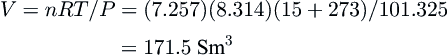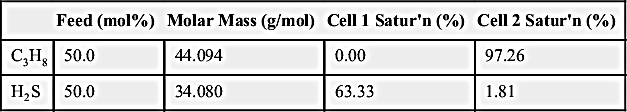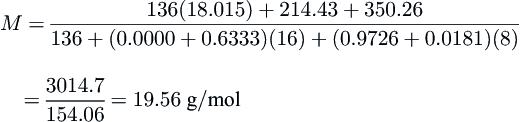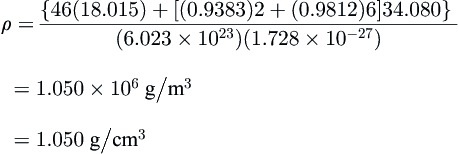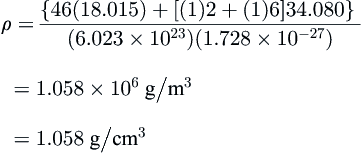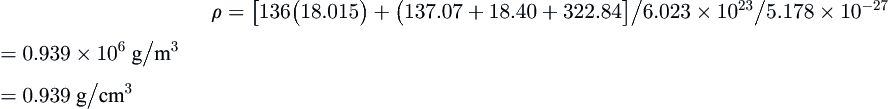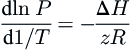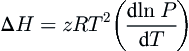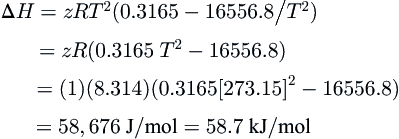In the design of processes, the physical properties are important. This is no less true when the processes involve hydrates. Some of the properties of hydrates were reviewed in the previous chapters. This chapter focuses on properties that were not covered earlier.
The estimation of the properties of hydrates is complicated by the fact that the properties depend on: (1) the type of the hydrate, (2) the guest molecule encaged in the hydrate, and (3) the degree of saturation (remember that hydrates are nonstoichiometric). It is unfortunate, but most hydrate programs do not give the saturation numbers as a part of their calculations. An exception is CSMHYD, which does give saturation values. The newer CSMGEM gives composition of the hydrate phase, but not specifically the cell saturation.
The heat capacity, electrical, and mechanical properties of hydrates are similar to those for ice. The thermal conductivity is unique because it is significantly different from that of ice (
Handa and Cook, 1987), as we shall see.
8.1. Molar Mass
The molar mass (molecular weight) of a hydrate can be determined from its crystal structure and the degree of saturation. The molar mass of the hydrate, M, is given by:
M=NWMW+∑cj=1∑ni=1YijνiMjNW+∑cj=1∑ni=1Yijνi
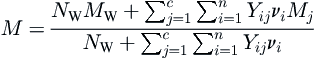 (8.1)
(8.1)
where NW is the number of water molecules per unit cell (46 for Type I, 136 for Type II, and 34 for Type H), MW is the molar mass of water, Yij is the fractional occupancy of cavities of type i by component j, νi is the number of type i cavities, n is the number of cavities of type i (two for both Type I and II, but is three for Type H), and c is the number of components in the cell.
Although this equation looks fairly complicated, it is just accounting for all of the molecules present and then using a number average to get the molar mass.
Table 8.1
Molar Masses of Some Hydrates at 0 °C
| Hydrate Type | Saturation | Molar Mass (g/mol) |
| Small | Large |
| Methane | I | 0.8723 | 0.9730 | 17.74 |
| Ethane | I | 0.0000 | 0.9864 | 19.39 |
| Propane | II | 0.0000 | 0.9987 | 19.46 |
| Isobutane | II | 0.0000 | 0.9987 | 20.24 |
| CO2 | I | 0.7295 | 0.9813 | 21.59 |
| H2S | I | 0.9075 | 0.9707 | 20.87 |
Note: calculated using Eqn (8.1). The saturation values were calculated using CSMHYD.
