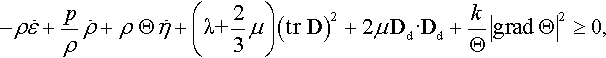Energy Formulations and Stability Conditions for Newtonian Fluids
In this section, we review and augment the thermomechanical theory for compressible Newtonian fluids presented in Chapter 7 and discuss the corresponding stability conditions.
E.1 Governing equations
Recall that the fundamental laws for a continuum in the thermomechanical theory are
which correspond to Eulerian statements of conservation of mass, linear momentum, and energy; refer to Section 5.3. In (E.1), ρ is the density, v is the velocity, T is the Cauchy stress, b is the body force per unit mass, ε is the internal energy per unit mass, r is the rate of heat absorbed per unit mass, q is the heat flux vector, and
is the rate of deformation. A compressible Newtonian fluid (refer to Section 7.2.1.2) is characterized by the constitutive equations
where p is the pressure, Θ is the absolute temperature, λ and μ are the dilatational and shear viscosities, respectively, and k is the thermal conductivity. The second law of thermodynamics in the form of the Clausius-Duhem inequality is
where η is the entropy per unit mass. It can be verified that use of the constitutive equations (E.2) in the second law (E.3), and subsequent decomposition of the rate of deformation D into its spherical (volumetric) and deviatoric (traceless) parts, i.e.,
leads to
where we have used
The mathematical model for a compressible Newtonian fluid is completed by specifying the equations of state. In the following sections, we present four equivalent formulations of the equations of state, each employing a different set of independent variables: density ρ or pressure p for the independent mechanical variable, and entropy η or temperature Θ for the independent thermal variable. (Recall that only the density-temperature formulation—with ρ as the independent mechanical variable and Θ as the independent thermal variable—was explored in Section 7.2.1.2.)
E.1.1 Density-entropy formulation
The system formulation (E.1) and (E.2) implicitly uses three choices: density ρ as the independent mechanical variable, entropy η as the independent thermal variable, and internal energy ![]() as the thermodynamic energy potential. Use of the chain rule
as the thermodynamic energy potential. Use of the chain rule
in the second law (E.4) gives
Using arguments similar to those employed in Section 7.2.1.2, we can prove that as a consequence of inequality (E.5),
and
With the internal energy ![]() a specified function of ρ and η, the equations of state (E.6a)1 and (E.6a)2 prescribe the dependent mechanical and thermal variables p and Θ in terms of the independent mechanical and thermal variables ρ and η. Equation (E.6b) demands nonnegativity of the bulk viscosity, shear viscosity, and thermal conductivity. Note that the material parameters λ, μ, and k may depend on the density ρ and entropy η. The conservation laws (E.1), constitutive equations (E.2), and equations of state (E.6a) constitute a closed system for the primitive quantities ρ, η, and v.
a specified function of ρ and η, the equations of state (E.6a)1 and (E.6a)2 prescribe the dependent mechanical and thermal variables p and Θ in terms of the independent mechanical and thermal variables ρ and η. Equation (E.6b) demands nonnegativity of the bulk viscosity, shear viscosity, and thermal conductivity. Note that the material parameters λ, μ, and k may depend on the density ρ and entropy η. The conservation laws (E.1), constitutive equations (E.2), and equations of state (E.6a) constitute a closed system for the primitive quantities ρ, η, and v.
E.1.2 Density-temperature formulation
In this formulation, density ρ is the independent mechanical variable, temperature Θ is the independent thermal variable, and the Helmholtz free energy ![]() is the thermodynamic energy potential. The Helmholtz free energy
is the thermodynamic energy potential. The Helmholtz free energy ![]() is defined by the Legendre transformation of internal energy
is defined by the Legendre transformation of internal energy ![]() with respect to the thermal variable, from η to Θ,
with respect to the thermal variable, from η to Θ,
See also (5.33). Taking the material derivative of (E.7), we obtain
so inequality (E.4) becomes
Use of the chain rule
in the second law inequality (E.8) leads to
Using standard arguments, we obtain
and
See also (7.32). Note that the material parameters λ, μ, and k may depend on the density ρ and temperature Θ. With the Helmholtz free energy ![]() a specified function of ρ and Θ, (E.1), (E.2), (E.7), and (E.9a) form a closed system for ρ, Θ, and v.
a specified function of ρ and Θ, (E.1), (E.2), (E.7), and (E.9a) form a closed system for ρ, Θ, and v.
