CHAPTER 8
Process Analysis for Defect Prevention
The problem is never how to get new, innovative thoughts into your mind, but how to get the old ones out
– Dee Hock
SYNOPSIS
Defects need to be classified as per their class irrespective of the nature of the process. Here, the class of defects are identified as human dignity, unhygienic factors, housekeeping, mistake proofing, process consumables, clearance gate, gaps in defect prevention measures, misinterpretation of work instructions and standard operating procedures, gaps in customer linkage, hidden defects and process capability. For a given process, identify the class of defects applicable to it; scrutinise each applicable one as per existing ground realities; and ensure that it has been eliminated, or prevented or rendered dormant. This is the process analysis for defect prevention.
Process review
Process review is an exercise wherein every process is reviewed against certain predetermined requirements/standards to ensure that the process is not a source of inefficiency and defects. Process review is equivalent to preventive action for a particular course of action—a team exercise. For example, a team for a manufacturing process can have personnel concerned drawn from production, quality and maintenance.
Process review technique hinges on the thought ‘look for defects and prevent them at source from occurring’ as against ‘get confronted by defects and then act upon to arrest them from occurring’. Prior to taking up any problem/project for continual improvement, the process associated with it is identified and subjected to review through a number of reference points to tie up the loose ends. This exercise is called process review.
Reference base—listing for review of process
Reference base for review is a collection of relevant issues against which a review has to be carried out. For example, reference base for review regarding a manufacturing process is given in Table 8.1.
TABLE 8.1 Reference for Review—Manufacturing Process
| Issues to be reviewed |
|---|
| Human dignity Unhygienic features Housekeeping Mistake proofing Process consumables ‘Clearance gate’ for process entry Misinterpretation in drawing, work instructions (WI), Standard operating procedures (SOP) Gaps in defect prevention measures Gaps in customer linkage Hidden defects Process capability |
Human dignity
Housekeeping, canteen, transport, material movement and dispatch jobs would have been designed and practiced in such a way that human dignity of personnel engaged in these jobs can be at stake, leading to a sense of apathy towards the job, resulting in defects, errors and poor performance. In all such jobs, the steps to be taken are as follows:
- Redesigning the work in such a way that it is perceived as an important and key work by those who are engaged in such jobs. One such redesigning measure is to build ‘autonomy’ in work by empowering them to plan, schedule and evaluate the quality of their own work including setting up of quality standards.
- Personnel apparel to wear and accessories to use while working should be arresting, attractive and dignified.
- Practices followed should make one recognise that upper management is concerned with maintaining high standards of performance and quality.
- Measures to improve the ergonomics to avoid awkward posture positions during work.
- Adopting ‘high technology’ to operate such jobs. This includes adoption of photosensitive devices in toilets, water closets and hand drying. Many canteens have gone hightech. The ISKCON canteen, manufacturing food for distribution to schools at Bangalore, is a remarkable example of designing mass cooking and its distribution in a respectful, dignified, hygienic and efficient manner.
Unhygienic features
The unhygienic features in a job include poor ventilation, poor lighting arrangements, dust–fume–smell–noise–heat–humidity beyond limits generated by the job, and working in awkward/uncomfortable positions which may impair the anatomy of workers in the long run. All these need to be mitigated even before encountering problems/difficulties (defects). This practice is defect prevention.
TABLE 8.2 Non-value Adding Activity: Few Examples
| Activity | Situation | Action |
|---|---|---|
| Fetching materials, tools | A distance of 10 ft has to be travelled four times per shift to get materials/tools | Keeping it close to the work point |
| Reorienting the material to feed | To reorient the work piece prior to feeding, 40 s are needed. Process time is 2 min | Supply reoriented components/install feeding bowls which correctly orients and feeds |
| Maintenance | Waiting for maintenance/follow-up with maintenance | Reverse the practice of maintenance seeking to serve |
| Activities external to main function | Non-nursing activities assigned to nursing personnel, likewise non-teaching activities to teaching personnel | Insulate from activities not related to one’s key function |
There is another type of unhygienic feature in a process. These are hidden and cannot be even recognised as unhygienic. These are non-value adding activities associated with a job. They are a source of distraction while on the job and hence a source of defect/error. This point is crucial. In certain jobs like surgery, driving, music and dance, such distractions of even few minutes duration can have devastating consequences. Such non-value adding jobs need to be meticulously identified through detailed activity analysis of a job and suitable action need to be taken to eliminate such activities. Certain examples in Table 8.2 illustrate the point. Chapter 14 also covers non-value adding jobs.
