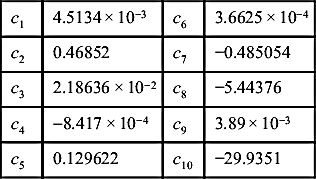Hand Calculation Methods
Abstract
The focus of this chapter is the simple methods for predicting the pressure and temperatures at which hydrates will form. These methods are: (1) a chart based on the gas gravity, (2) a K-factor method, and (3) a chart method for sour gases (those contain hydrogen sulfide). This chapter discusses how to use these methods and the approximate error associated with them and their limitations. Algorithms are provided to simplify the approaches. Several examples are given that demonstrate the accuracy (or perhaps the inaccuracy) of these methods.
Keywords
Baillie–Wichert chart; Gas gravity; K-factor method3.1. The Gas Gravity Method

![]() (3.1)
(3.1)

3.1.1. Verifying the Approach
3.1.1.1. Molar Mass
![]() (3.2)
(3.2)
3.1.1.2. Boiling Point
3.1.1.3. Density
![]() (3.3)
(3.3)
3.1.1.4. Discussion
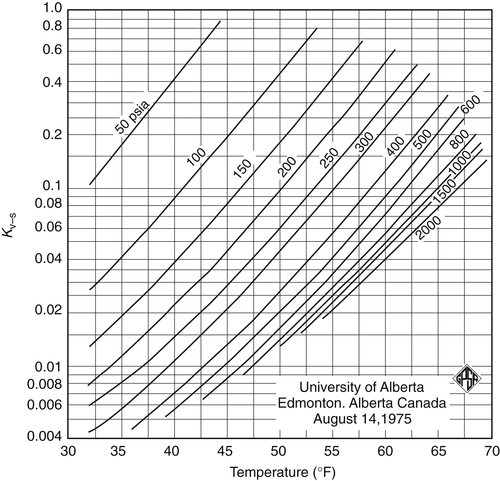
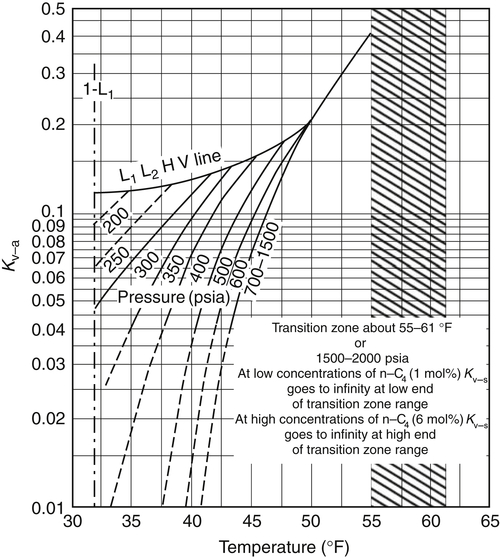
3.2. The K-Factor Method
![]() (3.4)
(3.4)
3.2.1. Calculation Algorithms
3.2.1.1. Flash
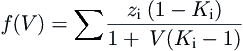 (3.5)
(3.5)
 (3.6)
(3.6)
 (3.7)
(3.7)
![]() (3.8)
(3.8)
![]() (3.9)
(3.9)
3.2.1.2. Incipient Solid Formation
![]() (3.10)
(3.10)
![]() (3.11)
(3.11)
3.2.2. Liquid Hydrocarbons
![]() (3.12)
(3.12)
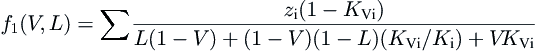 (3.13)
(3.13)
 (3.14)
(3.14)
![]() (3.15)
(3.15)
![]() (3.16)
(3.16)
3.2.3. Computerization
3.2.4. Comments on the Accuracy of the K-Factor Method

| 0 < t < 20 °C | 32 < t < 68 °F |
| 0.7 < P < 7 MPa | 100 < P < 1000 psia |
3.2.4.1. Ethylene
3.2.5. Mann et al
3.3. Baillie–Wichert Method


