Bt Crops
A Sustainable Approach towards Biotic Stress Tolerance
Mahmood-ur-Rahman, Muhammad Qasim, Shazia Anwar Bukhari and Tayyaba Shaheen
Insect pests are the main sources of biotic stress on crops. There are hundreds of insects that can cause serious damage to crops and are controlled by chemical pesticides, which are the main sources of pollution and cause the development and progression of a number of health problems in humans and animals. Because of these problems, scientists are always searching for alternative ways to control pests that impair sustained agriculture. Over a century ago, it was discovered that the Bacillus thuringiensis possesses insecticidal activity. Subsequently, several genes were identified that are insect resistant. In 1980, the technology was developed to transform these genes of Bacillus thuringiensis into crop plants. These genes were established to be resistant to the target insects and added desired resistance to crops capable of higher productivity and lower insect infestation. With the launch of the first transgenic plant varieties in 1996, this technology is becoming increasingly popular among farmers in both developed and developing countries. Now, 15 nations are growing genetically modified (GM) maize, especially in developed countries. This chapter describes the role of Bt genes for sustainable resistance against insect pests.
Keywords
genes; Bt cotton; insect resistance; transgenic plants; biosafety; risk assessment
6.1 Introduction
The transgenic plants harboring genes from Bacillus thuringiensis (Bt) produce crystal proteins causing resistance to insect pests harmful to crops and thus help to minimize the use of chemical insecticides. Since 1996, many plants have been genetically transformed with genes derived from Bt. Using this technology, plants acquire the ability to produce toxins and perform functions of self-pest resistance and eliminating the need to apply chemical sprays/synthetic pesticides. Genetically modified (GM) crops used to produce an insecticide were first marketed in the late 1990s.
Currently, all commercial Bt crops have genes that produce various forms of insecticidal toxins of Gram-positive bacteria (Federici, 2002). GM crops produce a variety of toxins that have specific effects on specific orders of insect pests (for example, Lepidoptera, Coleoptera, Diptera, etc.). Commercial Bt crops available today are cotton transformed with Bt genes (Cry1Ac, Cry2A, Cry1F, etc.), maize (Cry1Ab, Cry1Ac Cry1F, Cry3B, Cry9C, etc.), and potatoes (Cry3Aa) (Federici, 2002; Shelton et al., 2002). Some other crops, such as apple, broccoli, cabbage, tobacco, tomato, soybean, and rice, have been developed to express Bt genes but they have not yet been marketed (Table 6.1).
Table 6.1
Economics of Bt Cotton and Maize
| Crop | Country | Insecticide Reduction (%) | Increase in Yield (%) | References |
| Cotton | Argentina | 47 | 33 | Qaim and de Janvry (2005) |
| Australia | 48 | 0 | Fitt (2003) | |
| China | 65 | 24 | Pray et al. (2002) | |
| India | 41 | 37 | Sadashivappa and Qaim (2009) | |
| Mexico | 77 | 9 | Traxler et al. (2003) | |
| South Africa | 33 | 22 | Gouse et al. (2004) | |
| USA | 36 | 10 | Carpenter et al. (2002) | |
| Maize | Argentina | 0 | 9 | Brooks and Barfoot (2005) |
| Philippines | 5 | 34 | Yorobe and Quicoy (2006) | |
| South Africa | 10 | 11 | Gouse et al. (2006) | |
| Spain | 63 | 6 | Gomez-Barbero et al. (2008) | |
| USA | 8 | 5 | Fernandez-Cornejo and Li (2005) |

The first successful commercial Bt crop, Bollgard cotton, was marketed in the United States in 1996. In 2000, it occupied approximately 1.8 million acres (Perlak et al., 2001). It has an innate resistance to lepidopteran insects. Bollgard cotton contributed significant economic benefits to US agriculture. Studies have shown that US farmers receive an average benefit return of about $50/acre (Perlak et al., 2001). The introduction of Bt cotton drastically reduced the use of conventional chemical pesticides (Smith, 1997). Transgenic Bt crops are particularly protected against flies, worms, and beetles. Other benefits associated with Bt plants are:
• No need to use synthetic chemical pesticides.
• Increased opportunities for beneficial insects; Bt proteins do not kill non-target insects.
Important crops, which are damaged by the larvae and beetles, have been genetically modified to produce Bt toxin to control these pests. Genetically, modified Bt cotton contains a natural toxin released by the bacteria. In any case, the use of these systems means that many chemical pesticides will be eliminated. This practice is good for the consumer and for the environment and farmers.
