smith and Read, 2008; Brundrett, 2009). Thus, a mycorrhized plant has an additional mechanism of nutrient incorporation than non mycorrhized plant. For this reason, some authors consider this symbiotic association as a biofertilizer and recognize its usefullness in farming systems, remediating contaminated soils, among other applications (Barea et al., 2002a, 2013).
Symbiotic AM can contribute greatly to plant P nutrition and may be induced by P deficiency and the presence of organic matter in soil (López-Gutiérrez et al., 2004 a,b). AM contribution may be indirect due to AM hyphae exploring a larger soil volume and inducing physiological changes that favor the establishment of P solubilizing and mineralizing microorganisms in the mycorrhizosphere (Pineda et al., 2013). Alternatively, it may be directed as a consequence of the production of extracellular phosphatases such as acidic phosphatase activity (APA) and the access to distant P sources otherwise not available to the host plant (Li et al., 1991; Tarafdar and Marshner, 1994; Joner and Johansen, 2000; Shen et al., 2011).
It seems possible that some specific mechanisms must be operating in the plant rhizosphere to allow plant growth in savannas. Thus, AM, soil microorganisms, and APA in the rhizosphere are considered as crucial mechanisms for P availability and plant P uptake. López-Gutiérrez et al. (2004a,b) focus on the biochemical processes occurring in the rhizosphere of native plant species (Trachypogon plumosus) of P-deficient savanna soils. Therefore, microbial activity, as measured by viable plate counts and dehydrogenase activity (DHA), mineralization activity (APA), and AM dynamics was studied in the rhizosphere of T. plumosus growing on three seasonal savanna soils differing in P content and soil order (Table 15.6).
Table 15.6
General Characterization of Savanna Soils in Estación Experimental La Iguana
| Soil order | Entisol | Vertisol | Ultisol |
| pH | 5.4 | 5.7 | 5.4 |
| Sand (%) | 94.4 | 38.8 | 73.2 |
| Silt (%) | 1.6 | 29.2 | 13.2 |
| Clay (%) | 4.0 | 32.0 | 13.6 |
| Texture | Sand | Clay loam | Sandy loam |
| P-Total (mg kg−1) | 90.1 | 137.3 | 102.1 |
| P-NaHCO3 (mg kg−1) | 9.2 | 1.6 | 2.6 |
| N (%) | 0.08 | 0.11 | 0.11 |
| C (%) | 0.54 | 5.1 | 2.36 |
| Fe (mg kg−1) | 25.4 | 75.6 | 109.6 |
| Al (meq 100 g−1) | 0.85 | 0.20 | 0.70 |
| H (meq 100 g−1) | 0.20 | 0.15 | 0.20 |
| CEC | 2.42 | 15.66 | 8.28 |
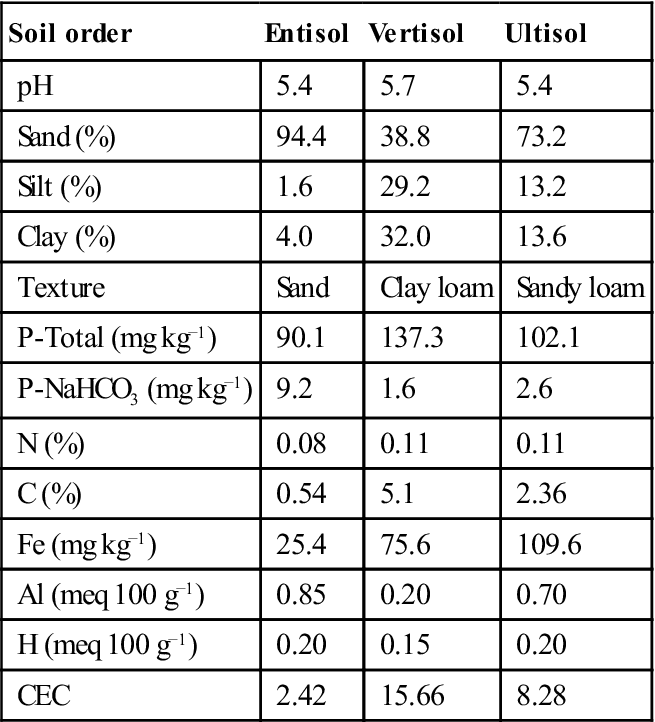
Source: Adapted from López-Gutiérrez et al., 2004b.