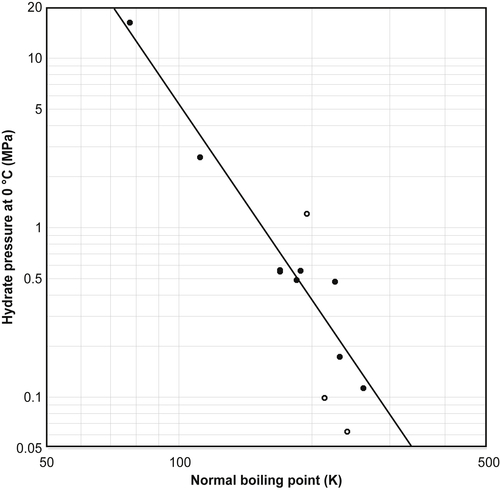
3.4. Other Correlations
3.4.1. Makogon
![]() (3.17)
(3.17)
![]() (3.18)
(3.18)
![]() (3.19)
(3.19)
3.4.2. Kobayashi et al
 (3.20)
(3.20)
3.4.3. Motiee
![]() (3.21)
(3.21)
3.4.4. Østergaard et al
![]() (3.22)
(3.22)
3.4.5. Towler and Mokhatab
![]() (3.23)
(3.23)
3.5. Comments on All of These Methods
3.5.1. Water
3.5.2. Nonformers
3.5.3. Isobutane vs n-Butane
3.5.4. Quick Comparison
3.5.4.1. Mei et al. (1998)
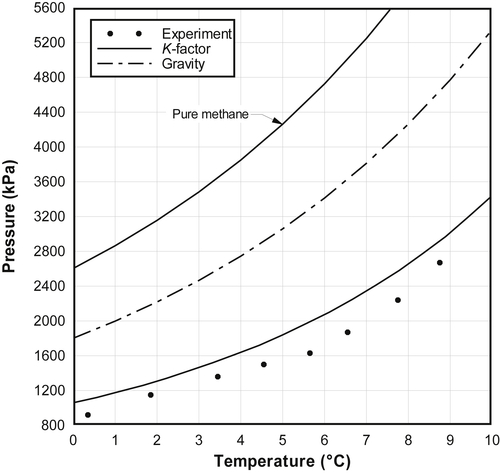
3.5.4.2. Fan and Guo (1999)
3.5.4.3. Ng and Robinson (1976)
3.5.5. Sour Natural Gas
3.6. Local Models
![]() (3.24)
(3.24)
![]() (3.25)
(3.25)
![]() (3.26)
(3.26)
![]() (3.27)
(3.27)
![]() (3.28)
(3.28)
3.6.1. Wilcox et al. (1941)
![]() (3.29)
(3.29)
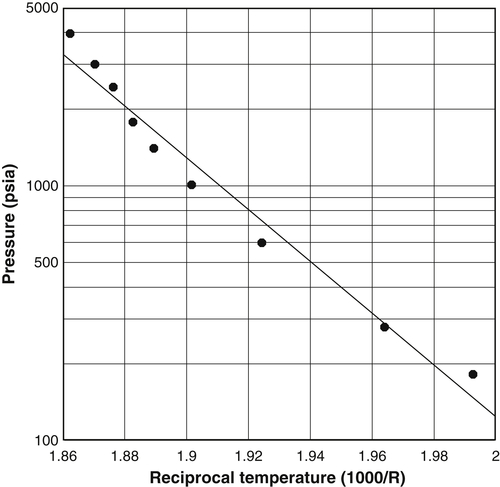
Table 3.2
Summary of Fitting Parameters for the Local Models for the Wilcox et al. (1941) Gas B
| Pressure Range (psia) | a | b | r2 | |
| Low Pressure | 180 – 1000 | 42.694 907 | −18.841 542 | 0.99690 |
| Intermediate | 600 – 1750 | 55.855 342 | −25.714 757 | 0.99698 |
| High Pressure | 1400 – 4000 | 80.912 574 | −38.987 619 | 1.00000 |
| All | 180 – 4000 | 51.667 820 | −23.424 368 | 0.98624 |