Table 8.1 summarizes the molar masses of a few hydrate formers. It is a little surprising that the molar masses of all six components are approximately equal (∼20
g/mol). This is because the hydrate is composed mostly of water (18.015
g/mol).
It is interesting that the molar masses of hydrates are a function of the temperature and the pressure, since the degree of saturation is a function of these variables. We usually think of molar masses as being constants for a given substance.
8.2. Density
The density of a hydrate, ρ, can be calculated using the following formula:
ρ=NWMW+∑cj=1∑ni=1YijνiMjNAVcell
 (8.2)
(8.2)
where
NW is the number of water molecules per unit cell (46 for Type I, 136 for Type II, and 34 for Type H),
NA is Avogadro's number (6.023
×
10
23 molecules/mole),
MW is the molar mass of water,
Yij is the fractional occupancy of cavities of type
i by component
j,
νi is the number of type
i cavities,
Vcell is the volume of the unit cell (see
Table 2.1),
n is the number of cavities types (two for both Type I and II, but is three for Type H), and
c is the number of components in the cell.
Equation
(8.2) can be reduced for a single component in either a Type I or Type II hydrate to:
ρ=NWMW+(Y1ν1+Y2ν2)MjNAVcell
 (8.3)
(8.3)
Again, although Eqns
(8.2) and
(8.3) look complicated, they are just accounting for the number of molecules in a unit cell of hydrate. The mass
of all of these molecules divided by the unit volume of the crystal gives the density of the hydrate.
Table 8.2
Densities of Some Hydrates at 0 °C
| Hydrate Type | Density (g/cm3) | Density (lb/ft3) |
| Methane | I | 0.913 | 57.0 |
| Ethane | I | 0.967 | 60.3 |
| Propane | II | 0.899 | 56.1 |
| Isobutane | II | 0.934 | 58.3 |
| CO2 | I | 1.107 | 69.1 |
| H2S | I | 1.046 | 65.3 |
| Ice | – | 0.917 | 57.2 |
| Water | – | 1.000 | 62.4 |
Note: calculated using Eqn (8.3). The saturation values were calculated using CSMHYD.
Properties of ice and water from Keenan et al. (1978).
When using these equations, be careful with the units. Follow the examples at the end of the chapter closely.
As noted earlier, most hydrate software packages do not give the degree of saturation, making it difficult to calculate the density of the hydrate. The K-factors from the Katz method (
Chapter 3) do not give the saturation, even though they have the appearance of doing so. Remember, the compositions thus calculated are on a water-free basis.
The densities of some pure hydrates at 0
°C are given in
Table 8.2. Note that the densities of the hydrates of the hydrocarbons are similar to ice. The hydrates of carbon dioxide and hydrogen sulfide are significantly denser. In fact, they are denser than water.
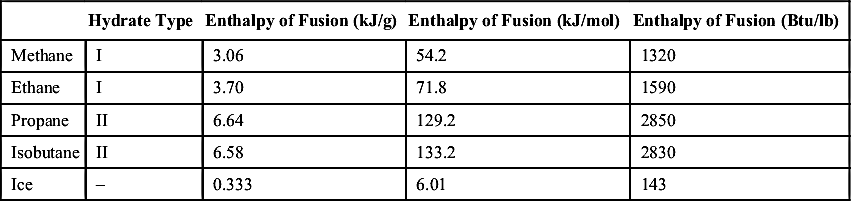
8.3. Enthalpy of Fusion
Another useful property is the enthalpy of fusion of the hydrate (sometimes called the heat of formation). From this, the amount of heat required to melt a hydrate can be estimated.
Table 8.3 lists some enthalpies of fusion for a few hydrates. Ice is included for comparison.