Housekeeping
Culture of discipline, orderliness, systematic thinking, enlivening environment, and building confidence in what an organisation does is built through housekeeping, complying with high standards. For example, can a prospective client get any confidence in a nursing home with an ill-kept reception area, no matter what its medical/surgical prowess may be? Tools/materials kept haphazardly at one machine will also make it happen at another. Poor practices and unhealthy attitudes get quickly noticed and replicated far and wide. This is a social phenomenon. Enforcing excellent housekeeping practices helps to develop healthy social attitudes in an organisation. Thus, housekeeping is not merely an exercise to keep things neat and clean but to develop a good organisational culture.
Housekeeping at a manufacturing operation is based on the model given in Figure 8.1. Similar models can be specified for any type of process.
Housekeeping ground rules should also be transparent. For example, cleaning schedules are displayed in organisations committed to maintaining high standards of housekeeping. Housekeeping is meant to improve work area. This requires effort on two fronts—involvement of people and support given to housekeeping. Model for improving the work area irrespective of its type is given in Figure 8.2.
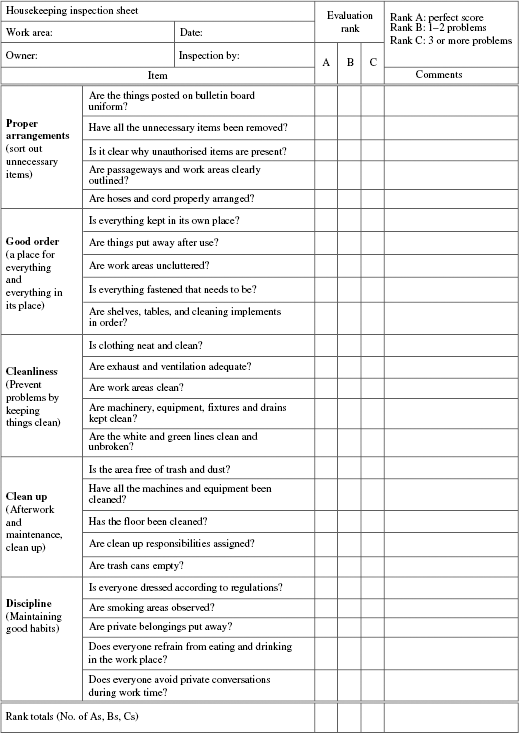
Housekeeping standards must be enforced and the format in Table 8.3 should be used for this purpose. Housekeeping inspection needs to be done jointly by the process owner and the housekeeping personnel.
Figure 8.1 Housekeeping at a process by a process owner
Figure 8.2 Model for improving the work area
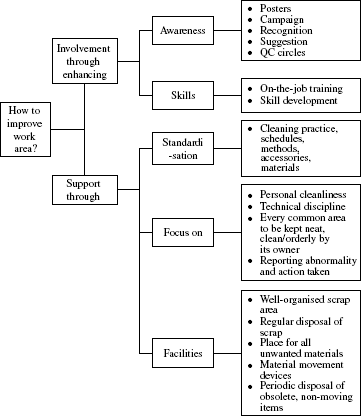
TABLE 8.3 Housekeeping Inspection Sheet
Mistake proofing
Mistakes (errors/defects) of certain types can be totally eliminated if a certain ‘discipline’ is followed. When the agency to enforce this discipline is ‘human-hand’, the possibility of occurrence of mistakes does exist. Instead, if the ‘discipline’ is built into the process itself then mistakes cannot occur. This is the logic of mistake proofing. It recognises that ‘to err is human’ but it is ‘superhuman’ to prevent error through built-in mistake proofing in the process itself. Table 8.4 gives an example of mistake proofing from manufacturing.