6.2 Bacillus thuringiensis
Bacillus thuringiensis is a Gram-positive bacterium. It lives in the soil and is a biological pesticide that has been known for more than a century. It is also found naturally in the gut of caterpillars and on dark areas of the surfaces of plants (Madigan and Martinko, 2005). Most of the strains of B. thuringiensis have cry genes which encode δ-endotoxin insecticidal proteins and are present on the plasmids (Xu et al., 2006). Previously, the presence of a plasmid in a strain of B. thuringiensis suggested its involvement in endospore and crystal formation (Cheng, 1984). See Table 6.2 for various milestones of Bt gene discovery.
Table 6.2
History of Bacillus thuringiensis and Bt Gene Discovery
| Year | Event |
| 1901 | Shigetane Ishiwatari isolated Bacillus thuringiensis |
| 1911 | Ernst Berliner isolated a bacteria that killed a Mediterranean flour moth and rediscovered B. thuringiensis |
| 1915 | Berliner reported the existence of a crystal within B. thuringiensis |
| 1920 | Farmers started to use Bt as a pesticide |
| 1938 | France started to make commercialized spore-based formulations of Bt called Sporine |
| 1956 | Hannay, Fitz-James, and Angus found that the main insecticidal activity against lepidoteran (moth) insects was due to the parasporal crystal |
| 1958 | Bt was started to be used commercially in USA |
| 1961 | Bt was registered as a pesticide to the EPA |
| 1977 | The first subspecies toxic to dipteran (flies) species was found |
| 1983 | First discovery of strains toxic to species of coleopteran (beetles) |
| 1980s | Routine use of Bt as pesticide |
| 1995 | The first genetically engineered plant, corn, was registered with the EPA |
The development of genetic transformation was an important milestone in the field of plant biotechnology. Now, scientists can introduce genes from different sources into the main crops, in order to induce resistance to various pests (Lycett and Grierson, 1990; Dhaliwal et al., 1998). B. thuringiensis has been widely known as a source of Bt genes for quite some time. These genes produce crystal proteins that are toxic to specific orders of various insect pests such as Lepidoptera (Whiteley and Schanept, 1986; Hoftey and Whiteley, 1989; Cohen et al., 2000), beetles (Krieg et al., 1983; Herrnstadt et al., 1986), and Diptera (Andrews et al., 1987). When insects feed on toxin crystals, the alkaline pH of their digestive system triggers the toxin. Cry toxin gets into the cell membrane of the insect gut and forms a pore. Pores lead to cell lysis and death of the insect (Dean, 1984). B. thuringiensis-based insecticides are generally used in the form of liquid sprays in plants. Insecticides are effective mainly when internalized. The toxins are soluble and are believed to produce pores in the epithelium of the midgut of the affected larvae. Some studies suggested the midgut bacteria of susceptible larvae are required for B. thuringiensis insecticidal activity (Broderick et al., 2006).
6.3 Transformation of crops with Bt genes
Genetic modification of plants developed into a new era with progress in the science of recombinant DNA technology in the 1970s. With this technology, genes from different heterologous systems, namely, animals, bacteria, or insects, can be transferred to plants (Deineko et al., 2007). Genetic engineering involves the transformation of a gene responsible for a particular trait into the plant genome and the subsequent development of a complete plant from the transformed tissue. Several methods of gene transfer in plants, such as the transfer of PEG-mediated genes (Uchimiya et al., 1986), microinjection (de la Pena et al., 1987), electroporation (Fromm et al., 1985, 1986; Lorz et al., 1985; Arencibia et al., 1995) and the method of particle bombardment (Sanford, 1988), were developed.
Currently, the methods used for the transformation of genes in crops are Agrobacterium-mediated transformation and transformation by particle bombardment (Deineko et al., 2007; Gelvin, 2012; Azad et al., 2013) (Figure 6.1). Transgenic plants have become increasingly popular among farmers in developed countries and also in the developing world. Millions (16.7 million) of farmers in 29 countries grew biotech crops on 160 million hectares in 2011, of which 90% (about 15 million) were small farmers in developing countries (James, 2012).