15.5.2.1.1 Mycorrhizal status in the studied savanna soils
AM colonization, expressed as a percentage of AM-colonized root length (%CRL) in T. plumosus, was higher during the rainy season in entisol and vertisol but not significantly different among seasons in ultisol (López-Gutiérrez et al., 2004b; Figure 15.4). The higher AM colonization as well as the increase in AM spore number during the rainy season (data not presented) concurs with the higher plant P demand. Nevertheless, seasonality, intrinsic characteristics of the plant–fungus combination and also soil properties can alter these parameters (Smith and Read, 2008). Moreover, AM spore number is relatively high in these soils since 3.6 spores g−1 dry soil is considered to be a high density (López-Gutiérrez et al., 2004b). Therefore, T. plumosus shows a particularly high mycorrhizal status for a grass in terms of AM infective potential spore density and root colonization when compared with reports from savannas of the Venezuelan Amazonia, which include values of 54.7% of CRL for Panicum pilosum (Poaceae) and only 29.8% for Andropogon bicornis (Poaceae) (St. John and Uhl, 1983). It has been reported that grasses with fine roots, abundant root hairs, and rapid growth are less sensitive to AM colonization (St. John, 1980).

The fact that AM colonization is, in general, high and increases during the rainy season (Figure 15.4), when P demand by plants also increases, suggests the importance of AM symbiosis as a mechanism for P uptake of T. plumosus in savanna ecosystems. This is emphasized when considering that one of the main strategies of grass species for P uptake in acid soils is a preferential distribution of resources to the roots and T. plumosus possesses a dense and fibrous radical system that allows an intensive exploration of the soil substrate (Medina and Bilbao, 1991).
Regardless of the presence or not of seasonal changes in the different mycorrhizal parameters studied, the sustained high level of AM colonization throughout the different seasons indicates that T. plumosus requires AM symbiosis to achieve an adequate P nutrition in savanna ecosystems.
15.5.2.1.2 Enzymatic activities in the studied savanna soils
Studies on the enzymatic activities of savanna soils in two contrasting seasons indicated that APA significantly increased during the rainy season in all soil orders examined (López-Gutiérrez et al., 2004a). This increase, however, was proportionally higher for entisol (Figure 15.5). These results suggest that P mineralization, as expressed by APA, increases during the rainy season, when plant nutrient uptake and nutrient leaching are higher.

Dehydrogenases are not expected to be free in soil; therefore, DHA expresses living cell activity and that is why it is an indicator of biological activity (López-Gutiérrez et al., 2004a,b). DHA significantly decreased during the rainy season in all soil orders (Figure 15.6). Even though several soil factors such as soil texture affect DHA, this enzymatic activity can be used to detect soil seasonal changes. Furthermore, DHA can correlate with changes in the microbial biomass due to long-term soil amendment. In Trachypogon savannas, DHA activities (Figure 15.6) appear to be well related to organic matter contents, which, in turn, control biological activities, thus DHA in entisol with poor soil carbon content (0.54%, Table 15.6) values was very low; on the contrary values increased in vertisol and ultisol with a higher soil carbon content (5.1 and 2.36%, respectively). In other studies done in ultisol and oxisol, extremely poor and acid soils of Uverito savannas evidenced an increase of APA, β-glucosidase and protease B-AA activity during the rainy season (Gómez and Paolini, 2011).

Furthermore, microorganisms in the rhizosphere may contribute to P nutrition through the synthesis and release of phosphatases when P is not available (Hayat et al., 2010; Shen et al., 2011). The decrease in DHA and bacterial counts during the rainy season, however, suggests that the increase in APA during that period is not mediated by rhizospheric microorganisms, i.e., soil extracellular phosphatases may have been already present in the soil or have plant root origin (Shen et al., 2011). López-Gutiérrez et al. (2004a) noted that in savanna ecosystems, as well as other tropical ecosystems affected by climatic seasonality, the APA appears to be an important mechanism of adaptation for the acquisition of the P. During the rainy season, the APA increased, but the rhizosphere microorganisms do not appear to be involved in the mineralization activity, but seem to contribute to the cycling of P by the immobilization and release P during the dry and rainy seasons, respectively. Native plants such as T. plumosus were highly colonized by mycorrhiza, suggesting that this may constitute an important adaptive mechanism of native species to soils deficient in P. A summary of the interrelationships among AM colonization, microbial biomass, and mineralization (APA production) in Trachypogon plumosus rhizosphere during wet and dry seasons is presented in Figure 15.7.
15.5.2.2 Releasing of organic acids and competition for phosphorus sorbing places in the rhizosphere
Many soil and rhizosphere microorganisms have the ability to solubilize phosphate (Richardson, 2001; Hayat et al., 2010). As early as the 1950s, Sperber (1958) found that organic acids, such as lactic, glycolic, citric, and oxalic, produced by microbial activities in soils were able to solubilize insoluble sources of phosphates. Moreover, it was noted that there is a relationship between the amount of phosphate solubilized and the soil production of organic acids (citric and oxalic) produced by the fungi Aspergillus niger, A. terreus, and Sclerotium rolfsu. Microorganisms can lead to the dissolution of insoluble phosphates by the production of inorganic or organic acids and/or the pH decrease, thus freeing available phosphate. In addition, these organic acids excreted by the microorganisms can act as chelating agents of metals present in insoluble sources as phosphate rock, also releasing soluble phosphates. The interaction between organic acids and sorbing places in soil materials has been very well studied by using the model of the adsorption isotherms (López-Hernández, 1977). Particular attention has been given to the competition between orthophosphate and organic anions in sesquioxides and clay materials (Nagarajah et al., 1968; Shen et al., 2011) and in soils (López-Hernández et al., 1986).