TABLE 8.4 Component Manufacture: Mistake Proofing Approach
| Error type | Mistake proofing |
|---|---|
| Component oversize or under size Component location in the machine is wrong Component with missed operation Wrong orientation of component |
Tool does not accept the component Machine does not start Machine does not start Feeder device that ensures correct orientation |
Mistake proofing is also referred to as foolproofing, fail-safe system and poka-yoke, a term coined by Dr. Shigeo Shingo which means avoid (Yoke) inadvertent error (Poka).
At design stage mistake proofing should be considered and built-in mistake proofing devices should be set up in the processes. The gaps are detected during the usage phase. Hence, as a rule, every process must be subjected to a scrutiny for introducing mistake proofing measures. This is a team effort involving all the key persons associated with the process and resource persons who are good at designing/fabricating and incorporating the mistake proof devices.
Every process benefits from mistake proofing measures. It is necessary to raise the following questions.
- Which defects can enter a process?
- Can they be detected/sensed?
- What screening/arresting device can be incorporated in the process to detect/screen?
- How to link the positive response (error found) to prevent the wrong one from entering into the process?
Examples of different types of possibilities that exist in a manufacturing operation are listed in Table 8.5 for the purpose of illustration. A similar attempt must be made for any other type of process.
There are two types of measures—passive and active. In Table 8.5, (1), (4), (5) and (6) are passive measures. They force the operator to look, listen, read and leave him to ‘act’. In (2), (3) and (7), process itself acts and thus compels the operator to restore the job. These types are called active devices. It is necessary to adopt active type.
There are devices which suffer from unfavourable conditions and fail; and thus protect the equipment, of which it is a part, from the damage due to unfavourable conditions like electrical surge, voltage fluctuation or over-load conditions. These can be called ‘mitigation devices’. Electrical/mechanical fuse is a common example of such a device. Crash helmet to be worn by a two-wheeler rider is another example of a mitigating device. Thus, it is not enough to have ‘mistake-proofing’ devices, but is more important to test, check and maintain the devices properly to ensure their functional integrity.
TABLE 8.5 Mistake Proofing Devices
| No. | Device |
|---|---|
| 1 | Guidelines to feed |
| 2 | Guide pin/locating pins with the provision machine does not start if the component is not properly placed |
| 3 | Stoppers to avoid oversize |
| 4 | Lining/cushioning |
| 5 | Counters, colour codes, checklists |
| 6 | Wake-up signals, light, buzzer, alarm |
| 7 | Limit switches, photo sensors, interlocks, use of mirrors |
Every device must have a documented procedure to ensure functional integrity. One simple method can be to have two sets—one set comprising all approved items which the device must pass after running through it and another all non-approved items, none of which can be allowed to pass by the device. As a routine practice at the start of the machine, the two sets should be passed and the mistake proofing device should be accepted only when the response is 100 percent correct in both the sets. The number of items in each set can be at least 50, as such mistake proofing devices are found commonly in high throughput operations.
Process consumables
Process consumables play a critical role in the prevention and control of defects. The type of consumables can change from process to process. The manner of storage and usage also vary from one type of consumable to another. For ease of handling and control, they can be classified into four categories as stated in Table 8.6.
TABLE 8.6 Classification of Consumables and their Control
| Category | Description and examples |
|---|---|
| ‘Critical’ consumables | There are items which fit the proverbial description ‘a drop to a can of milk’ category items. Wrong ink in a printing process, photo-tool error and a wrong drill are few examples of critical consumables. |
| ‘Reusable’ consumables | An item which after using over a certain period of time can be rejuvenated to render it fit for re-use. The item can be a source of defect when used without rejuvenation. |
| Expiry items | These are items which after using for the specified duration have to be discarded and cannot be reused. Stencil is an example. Medicines are another example. |
| Shelf-life items | These are a particular case of expiry items. When an item is kept with its seal unbroken, expiry rule is applicable. This may not be applicable when the item is opened for use. For such items, ‘shelf-life’ rule is used. It is the time stipulated for an item when its seal is opened after which it has to be discarded. |
Following examples illustrate how the same item is to be treated as an expiry item and shelf-life item.
| Item | An expired item | A shelf-life item |
|---|---|---|
| Disposable syringe | Throw it after one-use | When not opened throw it after the expiry date specified on it |
| Eye drops medicine | Throw it after 3 days of opening | When not opened throw it after the expiry date specified on it |
The control measures are given in Table 8.7.