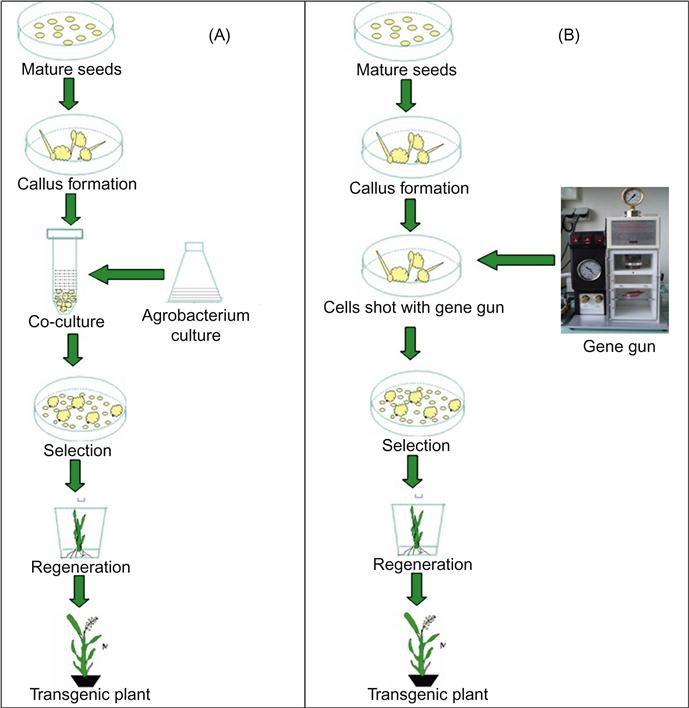
6.3.1 Agrobacterium-mediated genetic transformation
Agrobacterium tumefaciens is a bacterium that has the ability to transfer foreign genes in host plant cells. A. tumefaciens causes wound infections in dicotyledonous plants, and leads to the formation of root-knot (tumors). The first evidence that this bacterium was the causative agent of crown gall was found over a century ago (Smith and Townsend, 1907). It has the capability of transferring a DNA fragment of the carcinogenic (Ti) plasmid into the nucleus of infected cells, causing crown gall (Nester et al., 1984). Tumor formation is a transformation of plant cells due to the transfer and integration of T-DNA and the subsequent expression of the genes of T-DNA and foreign DNA between the T-DNA borders placed in plant cells (Deblaere et al., 1985; Hooykaas and Schilperoort, 1992; Hamilton, 1997; Torisky et al., 1997; Rao et al., 2009; Kumar et al., 2012; Anami et al., 2013).
The first report of a successful plant expressing foreign genes was available at the beginning of 1980 (Herrera-Estrella, 1983). A. tumefaciens naturally produces tumors in dicotyledonous plants only. This method of genetic transformation has many advantages compared to those of other methods. Low number of copies of the transgene is transformed in this process, which is desirable. The method has fewer problems of instability of the transgene and co-suppression (Koncz et al., 1994; Hansen et al., 1997). In addition, it takes a mutated gene in a single cell and not a patchwork of plants, which is very common when the direct gene transformation method is used (Enriquez-Obregon et al., 1997, 1998).
Agrobacterium-mediated transformation has been established in dicotyledous plants for many years and has recently been applied in monocotyledous plants (Hiei et al., 1994; Cheng et al., 1998). Previously, it was not possible in monocots. Now it is being used in various plants after the development of efficient and reproducible methods in banana (May et al., 1995), corn (Ishida et al., 1996), wheat (Cheng et al., 1997), sugarcane (Enriquez-Obregon et al., 1997, 1998), and chilli (Kumar et al., 2012). Now, the process has been used with success in several monocotyledonous crops (Chan et al., 1993; Hiei et al., 1994; Park et al., 1996; Rashid et al., 1996). Currently, Agrobacterium-mediated transformation is also being successfully used in rice transformation.
6.3.2 Transformation of plants by particle bombardment
Particle bombardment is a method of genetic transformation of crop plants by which the DNA is transformed directly into plant cells by physical or chemical method. The advantage of the direct transformation of genes is that each piece of DNA can be transferred and no special vectors are needed (Potter and Jones, 1997). There are many methods of direct transfer of plant-derived genes. The most promising approach is the “gene gun method” (Sanford, 1990; Jenes et al., 1992). This method involves high speed microprojectiles containing foreign DNA which penetrates through the cell wall and membrane. Thus, DNA can enter the plant genome.
Genetic transformation by particle bombardment makes use of different physical methods to obtain transformed plants. Currently, it is the most effective way to obtain transformed plants and is the only method previously used to achieve the transformation of chloroplasts and mitochondria (Altpeter et al., 2005). Successful and stable transformation of foreign genes is reported for rice, barley, and many other crops. Different types of plant tissues, such as floral tissues (microspores and anthers), tillers, and immature embryos, were successfully transformed by particle bombardment (King and Kasha, 1994; Stiff et al., 1995). Bt plants developed by both the Agrobacterium method and gene gun method, registered in the United States, are listed in Table 6.3.