E.1.3 Pressure-entropy formulation
In this formulation, pressure p is the independent mechanical variable, entropy η is the independent thermal variable, and enthalpy ![]() is the thermodynamic energy potential. The enthalpy
is the thermodynamic energy potential. The enthalpy ![]() is defined by the Legendre transformation of internal energy
is defined by the Legendre transformation of internal energy ![]() with respect to the mechanical variable, from specific volume 1/ρ to pressure p,
with respect to the mechanical variable, from specific volume 1/ρ to pressure p,
The rate of the Legendre transformation (E.10) is
Substitution of (E.11) into the second law inequality (E.4) gives
Subsequent use of the chain rule on ![]() leads to
leads to
from which it follows that
and
Note that the material parameters λ, μ, and k may depend on the pressure p and entropy η. With the enthalpy ![]() a specified function of p and η, the equations of state (E.12a) together with the conservation laws (E.1), constitutive equations (E.2), and Legendre transformation (E.10) form a closed system for p, η, and v.
a specified function of p and η, the equations of state (E.12a) together with the conservation laws (E.1), constitutive equations (E.2), and Legendre transformation (E.10) form a closed system for p, η, and v.
E.1.4 Pressure-temperature formulation
In this formulation, pressure p is the independent mechanical variable, temperature Θ is the independent thermal variable, and the Gibbs free energy ![]() is the thermodynamic energy potential. The Gibbs free energy
is the thermodynamic energy potential. The Gibbs free energy ![]() is defined by the Legendre transformation of internal energy
is defined by the Legendre transformation of internal energy ![]() with respect to the mechanical and thermal variables, from specific volume 1/ρ to pressure p and entropy η to temperature Θ,
with respect to the mechanical and thermal variables, from specific volume 1/ρ to pressure p and entropy η to temperature Θ,
The material derivative of (E.13) is
Use of (E.14) in the second law (E.4) yields
The chain rule
then implies that
Customary arguments allow us to conclude that
and
Note that the material parameters λ, μ, and k may depend on the pressure p and temperature Θ. With the Gibbs free energy ![]() a specified function of p and Θ, (E.1), (E.2), (E.13), and (E.15a) constitute a closed system for p, Θ, and v.
a specified function of p and Θ, (E.1), (E.2), (E.13), and (E.15a) constitute a closed system for p, Θ, and v.
E.2 Stability conditions
Stability of the rest state is required for a theory to be physically valid [22, 65]. For a compressible Newtonian fluid, a necessary and sufficient condition for stability of the rest state is that the internal energy ε is a convex function of entropy η and specific volume τ = ρ−1 [66, 67]:
where subscripts denote partial differentiation, e.g.,
With a change of independent variable from specific volume τ to density ρ, condition (E.16a) becomes
Note that one need monitor only two of these inequalities, since (E.16b)1 and (E.16b)3 imply (E.16b)2, and (E.16b)2 and (E.16b)3 imply (E.16b)1. It can be verified that the equivalent conditions on the Helmholtz free energy are
or, upon a change of independent variable from specific volume τ to density ρ,
Similarly, we have
for the enthalpy and
for the Gibbs free energy.
It can be shown (see, for instance, [68]) that for a compressible Newtonian fluid, the convexity conditions (E.16b) on the internal energy are equivalent to (1) nonnegativity of the specific heat at constant volume CV and the isothermal bulk modulus κ, i.e.,
and (2) an absence of negative decay rates in the linearized stability analysis of the the rest state. Thus, for a compressible Newtonian fluid, there are three equivalent conditions for stability of the rest state.