TABLE 8.7 Consumable Items: Control Measures
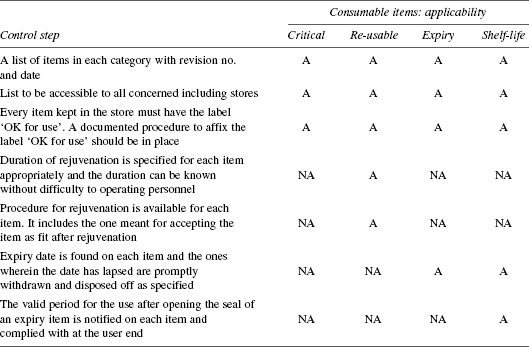
Consumables—control document needs to be available for each process.
‘Clearance gate’ for process entry
Refer process model in Figure 4.1. There are a number of streams represented by (A), (C) and (D) which enter the conversion stream (B). Each of the entry stream (A), (C) and (D) can be a potential source of defects. Hence a ‘clearance’ mechanism is needed whereby the stream entering (B) is assessed and found defect free. This is the concept of clearance gate.
Following are some of the typical examples taken from manufacturing activity:
- Tools, jigs and fixtures kept ready for issue to manufacturing have the label ‘OK’ and it is affixed only after finding it fit-for-use by ‘try out’.
- Drawings have the latest revision number with date and authorised signatory. The fact of being latest can be verified with ease at any time.
- Machine after maintenance is cleared only when it is certified to be fit for production.
- Instrument used for inspection/checking has a ‘Calibration—OK’ label with next date for calibration.
- Product identification with indication of fitness for use.
- Acceptance of an order for execution only after assessing the technical and commercial capability to execute it.
Identification of all the ‘clearance’ gates is required for a process and it should be ensured that all are in place.
Misinterpretation of drawing, SOP, WI
‘Misinterpretation’ is also an error; hence misinterpretation should not be looked upon as a trivial issue that can be disposed off by providing the correct interpretation as and when the need arises. This approach is not even in tune with the principle of ‘defect prevention’. For this it is necessary to log every instance of misinterpretation along with the corresponding correct interpretation through
- precise definitions.
- simple, straight, clear and unambiguous instructions.
- diagrams and pictures that bring in greater clarity.
- use them to update the relevant documents from time to time as well as knowledge of all persons concerned.
Gaps in defect prevention measures
In a process, gaps in defect prevention measures need to be found at regular intervals of time, at least once a year. Gaps can be found by a review/scrutiny of the process to ensure that
- all the defects of the process are indeed identified and detected;
- control measures are finely tuned; and
- corrective measures on defect prevention are in harmony with the dominant pattern of the process.
These reviews are carried out in a systematic way through the following four methods.
- Defect identification detection analysis.
- Defect detection and control by operator.
- Defect control review.
- Defect prevention and dominant pattern.
Illustrations concerning each are drawn from manufacturing operations.
1) Defect identification and detection analysis
Identification List all the defects that are currently identified in a process. Check that the list includes those relating to customer/field complaints also. Once this is done, defect identification task is completed.
Detection Next check whether each defect can be detected using the format as given in Table 8.8. In this format all the defects identified must find a place.
TABLE 8.8 Defect Detection Analysis

2) Defect detection and control by operator
Next, assess the responsibility of the operator to detect, stop and rectify the defect on the basis of response to the following queries and take suitable action where the response is in the negative.
- Does the operator know the defects for which he/she is responsible?
- Can he/she detect the defect while processing?
- Can he/she take action to correct the defect himself/herself? d) Does he/she know the type of defect for which he/she has to stop the process when it occurs?
- Which are the defects which he/she cannot detect?
- What is the action plan on (e) to make it controllable by the operator?
Actions to achieve affirmative status for each question brings in ‘self-control’—a status wherein the one who does the work (operator) controls quality. The word ‘operator’ does not refer to the one at the shop floor but anyone who is at the cutting edge where defect can occur. Annexure 8A gives a list of questions through which the status of ‘self-control’ can be assessed.
For the defects to be detected as per the analysis detailed earlier, understand the strength of association between the defect and process factor as Strong (S), Good (G) and Weak (W). Review the control measures pertaining to the parameters found in S and G, and take suitable measures to improve/refine to eliminate/reduce defects. Mode of carrying out this exercise is set out as given in Table 8.9.