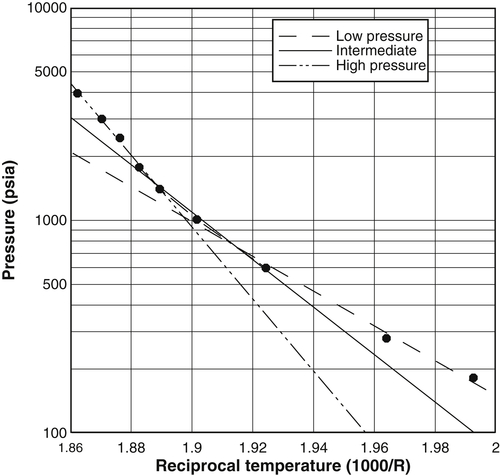
3.6.2. Composition
![]() (3.30)
(3.30)
![]() (3.31)
(3.31)
![]() (3.32)
(3.32)
![]() (3.33)
(3.33)
3.6.2.1. Sun et al
![]() (3.34)
(3.34)
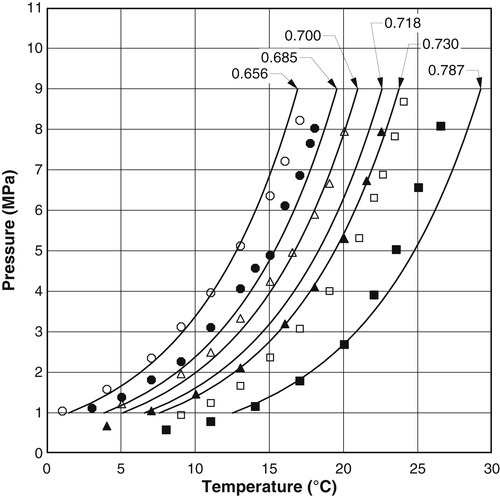
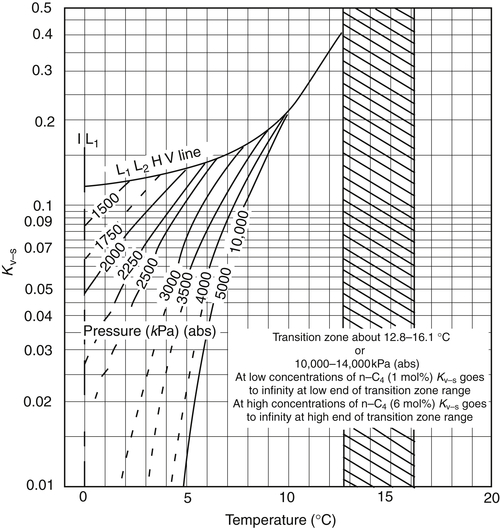
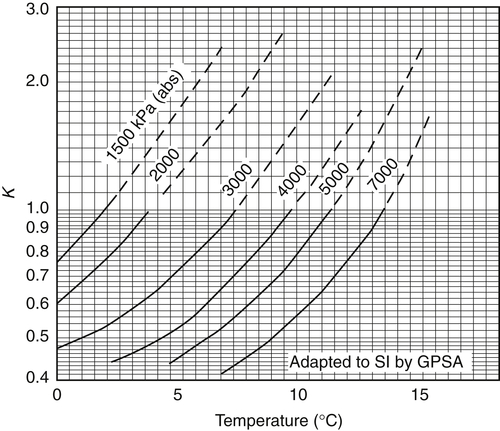
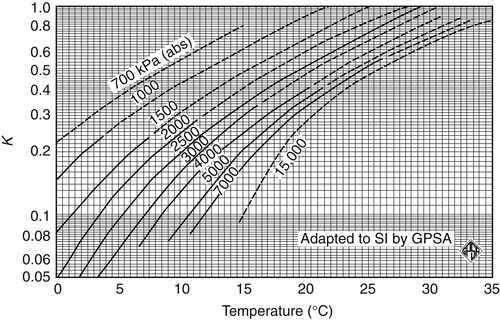
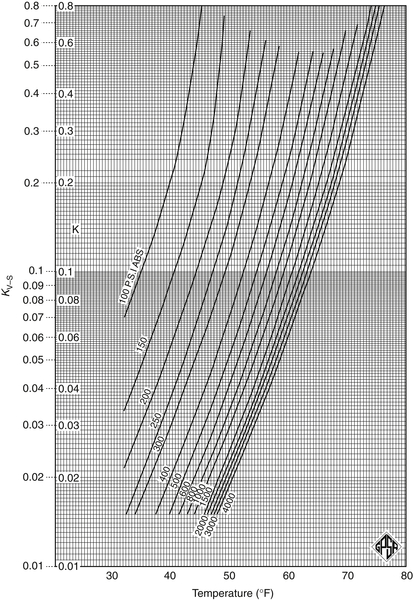
Appendix 3A Katz K-Factor Charts