CHAPTER 21
Measuring System
Measure what is measurable; And make measurable what is not so
– Galileo
Italy’s Ivano Brugentti walked away with Gold in men’s 20 m walk by a narrow margin of 0.50 seconds
– 2004, Athens Olympics
SYNOPSIS
The quality of continual improvement is much influenced by the quality of data used in the study. Quality of data is dependent on the quality of the measurement system that exists. Measurement system is a combination of measuring equipment and other external factors such as procedure for collecting sample, its identification, preservation and preprocessing prior to its analysis extending up to recording of the results and its reporting and sample disposal. One should have a sound knowledge of these aspects of total measurement system that precede and follow measurement and the measuring device. Any deficiency in these by itself may be a source of defect.
Importance of the measuring system
Data is the sheet anchor of continual improvement. Collection of data means taking measurements. Measuring gauges are the means to take measurements. Measurements by counting also depend on measuring gauges such as counters, go and no go gauges.
Integrity and reliability of data, therefore, depend upon the integrity and reliability of measuring gauges. Importance of measurements and integrity of measuring gauges can be recognised by the quotation stated above.
Integrity and reliability of measuring gauges are achieved by selecting the one that is appropriate for the application and maintenance of the gauge properly through calibration. In Chapter 22 and in this chapter, certain aspects of selection and calibration of measuring gauges are covered. A knowledge of these is essential for those concerned with continual improvement. The expression measuring gauges is a generic one and it includes all types of measuring instruments and devices.
Measuring system: illustration
The measuring gauge is one of the most important components of the total measurement system which includes the instrument, standards, operations, methods, fixtures, software, if any, personnel and environment. The measurement system can be compared to a manufacturing process with numbers (measurements) as its output.
The essentials of a measuring system are
S – Standard; it is an accepted basis for comparison
W – Work piece, part, sample for analysis/measurement
I – Measuring instrument/gauge/device
P – Personnel and procedure
E – Environment
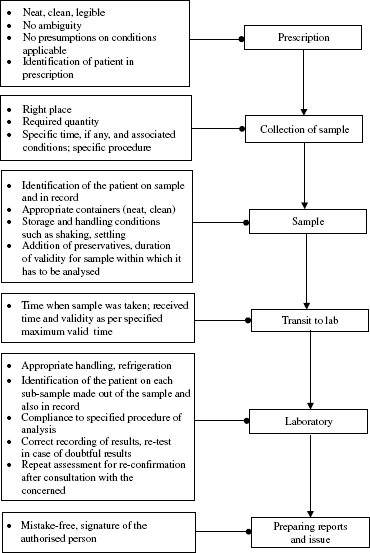
Illustrations of the tasks involved in taking measurements drawn from healthcare and a typical chemical analysis in a laboratory are in Figures 21.1 and 21.2, respectively. From these figures, it can be seen that errors can arise (a) before analysis, (b) in the measuring apparatus and (c) while reporting the results. Hence, to control errors, a total view of the measurement system needs to be taken.
It is a common experience that the value of even successive measurements, do differ from one another. This phenomenon is dealt within Chapter 22. The differences in the measurements are commonly termed as ‘error’ in the context of taking measurements and instrument calibration. Infact, the differences are to be understood as variation. Hence, error in the context of measurement is not to be mistaken as error, the mistake, but as variation in measurement. Built-in design features of an instrument control certain types of variation. But, every instrument has certain inherent variation and is equivalent to process capability explained in Chapter 12. Thus, assessing built-in error of an instrument is a statistical exercise and is a central point of calibration of the instrument. These aspects are discussed in Chapter 22.
In the total measuring systems outlined in Figures 21.1 and 21.2, it can be appreciated that there are certain activities that precede and follow the actual task of taking measurements such as the method of taking samples, pre-treatment of the sample, if any, its identification, recording and reporting of the results, preservation or disposal of the sample besides unique classification of every measuring device, provision to know the calibration status while in use and recall for calibration. One engaged in continual improvement must have a prior knowledge of a good system to find out the deficiencies in the system, if any. An outline of a good system is given in Annexure 21A.
Certain fundamental properties that define a ‘good’ measurement system
Adequate discrimination and sensitivity
Discrimination comprises of two elements:
- The value of the smallest graduation on the scale of the instrument.
For example, there can be three types of vernier, where the minimum readings that can be read, are as follows:
Case A: 0.1 mm Case B: 0.01 mm Case C: 0.001 mm
Among the three, the one best in discrimination is ‘C’ and the one that is coarse is ‘A’. - The readability or resolution of the instrument is the amount of change from a reference value that it can detect and faithfully indicate. It is also referred to as sensitivity—the smallest input that results in a detectable (useable) output signal.
Figure 21.1 Measurement task: Healthcare
For example, there are two balances B and C. In each, a certain material is weighed and balanced at 2 g. A pre-weighed weight of 0.01 g is added to the material in B and C. There is no deflection in B as evidence of detection of a change of 0.01 g in input. In ‘C’, there is deflection and it is also possible to read the change of the input in the output. Thus, ‘C’ is more sensitive compared to ‘B’.
Figure 21.2 Measurement task: Chemical analysis—estimating proportion of chlorine in a material
The rule to be complied with is the rule-of-ten. If the measurement is required to the first decimal point, then use the instrument that can read to the second decimal point (one-tenth).
These two aspects can be easily verified in an instrument and their details may also be found in the user’s manual. This is not the case for the remaining three aspects covered in the following sections.
Measurement system ought to be in statistical control
The measurement system ought to be in statistical control. This means that under repeatable conditions, the variation in the measurement system is due to common causes only and not due to special causes. This can be referred to as statistical stability and is best evaluated by graphical methods. The concept of statistical control is covered in Chapter 22.