These values represent the formation of a hydrate from liquid water and a gaseous guest molecule. This explains why they are significantly larger than the heat of fusion of water. For pure water, the ice is becoming liquid. When a hydrate melts, it forms a liquid and a gas and the gas is a more highly energetic state.
On the other hand, the enthalpies of fusion are comparable to the enthalpy of sublimation of ice (the phase change going from a solid directly to a gas).
For water, this is 2.83
kJ/g or 51.0
kJ/mol. This process is probably more comparable to the formation of a hydrate than is the simple melting of ice.
Table 8.3
Enthalpies of Fusion for Some Gas Hydrates
| Hydrate Type | Enthalpy of Fusion (kJ/g) | Enthalpy of Fusion (kJ/mol) | Enthalpy of Fusion (Btu/lb) |
| Methane | I | 3.06 | 54.2 | 1320 |
| Ethane | I | 3.70 | 71.8 | 1590 |
| Propane | II | 6.64 | 129.2 | 2850 |
| Isobutane | II | 6.58 | 133.2 | 2830 |
| Ice | – | 0.333 | 6.01 | 143 |
Note: Molar enthalpies of fusion converted to specific values (i.e., per unit mass) by using the molar masses from Table 8.1.
Original values from Sloan (1998). Properties of ice and water from Keenan et al. (1978).
One method for estimating the effect of temperature on the heat of fusion is the so-called Clapeyron approach. A Clapeyron-type equation is applied to the three-phase locus. The Clapeyron-type equation used in this application is:
dlnPd1/T=−ΔHzR
 (8.4)
(8.4)
where Δ
H is the enthalpy of fusion,
z is the compressibility factor of the gas at the conditions of interest, and
R is the universal gas constant. Inherent in this equation is the assumption that the molar volume of the liquid and the hydrate are insignificantly small in comparison to that of the gas; also, this is the only assumption in Eqn
(8.4).
From the correlation provided in
Chapter 2, the derivative required for Eqn
(8.4) is obtained as follows:
ⅆlnPⅆ1/T=BT2−C+DT
 (8.5)
(8.5)
Therefore, to calculate the heat of fusion, an analytical expression is required for the three-phase locus. This expression is then differentiated and the enthalpy of fusion is calculated.
8.4. Heat Capacity
There are limited experimental data for the heat capacity of hydrates.
Table 8.4 lists some values. For comparison, ice is also included in this table. Over the narrow range of temperatures that hydrates can exist, it is probably safe to assume that these values are constants.
Table 8.4
Heat Capacities for Some Gas Hydrates
| Hydrate Type | Heat Capacity (J/g °C) | Heat Capacity (J/mol °C) | Heat Capacity (Btu/lb °F) |
| Methane | I | 2.25 | 40 | 0.54 |
| Ethane | I | 2.2 | 43 | 0.53 |
| Propane | II | 2.2 | 43 | 0.53 |
| Isobutane | II | 2.2 | 45 | 0.53 |
| Ice | – | 2.06 | 37.1 | 0.492 |
Original values from Makogon (1997). Properties of ice and water from Keenan et al. (1978).
8.5. Thermal Conductivity
There have been limited studies into the thermal conductivity of hydrates. However, they show that hydrates are much less conductive than ice. The thermal conductivity of ice is 2.2 W/m K, whereas the thermal conductivities of hydrates of hydrocarbons are in the range 0.50 ± 0.01 W/m K.
The thermal conductivity is a key parameter in the process to melt hydrates. This relatively small value is one of the reasons why hydrates take a long time to melt.
8.6. Mechanical Properties
In general, the mechanical properties of hydrates are comparable to those of ice. In the absence of additional information, it is safe to assume that the mechanical properties of the hydrate equal those of ice.
One should not assume that hydrates are soft, slushy material. Hydrate blocks can be as hard as ice. When projected from a pipe under high velocity they can do significant damage.
8.7. Volume of Gas in Hydrate
The purpose of this section is to demonstrate the volume of gas encaged in a hydrate. For the purposes of this section, we will examine only the methane hydrate.