Table 6.3
Bt Crops Registered in USA up to 2009
| Crop | Bt Toxins | Target Insects | Registered |
| Corn | Cry1Ab | Lepidoptera | 1995 |
| Cry1F | Lepidoptera | 2001 | |
| Cry3Bb1 | Coleoptera | 2003 | |
| Cry1Ab+Cry3Bb1 | Lepidoptera and Coleoptera | 2003 | |
| Cry34Ab1+Cry35Ab1 | Coleoptera | 2005 | |
| Cry1F+Cry34Ab1+Cry35Ab1 | Lepidoptera and Coleoptera | 2005 | |
| Modified Cry3A | Coleoptera | 2006 | |
| Cry1Ab+Modified Cry3A | Lepidoptera and Coleoptera | 2007 | |
| Cry1A.105+Cry2Ab2 | Lepidoptera | 2008 | |
| Cry1A.105+Cry2Ab2+Cry3Bb1 | Lepidoptera and Coleoptera | 2008 | |
| Vip3Aa20 | Lepidoptera | 2008 | |
| Cry1Ab+Vip3Aa20 | Lepidoptera and Coleoptera | 2009 | |
| Cry1Ab+Vip3Aa20+modified Cry3A | Lepidoptera and Coleoptera | 2009 | |
| Cry1A.105+Cry2Ab2+Cry1F+Cry3Bb1+Cry34Ab1+Cry35Ab1 | Lepidoptera and Coleoptera | 2009 | |
| Cotton | Cry1Ac | Lepidoptera | 1995 |
| Cry1Ac+Cry2Ab2 | Lepidoptera | 2002 | |
| Cry1Ac+Cry1F | Lepidoptera | 2004 | |
| Modified Cry1Ab+Vip3Aa19 | Lepidoptera | 2008 |
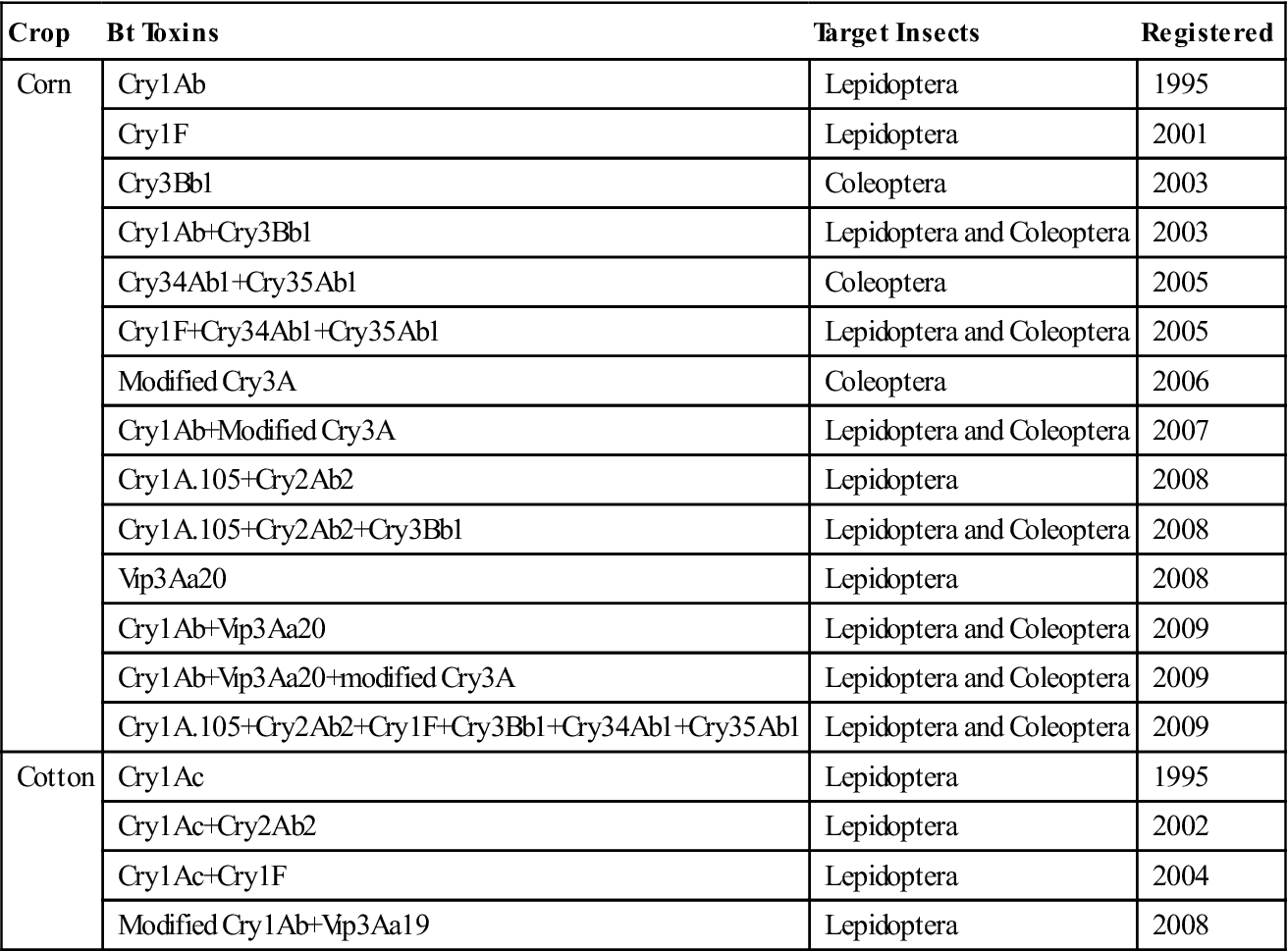
Source: Tabashnik et al., 2009.
6.4 Molecular analyses of putative transgenic plants
Detection of genetically modified organisms (GMOs) is possible through biochemical and molecular methods. It can be qualitative or quantitative. The following are the procedures to detect the presence and expression of Bt genes in the host genome.
6.4.1 Selection marker
A selectable marker gene in a GM organism develops a characteristic that can be used for artificial selection; this attribute is called a reporter gene/s. They are often genes that confer antibiotic resistance (Tuteja et al., 2012; Bala et al., 2013).
6.4.1.1 Types of selection marker
6.4.1.1.1 Antibiotic-resistant marker genes
Marker genes cause resistance patterns in a transgenic organism, an antibiotic to which the gene has been introduced. For example, neomycin phosphotransferase II (NPT-II gene) is typically being used in transgenic technology and results in resistance to the antibiotics kanamycin and neomycin (Tuteja et al., 2012).
6.4.1.1.2 The marker genes for herbicide tolerance
Transgenic plants resistant to herbicides have marker genes specific to chemical herbicides. The gene for tolerance to the herbicide glufosinate-ammonium is often used as a marker in the technology of GM plants. Those cells that survive exposure to the herbicide are selected and regenerated into a whole organism (Dale et al., 2002). The ALS gene is also being used as a selectable marker and is gaining popularity for several reasons. It is present in all plants, so there is no potential food safety concern (Yao et al., 2013).