The rock phosphates are natural phosphate sources that are a more economical option for agriculture use, particularly in countries where the availability of raw material for the production of soluble phosphate is complicated (Toro et al., 1997). Leon et al. (1986) showed an increase in the number of soil microbial solubilizers after fertilizing with rock phosphate. Toro et al. (2008) also obtained a similar effect by applying phosphate rocks in a system managed with conservation techniques in savanna soils. These results suggest that the use of rock phosphate as fertilizer is favored by the proliferation of microbiota with an ability to solubilize P-insoluble sources. The interaction of phosphate microbial solubilizers and the arbuscular mycorrhizal fungi is positive and helps plant nutrition; when the source used is the rock phosphate, both microorganisms act synergistically (Toro et al., 1997, 1998) emphasizing the promising use of these organisms for agricultural management (Barea et al., 2002b; Richardson et al., 2009; Bhattacharyya and Jha, 2012).
In a study to characterize the ability of phosphate solubilizing bacteria to dissolve insoluble phosphate compounds and rock phosphate from native acidic soil of the savannas of Guárico State, Venezuela, it was found that these bacteria showed high ability to solubilize phosphate from Fe and Al compounds, and, to a lesser extent, from phosphate rock (Osorio, 2007). The species of bacteria found in the rhizospheres studied were mainly: Bacillus pumilus, Burkholderia cepacia, Bacillus circulans, and Pseudomonas fluorescens. By using the technique of high resolution liquid chromatography it was noted that the most active bacteria in the solubilization process generated acetic, tartaric, malic, butyric, and oxalic acids.
15.6 Conclusion and future prospects
Savannas from the Orinoco basin have acid soils with low nutrient content, particularly N and P deficiencies, which together with the marked climatic seasonality reduce the spectrum of possibilities for agricultural production. Despite the environmental constraints, these ecosystems are important centers of agricultural and forestry activities in Venezuela and Colombia and represent the main future alternative to prevent expansion into tropical areas of greater ecological fragility as mountainous landscapes and the Amazonian rainforest. Plants growing on limited N and P soils are usually adapted through two strategies: (1) enhancing N and P conservation and use efficiency and (2) enhancing N fixation and P incorporation and uptake. Vegetation of tropical savannas of the Orinoco basin usually have low nutrient requirement and therefore low nutrient content, but a high use efficiency of N and P. On the other hand, soil biota such as termites, earthworms, arbuscular mycorrhiza, phosphate solubilizing bacteria, and symbiotic N-fixing bacteria as Rhizobium can play a key role in nutrient cycling and in the protection of the plant to environmental stress. These microorganisms have great future potential to contribute to improving local fertility problems and consequently they might have a potential use to generate biofertilizers to be used once the savanna is transformed in more intensive agricultural systems.
Savannas from the Orinoco basin are under the influence of different factors, which affect nutrient cycling and should be investigated. Natural herbaceous vegetation with low nutrient requirements has been replaced by exotic and more productive pastures for cattle raising. Also, large-scale plantations of pine (Pinus caribea) and eucalyptus (Eucalyptus spp.) have been established since the end of the 1960s (Rondón et al., 2006). Vegetal production intensification results in net changes in the amounts of C stored in the soil and the introduction of African grasses have displaced native species, converting relatively diverse and open savanna communities into monospecific grassland stands (Williams and Baruch, 2000). New questions arise from the influence of different fire regimes (Hutley et al., 2013) and changes in land use (San José et al., 2008; Groover et al., 2012) on the role of savannas as a sink or source of C, and how higher temperatures and inputs of C and N through atmospheric fertilization modify ecosystem structure and function (Higgins and Scheiter, 2012; Wake, 2012), particularly on biomass production and nutrient cycling.
Acknowledgments
We thank Fondo Nacional de Ciencia y Tecnología (Proyecto S1-2000000649) and Consejo de Desarrollo Científico y Humanístico-UCV (Proyecto 03.31.4109.98) for partially financing the research involved in this chapter. Thanks to M. Auxi Toro for the English revision of the manuscript. We are grateful to the personnel at the Estación Biológica los Llanos (SVCN) and Estación Experimental La Iguana (USR) who allowed us to use their installations, gave us technical assistance during field sampling, and provided us with information pertinent to the studied subjects.