TABLE 8.9 Strength of Association between Process Factors and Defect Detected

4) Defect prevention and dominant pattern
Processes are influenced by numerous variables: input materials, physical facilities, skills, environmental conditions, etc.
All these variables are not equally important from the point of view of defects of the process. Often one or two variables may be more impacting than all the rest combined. Such a variable is called a dominant variable. Priority on control and mastery over the dominant variable helps in defect prevention. Different dominant patterns and the control measures for each are as given in Table 8.10.
TABLE 8.10 Dominant Pattern and Preventive Measures
| Dominant pattern | Defect prevention measures |
|---|---|
| Setup dominant High stability and reproducibility over many cycles of operation |
Provide means for
|
| Time dominant Process is known to change with time—heating up, tool-wear, depletion of inputs |
Provide means for
|
| Component dominant Quality of input materials and inputs; determine defect-free output Example: an assembly operation |
Provide means for
|
| Worker dominant Quality depends on knack/skill of workers |
Provide means for
|
| Information dominant Processes are of a ‘job shop’ nature |
Provide means for
|
| Preventive maintenance dominant Equipment maintenance |
Provide means for
|
Integration of review results
For a given process arrive at the results on defect identification, detection and control on the basis of the methodologies described earlier. Study the results and integrate them together as defect prevention plan as per Table 8.11 to monitor the progress of prevention and control measures.
TABLE 8.11 Defect Prevention and Control Measures

Gaps in customer linkage
Every process has its customer. The kind of customer with which a process is linked can vary as depicted in Figure 8.3 and accordingly proper linkage has to be established. It should be remembered that improper linkage is a critical source of defect and hence linkage has to be properly specified. The various aspects of linkage that need to be looked into are stated in Table 8.12.
Figure 8.3 A process and its type of customers
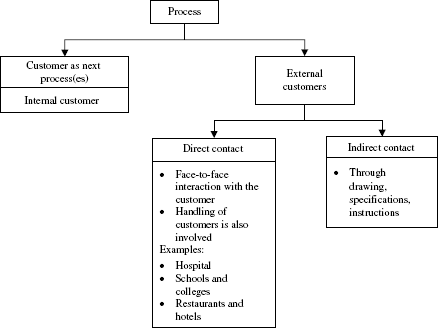
TABLE 8.12 Criteria for Customer Linkage
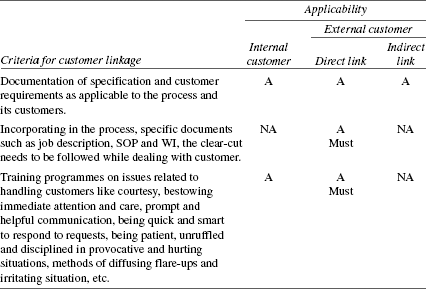
For every process, the type of linkage that exists has to be known and a review of the linkage has to be carried out to ensure that it is properly specified according to the criteria in Table 8.9. This will ensure defect-free input from supplier as well as output to customer.
Documents on specification and customer requirements need to be screened to ensure that nothing is omitted and everything stated in the document is correct. This is ensured by systematic analysis of customer needs and specifications. The method of analysis is dealt in Chapter 9. Table 8.13 gives a few examples of incorporating the customer requirements in process-specific documents.
Hidden defects
The two important hidden defects are ‘white collar defects’ and ‘institutionalised’ undesirable practices. Some of these are not even perceived as defects. These are not captured by any routine data. It is for these reasons, special effort is needed to capture these defects to plan preventive action. These defects contribute to significant loss of time and money.
White collar defects White collar measurements of quality loss are as follows:
- Number of reviews before plan is approved
- Letters re-typed
- Personnel turnover
- Absenteeism
- Purchase orders changed
- Interest lost on receivables
- Interest lost on delays in invoicing
- Excess inventory carrying costs
- Recording errors
- Search time for suppliers, tools, drawings, etc.