6.4.1.1.3 Metabolic markers genes
Metabolic or auxotrophic marker genes allow transformed cells to synthesize an essential component, usually an amino acid, which is not produced by the application in any other way to synthesize the cells. The surrounding medium intentionally does not have the essential component that cells need to grow. Cells that have successfully incorporated the selection marker and the rest of the gene construct will be able to produce the principal components in the cells and therefore survive. Such cells are selected and regenerated into whole organisms.
6.4.1.1.4 Screenable marker genes
Detectable marker genes encode a protein that can be identified by various laboratory tests. The presence of the protein confirms that the transformation has successfully occurred. Detectable marker is used as an alternative to a selectable marker as an aid for researchers who intend to distinguish between wanted and unwanted cells, based on the phenotype (Miki and McHugh, 2004). There are different types of methods of detection:
1. Green fluorescent protein makes the cells glow green under UV light. Yellow fluorescent protein and red fluorescent protein are also available. Using different colors, scientists can easily study multiple genes simultaneously. The technology is widely used in transgenic crop technology (e.g., maize, Kirienko et al., 2012; Coussens et al., 2012).
2. GUS assay (using β-glucuronidase) is a method for the detection of a single cell for blue stain without requiring complicated equipment.
6.4.2 Molecular analyses based on DNA
6.4.2.1 Polymerase chain reaction (PCR)
Polymerase chain reaction (PCR) is a method for the molecular amplification of a fragment of DNA. It allows the detection of specific DNA strands possessing millions of copies of a sequence of the target gene. The method works by synchronizing the sequence of the gene-specific DNA fragments with complementary primer. Specific genetic primers can be used to detect the presence of the genes that can be studied by nucleotide sequences of Bt genes in the presence of the target sequence. The primer matches the newly inserted gene with the assistance of such primer and starts a chain reaction. The process is repeated several times by sequential heating and cooling, and the target sequence is multiplied several million times. The millions of identical fragments are dyed to identify a segment of the primer-amplified-specific DNA on the gel under UV light (Figure 6.2A).