The following are the properties of the methane hydrate at 0
°C: the density is 913
kg/m
3, the molar mass (molecular weight) is 17.74
kg/kmol, and methane concentration is 14.1
mol percent —this means there are 141 molecules of methane per 859 molecules of water in the methane hydrate. The density and the molar mass are from earlier in this chapter and the concentration is from
Chapter 2.
This information can be used to determine the volume of gas in the methane hydrate. From the density, 1 m3 of hydrate has a mass of 913 kg. Converting this to moles, 913/17.74 = 51.45 kmol of hydrate, of which 7.257 kmol are methane.
The ideal gas law can be used to calculate the volume of gas when expanded to standard conditions (15 °C and 1 atm or 101.325 kPa)
V=nRT/P=(7.257)(8.314)(15+273)/101.325=171.5Sm3
Therefore, 1 m3 of hydrate contains about 170 Sm3 of methane gas.
Or, in American Engineering Units, this converts to 1 ft3 of hydrate contains 170 SCF of gas—not a difficult conversion. And 1 ft3 of hydrate weighs about 14.6 lb, so 1 lb of hydrate contains 11.6 SCF of methane.
By comparison, 1 m3 of liquid methane (at its boiling point 111.7 K or −161.5 °C) contains 26.33 kmol, which converts to 622 m3 of gas at standard conditions.
Alternatively, 1
m
3 compressed methane at 7
MPa and 300
K (27
°C) (1015
psia and 80
°F) contains 3.15
kmol or 74.4
Sm
3 of methane gas. The properties of pure methane are from
Wagner and de Reuck (1996).
To look at this another way, to store 25,000 Sm3 (0.88 MMSCF) of methane requires about 150 m3 (5300 ft3) of hydrates. This compares with 40 m3 (1400 ft3) of liquefied methane, or 335 m3 (11,900 ft3) of compressed methane.
8.8. Ice versus Hydrate
The properties of hydrates over wide ranges of conditions are not readily available. In the absence of additional information, one might be tempted to assume that the property of the hydrate is the same as that for ice. However, as some of the examples have shown, this may lead to significant errors.
Examples
Example 8.1
Calculate the molar mass of a hydrate from an equimolar mixture of hydrogen sulfide and propane at 0
°C. This is a Type II hydrate and the saturations are given in the table below, which are from
CSMHYD.
| Feed (mol%) | Molar Mass (g/mol) | Cell 1 Satur'n (%) | Cell 2 Satur'n (%) |
| C3H8 | 50.0 | 44.094 | 0.00 | 97.26 |
| H2S | 50.0 | 34.080 | 63.33 | 1.81 |
Answer: Calculate the contributions from the propane and hydrogen sulfide first:
C3H8:[(0.0000)16+(0.9726)8]44.094=214.43
H2S:[(0.6333)16+(0.0181)8]34.080=350.26
Substitute into Eqn
(8.1):
M=136(18.015)+214.43+350.26136+(0.0000+0.6333)(16)+(0.9726+0.0181)(8)=3014.7154.06=19.56g/mol
Again, the resulting molar mass is approximately 20 g/mol.
Example 8.2
From CSMHYD, the hydrate of hydrogen sulfide, a Type I former, at 10 °C has 93.83% of the small cages occupied and 98.12% of the large ones. Calculate the density of the hydrate. Compare this with the density if it was assumed that the hydrate was 100% saturated.
ρ={46(18.015)+[(0.9383)2+(0.9812)6]34.080}(6.023×1023)(1.728×10−27)=1.050×106g/m3=1.050g/cm3
The density of this hydrate is slightly greater than that of pure water and, unlike ice, it will not float on water.
For comparison, assuming 100% saturation:
ρ={46(18.015)+[(1)2+(1)6]34.080}(6.023×1023)(1.728×10−27)=1.058×106g/m3=1.058g/cm3
This is an error of less than 1%.