- Management approvals beyond two levels
- Down time
- Number of engineering changes per part number
- Man-hours lost due to meetings starting late
- Number of schedules delayed
- Items purchased on emergency at extra cost but not delivered to point of use
- Demurrage
- Design errors found while in use—in-house, field
- Cancellation of travel arrangements
TABLE 8.13 Illustrations of Linking Customer to Process
| Process | Means of linking |
|---|---|
| ‘Prescription process’ to be followed by doctor | The WI specifically states the following.
|
| ‘Scrutiny process prior to scanning’ to be followed by scanning operator | The WI to specifically state the following.
|
| ‘Patient sent to surgery’ to be followed by surgeon | Include in the WI the following. Mark and initial with a marker the body part of the patient to be opened before sending the patient to the theatre. |
Institutionalised defects Loss due to ‘institutionalised’ practices are not even seen as ‘quality loss’ and hence they are not questioned.
- Cost of re-design due to quality reasons.
- Cost of changing processes because of not meeting quality requirements.
- Cost of ‘allowances’ included in ‘standards’.
- Extra quantities purchased.
- Allowances for scrap and rework during production.
- Allowance in time standards for scrap and rework.
- Allowances as down time.
- Extra manufacturing costs due to excess ‘give away’, and additional operations taken as normal such as rust removal or operation to open up the poly bags individually prior to use.
- Extra manufacturing costs due to non-value addition jobs/activities.
- Not assessing execution efficiency tenderwise in terms of cost escalation, time escalation and actual profit against the budget as per tender offer made.
- Cost discrepancy for items where unit of purchase is by weight and that of use is by numbers.
It is worthwhile if a continual improvement project is taken up on ‘hidden defects’ in sections where they are presumed to be high.
Process capability
The meaning and significance of process capability and process capability index are explained in Chapter 12.
Conclusion
The technique of process review to spot out the sources of defects in a process to take suitable corrective measures on defect prevention has been explained with the help of examples mostly drawn from manufacturing process. However, the methodology of process review is applicable to any process.
Annexure 8A
Self-control: an evaluation as applicable to manufacturing
- Does the operator know what he/she is supposed to do?
- Are there specifications or instructions which apply to this operation?
- Are they written down? If applicable in more than one place, do all agree?
- If visual defects, are there standard samples?
- Are they complete?
- Does an operator have access to them?
- Does he/she actually refer to them in practice?
- Does he/she really understand them? How is it ensured?
- Are these specifications the sole criterion of acceptability?
- Does an operator know whom to consult to give an official interpretation of the specifications in doubtful cases?
- Does an operator know how the product is used, where it is used? Has he/she seen it used?
- Does an operator know the full consequences of his/her failure to meet the specifications?
- Does an operator know the effect of not following the WI?
- Does an operator receive specification changes automatically and promptly?
- Does an operator know what to do with a defective raw material?
- Does an operator know what to do with a defective finished product?
- Are there specifications or instructions which apply to this operation?
- Does an operator know what he/she is doing?
- Are gauges or measuring equipments provided?
- Do they show how the process is doing rather than sort good from bad?
- Are they available to the operator?
- How does he/she know it is calibrated?
- How does he/she know that it is meant for the job on hand?
- Is the operator told how often to sample his work? Is the time allowed sufficient?
- Is an operator told how many pieces (or readings) to sample?
- Is the operator told the criteria on which he/she should decide to correct the process?
- In case an automated process covers (A), (B) and (C), is the operator fully aware of all the relevant details?
- Is there any independent check as to whether the operator actually follows the specified sample size, frequency and adjustment criteria?
- Is an operator required to record the results of his checks?
- Does anyone verify the accuracy of these records?
- Are inspection data fed back to the operator for his/her use?
- Does an operator know of his/her quality performance?
- Are gauges or measuring equipments provided?
- Can an operator regulate the process?
- Has the quality capability of this process been measured?
- Is the quality capability within the tolerances allowed by the specification?
- Does an operator make his own decisions as to when the process requires correction (as opposed to having them made by a foreman or patrol inspection)?
- Is there a swift, sure adjustment an operator can make to eliminate defects when they occur?
- Does an operator know
- Under what conditions he/she takes corrective action?
- What action?
- Under what conditions he/she shuts down and seeks help?
- Whose help?
- Have the operator’s actions, which cause the defect, been written down and given to him?
- Have the operator’s actions, which can prevent the defect, been written down and given to him?