6.4.2.1.1 Qualitative detection
B. thuringiensis genes in a sample can be analyzed by qualitative PCR and multiplex PCR. Multiplex PCR uses multiple, unique primer sets within a single PCR. Amplicons of varying sizes specific to different DNA sequences that produce different transgenes are detected on agarose gel. By targeting multiple genes at once, additional information from a single test cycle is obtained, which saves time and the reagents (Figure 6.2A).
6.4.2.1.2 Quantitative detection
Quantitative PCR (Q-PCR) was used to measure the amount of PCR product. It is the preferred method to measure quantitatively the levels of transgenic DNA. Q-PCR is often used to determine the number of copies in the sample. The method is endowed with the highest accuracy of real-time quantitative PCR. Methods of QRT-PCR use fluorescent dyes such as SYBR Green or DNA probes containing a fluorophore, such as TaqMan, to measure the amount of amplified color product in real time (Figure 6.2B).
6.4.2.2 Southern blot
This technique was developed by Southern (Southern, 1975) to detect a complementary known DNA sequence used for the identification of desired DNA fragments using a specific probe. Southern blot allows a comparison of the genome of a particular organism of which a fragment of a gene or genes is available (the probe). One can tell if an organism contains a particular gene, and provide information on the organization and restriction map of the gene. The chromosomal DNA isolated from the microorganism of interest is completely digested with restriction endonucleases. The restriction fragments are separated by electrophoresis on agarose gel. The next step is the transfer of fragments of gel to a nitrocellulose filter or nylon membrane. This technique is dependent on the size and the specific activity of the probe and requires a large amount of DNA. Short probes are more accurate in general.
6.4.2.3 Fluorescent in situ hybridization
In situ hybridization, as the name implies, is a method for locating any mRNA or chromosomal DNA in the cell cytoplasm by hybridization of the sequence of interest in a free strand of a nucleotide probe. It is a powerful tool for the physical mapping of genes (Jiang et al., 1995) and target nucleic acid sequences that can be detected on the chromosomes, cells, or tissue sections. The technique was originally developed by Pardue and Gall (1969). They observed sequences hybridized with radioactive probes, because at that time only radioisotope-labeled nucleic acid materials were available.
Several disadvantages of isotopic hybridization inspired the development of new techniques. The dangers of using radioactive probes are: (1) the probes are unstable due to isotope decay over time, and, therefore, the specific activity of the probes is not constant; (2) sensitivity to X-rays is generally high and resolution is limited; (3) they require long exposure times to produce measurable signals on X-ray film; and (4) probes of radioactive materials are relatively expensive and dangerous, and must be transported, processed, stored, and disposed of with great care (Jeffrey and Singer, 2003).
Now, fluorochrome is used for labeling the probe for the detection of nucleic acid complementary sequence; the method is known as “fluorescent in situ hybridization” or FISH. The first fluorescence detection of antibody-dependent nucleic acid hybrids was by Rudkin and Stollar (1977), but this technology has been replaced with the advent of nucleic acid probes labeled with fluorescence. The technique of FISH was introduced for the first time in 1980. FISH allows (1) high sensitivity, (2) a short measurement period, and (3) to reach the manipulation of the sample (Ohmido and Fukui, 1997). Using this technique, a specific transgene was detected (Mahmood-ur-Rahman et al., 2010) (Figure 6.2C and D).
6.4.3 Molecular analyses based on protein
6.4.3.1 Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) is a method for detecting the presence of a particular protein in a sample. The technique requires a binding reagent that can be immobilized on the solid phase with a detection reagent that is specifically used for an enzyme and generates a signal that can be quantified correctly. Specifically, only the required protein and its specific interactions of antigen–antibody binding to the solid phase are detected, while the non-specific components are unbound and are washed.
6.4.3.2 Western blotting
The Western blot technique is widely used to detect proteins in a given sample. In this technique, gel electrophoresis is used to separate proteins. These proteins are transferred onto a nitrocellulose membrane, where they are examined by the use of antibodies specific for the transferred target protein.