Measurement system fit for product control
The measurement capability must be superior to product tolerance. That is, the variability of the measurement system must be small compared to the product specification limits.
Measurement system fit for process control
The measurement capability must be superior to process capability. That is the variability of the measurement system must be small compared to process variation.
The last three aspects of measurement system are covered in Chapter 22. Their details will not be found in the user’s manual on measuring devices and have to be assessed. These three are the core of measurement system analysis and their foundation is in statistical thinking, statistical logic and statistical techniques. No other methodology is applicable to handle the issue of control and capability.
Traceability
Purpose
Traceability is an important concept in the trade of goods and services. Measurements that are traceable to the same or similar standards will agree more closely than those that are not traceable. This helps to reduce the need for re-test, rejection of good product and acceptance of bad product.
Definition
Traceability is defined by the ISO International Vocabulary of Basic and General Terms in Metrology (VIM) as
The property of a measurement or the value of a standard whereby it can be related to stated references, usually national or international standards, through an unbroken chain of comparisons all having stated uncertainties.
Mechanics
The traceability of a measurement will typically be established through a chain of comparisons to the National Measurement Institute (NMI). However, in many instances in the industry, the traceability of a measurement may be linked to a previously agreed reference value or consensus standard between a customer and a supplier.
The traceability linkage of these consensus standards to the NMI may not always be clearly understood, so ultimately it is critical that the measurements are traceable to the extent that satisfies customer needs. With the advancement in measurement technologies and the usage of state-of-the-art measurement systems in industry, the definition as to where and how a measurement is traceable is an ever-evolving concept.
This is the view expressed in Youden (1962).
Conclusion
In conclusion one should recognise that to control/ improve one should "know" the status; 'measure' the entity that is the subject of study, investigation and/ or control. Hence measuring system and its reliability are of fundamental importance to control/ improve.
Annexure 21A
Framework of a system of control on measurements and measuring devices
This section includes the frameworks
- Table 21A.1: Individual test, analysis and measuring devices.
- Table 21A.2: Issues common to all the devices.
- Table 21A.3: History card, ‘fields’, to be covered.
- Table 21A.4: Checklist for preparing test/analysis in a laboratory (hospital).
TABLE 21A.1 Individual Test, Analysis and Measuring Devices
| Sl. no. | Issues |
|---|---|
| 1. | Master matrix of measurements made in the laboratory and the instruments used for each measurement |
| 2. | Schedule for assessing ‘bias’, ‘linearity’, ‘gage R&R’ (all defined later) appropriate to each measurement. It is preferable to have the schedule—calibration frequency—in terms of time: everyday, once a week/month, etc. |
| 3. | WI/SOP for conducting tests in a comprehensive manner. Table 21A.4 gives the checklist for preparing WI for a test conducted in a hospital laboratory |
| 4. | Unique identification code of each measuring device reflected on the device plus each format/record associated with it |
| 5. | Calibration code, proof of having been calibrated and certified fit for use, reflected on the measuring device along with the next due date plus its link to the calibration results recorded |
| 6. | No. (5) made applicable to each test. This would be necessary when the same measuring device is used for more than one type of test simultaneously |
| 7. | History card for each measuring device. The ‘fields’ to be covered in the history card are in Table 21A.3 |
| 8. | Calibration agency: internal and external. Address of external agency |
| 9. | Tamper proof arrangements, if any, against unauthorised adjustments |
Tables 21A.1 and 21A.2 summarise the issues related to calibration control of measuring devices. Where tests to be conducted are subcontracted, check if the points stated in Tables 21A.1 and 21A.2 are complied to. More importantly, the identification of the sample and its analysis are correctly maintained at all points of the transaction receipt, its handling at different stages, recording of results and reporting by the subcontractor without any mix up. This has to be physically examined and assurance to the same ensured.
TABLE 21A.2 Issues Concerning All Measuring Devices and Laboratory
| Sl. no. | Issues |
|---|---|
| 1. | Device designed as ‘Mother instrument’
|
| 2. | Internal calibration
|
| 3. | Instruction and training on handling, usage and storage of devices for users and enforcing the following:
|
| 4. | Positive recall procedure to enforce calibration schedule |
| 5. | Follow-up scheme on devices loaned, given for repair/maintenance, calibration |
| 6. | Down-grading of a device, amending its history card |
| 7. | Discarding a device, withdrawal of its history card, scrapping it after mutilating it |
| 8. | Review of calibration certificate of the external agency for its correctness and completeness prior to releasing the device for use |
| 9. | Skill matrix of individuals tuning different type of devices and conducting different types of tests |
| 10. | Knowledge of using the statistical methods in calibration |
| 11. | First-aid, safety measures, fire prevention measures |
| 12. | Antidotes availability for ready use |
| 13. | List of non-compliable items which should not be stored together |
| 14. | List of poisonous substances, hazardous substances and their safety data |
| 15. | Use of neat, clean, dry and glassware with clear graduation at all times |
| 16. | Label control—complete designation of the content, expiry date, signature with date |
| 17. | Self-speaking identification on each and every item |
| 18. | Purchasing branded items—glassware, reagents, chemicals—from authorised accredited agents/direct manufacturers |
| 19. | Everyone in the laboratory being aware of (11) to (14) |
| 20. | All records maintained are free from overwriting, unauthorised corrections, incomplete information fields or dates or no signature of authorised person(s) |
TABLE 21A.3 Particulars to be Covered in the History Card

TABLE 21A.4 Checklist of Planning for Test/Analysis in the Laboratory (Hospital)