Example 8.3
From CSMHYD a mixture of 50% methane, 30% ethane, and 20% propane forms a Type II hydrate at 10 °C and 1617 kPa. The saturation is given in the table below. Calculate the density of this hydrate.
| Feed (mol%) | Molar Mass (g/mol) | Cell 1 Satur'n (%) | Cell 2 Satur'n (%) |
| CH4 | 50.0 | 16.043 | 53.06 | 0.68 |
| C2H6 | 30.0 | 30.070 | 0.00 | 7.65 |
| C3H8 | 20.0 | 44.094 | 0.00 | 91.52 |
Answer: Perform the inner summations first:
CH4:[16(0.5306)+8(0.0068)]16.043=137.07
C2H6:[16(0.0000)+8(0.0765)]30.070=18.40
C3H8:[16(0.0000)+8(0.9152)]44.094=322.84
ρ=[136(18.015)+(137.07+18.40+322.84]/6.023×1023/5.178×10−27=0.939×106g/m3=0.939g/cm3
This hydrate is less dense than water.
Example 8.4
The hydrate locus for methane can be represented by the following equation:
lnP=−146.1094+0.3165T+16556.8/T
where P is in MPa and T is in K. Differentiate this equation to estimate the enthalpy of fusion for the hydrate at 0 °C. Assume that methane is an ideal gas (z = 1).
Answer: First, differentiate the expression for the hydrate locus:
ⅆlnPⅆT=0.3165−16556.8/T2
Make a small transformation of Eqn
(8.3)ⅆlnPⅆ1/T=−ΔHzR
ⅆlnPⅆT=ΔHzRT2
ΔH=zRT2(ⅆlnPⅆT)
Make the appropriate substitutions:
ΔH=zRT2(0.3165−16556.8/T2)=zR(0.3165T2−16556.8)=(1)(8.314)(0.3165[273.15]2−16556.8)=58,676J/mol=58.7kJ/mol
This is comparable to the value of 54.2
kJ/mol from
Table 8.3.
It was assumed that methane was an ideal gas. The reader should estimate the effect of this assumption on the calculated results. That is, determine the compressibility factor for methane at 0 °C and 2.60 MPa and substitute it into the above equation.
Example 8.5
Calculate the volume of propane released (at standard conditions, 60 °F and 1 atm) when 1 kg of propane hydrate is melted at 0 °F. Use the information provided in the tables in this chapter (or elsewhere in this book) and, where necessary, assume the properties are independent of temperature.
Answer: Calculate the moles of hydrate by using the molar mass from
Table 8.1 1kg/19.46kg/kmol=0.05139kmol=51.39mol
Use the composition of the hydrate from
Table 2.4 to determine what fraction of this is propane:
51.39×(5.55/100)=2.85mol
Use the ideal gas law to convert this to a volume at standard conditions (note 60 °F = 15.56 °C and 1 atm = 101.325 kPa) and R = 8.314 × 10−3 kPa m3/mol K.
V=nRT/P=(2.85)(8.314×10−3)(15.56+273.15)/101.325=67.5×10−3m3=67.5L[std]
So 1 kg of propane hydrate releases almost 70 L[std] of gas.
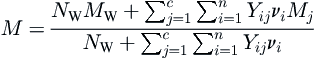 (8.1)
(8.1)
 (8.2)
(8.2)![]() (8.3)
(8.3)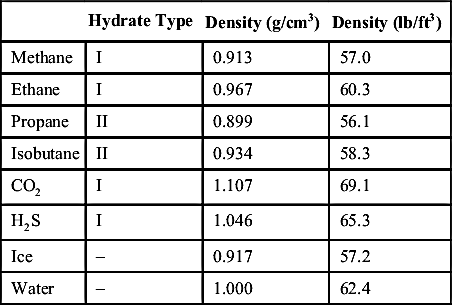
 (8.4)
(8.4) (8.5)
(8.5)