6.5 Greenhouse and field experiments
The development of resistance in insects against Bt toxins is a major problem after prolonged exposure in the field. The most important technologies to increase the effectiveness of the Bt plant is a pyramid of two or more genes in crops (Cohen et al., 2000), observed in field conditions by evaluation over several years. Several transgenic lines of Basmati rice with Bt genes were evaluated under field conditions over several generations (Bashir et al., 2005; Mahmood-ur-Rahman et al., 2007) and sustainable resistance was monitored. These transgenic lines have shown resistance to target pests at all stages of growth and development of plants. After five years of field exposure, it has been observed that insect resistance was very stable over the years and the lines were significantly better as compared to untransformed control plants under field conditions (Bashir et al., 2005; Mahmood-ur-Rahman et al., 2007). These lines have shown resistance to advanced generations and there was no problem of gene silencing or gene segregation. Although these lines were significantly different from non-transformed control plants in height and days to maturity, these features have been observed up to the eighth generation, with trouble-free operation or segregation associated with these inherited lines.
Resistance against various lepidopteran pests in rice has been studied with the Bt genes (Bashir et al., 2004a, 2005; Breitler et al., 2004; Ye et al., 2001a,b, 2003). Zhau et al. (2003) reported that in the greenhouse, pyramidal two toxin genes with different mechanisms of cultivated plants show delays in the development of resistance. Toxin titers varied between the different lines, the plant with the same Bt gene construct (Cheng et al., 1998; Datta et al., 1998), and transformed assumes substantially in the later stages of development, particularly during the reproductive phase in rice (Alinia et al., 2000) (Table 6.4).
Table 6.4
Field Evaluation of Transgenic Rice
| Gene | Trait | Location | References |
| cry1Ab and cry1Ac | Insect resistance | China | Tu et al. (2000) |
| cry1Ab | Insect resistance | China | Shu et al. (2000) |
| cry1Ab | Insect resistance | China | Ye et al. (2001a) |
| cry1Ab | Insect resistance | China | Shu et al. (2002) |
| cry1Ac and cry2A | Insect resistance | Pakistan | Bashir et al. (2004a,b) |
| cry1Aa and cry1B | Insect resistance | Spain | Breitler et al. (2004a) |
| cry2A | Insect resistance | China | Chen et al. (2005) |
| cry1Ac and cry2A | Insect resistance | Pakistan | Mahmood-ur-Rahman et al. (2007) |
| cry1Ac and CpT1 | Insect resistance | China | Han et al. (2006) |

It is already documented that the transgenic lines could be early or late maturation (Jiang et al., 2000). There are several possible reasons for the morphological changes in transgenic plants, including somaclonal variations (Larkin and Scowcroft, 1981), disruption of indigenous genes in transgenic plants (Van et al., 1991), pleiotropy, and gene silencing (Matzke et al., 2000). Somaclonal variations may be one of the main reasons, as it took more time for the production of transgenic plants, compared to normal tissue culture techniques. The longer the time in tissue culture, the higher the frequency of somaclonal variations in plants (Kaeppler et al., 2000). The antibodies used as selectable markers can induce mutations in rice (Wu et al., 2000).
There are several studies of genetic transformation and evaluation of its effectiveness in the greenhouse and field (Bashir et al., 2004a, 2005; Breitler et al., 2004; Shu et al., 2000; Ye et al., 2001a,b, 2003). Most of the transgenic lines used in the field tests expressed Cry1Ab, Cry1Ac Cry2A, or Cry1Ab/Cry1Ac genes. With the passage of time, over 500 species of insects have acquired resistance to the conventional insecticides. So far, the history of Bt is better. In the field, only the diamondback moth is reported to have developed resistance against Bt sprays but has not acquired resistance to Bt crops (Ferre and Rie, 2002).
6.6 Biosafety and risk assessment studies
A series of problems associated with the development of resistance in target pest species, such as horizontal gene transfer, impact on non-target invertebrates, and morphological and agronomic variations, are being discussed globally. It is also important to consider if advanced generations of these lines are also resistant to insects in the field or lose their effectiveness over time, which could lead to a more rapid development of resistance in target pests. So, it is recommended that not all common varieties should be transformed with Bt genes to maintain refugia (Cohen et al., 2000). The potential of gene flow via pollen from Bt lines and other commercial varieties is an important consideration. The response of non-target insects on transgenic plants is well studied for herbivorous insects, insects, and invertebrates (Bashir et al., 2004a,b; Noor et al., 2010).
The specificity of cry genes to some orders of insects is its most important feature. Bt sprays do not disrupt the overall structure of a community’s predators or parasitoids, or population trajectories of non-target herbivores (grasshoppers and crickets; Schoenly, 2004). Several researchers have tried to determine the number of non-target insects in transgenic and control fields (Fitt et al., 1994; Sims, 1995; Orr and Landis, 1998; Bashir et al., 2004a,b). The results showed that Bt crops are safe for non-target insects pests and visiting insects (Hendriksma et al., 2012, 2013).
There is some concern that purified Bt toxins can pose risks to the soil environment. The fate of Bt protein in earth is an important parameter (Saxena et al., 1999; Saxena and Stotzky, 2001; Stotzky, 2001). Bt concentrations could accumulate in the soil and pose a threat to earthworms, nematodes, protozoa, bacteria, and fungi. Contrasting results were obtained for evaluating the persistence of Bt protein. Donegan et al. (1995) observed immunological activity of the protein up to 28 days and for proteins Cry1Ab and Cry1Ac up to 56 days in the soil.
There are two types of gene flow: horizontal gene transfer and vertical gene flow. Horizontal gene transfer (HGT) is the transfer of genetic material from one organism to another organism, which is not sexually compatible with the donor (Gay, 2001). HGT is based on the known mechanisms in bacteria, including transduction, conjugation, and transformation. Accidental transfer of DNA between bacterial cells by bacteriophage infection has been studied (Kidambi et al., 1994; Herron, 1995). Ochman et al. (2000) have reported that it has been detected in up to 16% of the protein coding DNA in bacteria in the vicinity. Transfer of mobile sequences (plasmids, transposons, and chromosomal genes mobility) between the bacterial cells capable of mediating between the bacterial population in the rhizosphere soil and plant surfaces and water is also reported.
Non-target effects are no adverse effects of GM plants on friendly organisms in the environment (Dale et al., 2002). Non-target organisms are pollinators and herbivores feed on the affected and surrounding vegetation. It is important to remember that every human intervention to protect crops from pests has some negative effect on non-target organisms (Schuler, 2000) and on the entire community of life (Shelton et al., 2002). The impact of GM crops on the population dynamics of natural enemies depends on several factors, including the expression of the transgene, the specificity of the transgene product, and the tissue specificity of the transgene (Schuler, 2000). The populations of many non-target species were higher in Bt cotton fields as compared to the field sprayed by conventional pesticides (Xia et al., 1999; Head et al., 2001). Some insects and spiders were strongly influenced by insecticide sprays (Candolfi et al., 2004).
Some chemicals, known as allelochemicals, released from certain plants are known to have inhibitory or stimulatory effects on the growth of other crops (Torres et al., 1996). GM crops could have the potential to suppress other cultures, which decreases biodiversity in an ecosystem. Some allelochemicals isolated from the transgenic rice hull and identified as momilactone A and momilactone B have no allelopathic effects, while allelochemicals from barnyard grass have inhibitory effects on the germination and growth of plants (Kato-Noguchi, 2004; Xu et al., 2005; Chung et al., 2006).
6.7 Conclusion and future prospects
The year 2011 was the 16th year of the commercialization of GM crops (James, 2012). An increase of 94 times in the area of 1.7 million hectares in 1996 to 160×106 hectares in 2011 makes biotech crops the fastest adopted agricultural technology in the history of modern agriculture (James, 2012). In 2011, a record number of GM plants were grown by 16.7 million farmers. Over 90% or 15 million of these farmers were small resource-poor farmers in developing countries. Bt cotton farmers’ income has increased significantly while their exposure to pesticides has decreased (James, 2012). There are 13 nations, both developed and developing, growing Bt cotton today, including the United States, Brazil, Argentina, India, China, Pakistan, and Australia.
The world needs at least 70% more food by 2050. For developing countries, food production must double by 2050, where 2.5 million poor small farmers need to survive. GM crops have had an exemplary safety record because they benefit farmers, the environment, and consumers. GM crops have been adopted faster than any other agricultural advance in the history of humanity (Alberts et al., 2013). The adoption of GM crops should be increased while keeping in view the following three factors: (1) timely implementation of appropriate, responsible, and cost/time-effective regulatory systems; (2) a strong political will and financial support; and (3) constant improvement in biotech crops in Asia, Latin America, and Africa (James, 2012), to meet the requirements of developed as well as developing countries.
Acknowledgments
The authors acknowledge the financial support of the Higher Education Commission, Government of Pakistan. The authors are also thankful to Prof. Dr. Muhammad Ibrahim Rajoka, Department of Bioinformatics and Biotechnology, GC University, Faisalabad (Pakistan) who read the manuscript critically.
